Zinc Methionine Sulfate: A Grounded Commentary on Development, Applications, and Future Pathways
Historical Development
Knowledge of trace minerals in animal nutrition took off after mid-twentieth century, and among a wave of new organic trace minerals, zinc methionine compounds landed as a practical advancement. Farmers and feed makers spent years wrestling with mineral absorption, knowing sick animals slow down entire operations. Over time, researchers noticed that combining essential amino acids with minerals led to better uptake, and methionine—an amino acid vital for protein building—earned its place. Zinc methionine sulfate grew out of efforts to sharpen absorption in livestock, translating into healthier animals and less waste. Patent filings and scientific articles started picking up in the 1970s and 80s, showing this compound could help animal production keep up with rising global demands for meat, eggs, and milk without pilling on unabsorbed zinc in manure. This direct, needs-based historical arc shows why innovation in vitamin and mineral chemistry is not just lab trivia.
Product Overview
Zinc methionine sulfate blends zinc sulfate with L-methionine in a complex that delivers zinc in a more accessible form for biological systems. Unlike raw zinc salts, this bond makes the mineral readier for transport across cellular barriers. The supplement comes in the form of whitish to off-white powder or granules. Feed premix manufacturers appreciate its handling qualities—less dust, more stability, good shelf life—though the target is always physiological value, not convenience. Product variants may differ in the ratio of methionine to zinc, a detail that helps feed specialists dial in specific dietary requirements for different livestock groups.
Physical & Chemical Properties
Zinc methionine sulfate holds together as a stable, non-hygroscopic compound under normal storage conditions. Its fine powder dissolves easily in water, which lends itself to mixing in feed or liquids. The compound resists breaking down until it passes into the animal’s digestive tract. Molecularly, the average product weighs around 337 g/mol, and its formula features zinc cation paired to methionine via sulfur and an extra bit of sulfate for stabilization. This chemical handshake unlocks both zinc and methionine at the cellular level. Color, bulk density, and solubility will shift a bit among suppliers, but the core properties serve feed, pharma, and even fertilizer industries.
Technical Specifications & Labeling
Industry analysis, including HPLC and ICP-OES techniques, confirms zinc and methionine content batch by batch. Spec sheets from reputable suppliers point unmistakably to minimum zinc concentrations—often 20% or higher—and clear lower limits for methionine. Moisture, heavy metals, and microbial contamination get attention in certificate of analysis documents. Labels state all active components by percentage, show batch numbers, and feature storage directions that keep the compound away from sunlight and humidity. For feed use, labeling must meet local regulations such as AAFCO guidelines in the U.S. and similar standards across Europe and Asia. These antic corners on labeling help keep animal industries from running afoul of trace mineral overdosing, a real concern as regulatory bodies press for more transparent, responsible feed additives.
Preparation Method
The main synthesis route involves reacting zinc sulfate heptahydrate with L-methionine in water, under neutral or slightly acidic pH. Temperature, mixing time, and purification steps can boost yield and quality. Reaction conditions strip away excess water to get the finished compound, which dries into powder or granule form. The process unites zinc and methionine through gentle heating, then precipitates out the complex, later filtered, dried, and inspected. Some producers tweak pH or throw in binding agents to improve final flow or reduce caking. No multi-step chemistry marathon here, but small adjustments in temperature or reagents separate a premium-quality product from a middling one.
Chemical Reactions & Modifications
Researchers have dug into how zinc methionine sulfate interacts in blends with other feed minerals or vitamins, and small tweaks to its molecular balance yield differences in animal uptake. For example, swapping L- for DL-methionine shifts product purity and absorption. The compound can react with strong acids or bases, but feed use rarely walks near those extremes. In aqueous solutions, it holds up well across a moderate pH range. Chemists have tried modifying the molecule with extra functional groups, though most feed and pharma uses settle on the original build for safety and regulatory simplicity. In lab work, isolating the compound has also led to slow-release formulas, aimed at reducing excretion rates in livestock and trimming mineral runoff into the environment.
Synonyms & Product Names
Technical catalogs and procurement systems list zinc methionine sulfate under several variations: zinc bis(methionine) sulfate, zinc methionine chelate, or simply Zn-MetSO4. Commercial names drift based on supplier, but nearly always include a nod to methionine or chelate for market recognition. International buyers may see it listed as 'zinc-methionine sulfate complex’ or 'organic zinc supplement’. These product names do more than fill a label—livestock nutritionists use them to trace which chelated mineral will best support a specific animal system without running up over-supplementation risks or cross-contaminating sensitive feed blends.
Safety & Operational Standards
Strict guidelines drive every batch of zinc methionine sulfate made for animal feeds or pharmaceuticals. Approved sources of raw zinc must meet industry and government standards on heavy metals and dioxins. Plant workers take care with dust control and skin contact, since zinc and methionine powders can irritate eyes and throats. Good Manufacturing Practice (GMP) sites use sealed reactors, stainless-steel tanks, and positive-pressure clean rooms to keep cross-contamination at bay. Finished products move through third-party labs for quality checks, including microbe content and purity. These controls matter: the smallest slip in safety protocols risks animal health, damages brand trust, and can cost producers their export licenses.
Application Area
The largest volumes go directly into feed mixes for livestock—poultry, swine, cattle, even aquatic species aiming for lean, fast growth with less disease loss. By providing zinc in chelated form, animal diets can push performance and fertility benefits even at lower total dosages than old-school zinc oxide or sulfate. In specialty pet nutrition, this same compound helps maintain skin, immune, and joint health in cats and dogs. Niche use cases sprawl into fertilizers designed for zinc-deficient soils as well as human supplements in markets with documented dietary zinc shortages—though regulatory hurdles require extra documentation for pharmaceutical-grade products.
Research & Development
Zinc methionine sulfate often ends up in feed trial projects and university studies, especially as producers search for sustainable ways to cut mineral waste and environmental footprint. Decades of studies find that this chelated mineral brings higher bioavailability compared with inorganic sources, with fewer side effects or residue concerns. Ongoing research tests it against emerging animal health threats, and scientists are tweaking dosing curves to minimize excretion. Silage and mixed ration studies probe for new interactions with probiotics, enzymes, or mycotoxin binders. The feed field moves fast, and data from peer-reviewed studies gets rolled into new product registrations, patent filings, and cross-continental approvals.
Toxicity Research
Toxicologists warn about the narrow path between zinc sufficiency and overload. Animals given doses well above NRC and EFSA guidelines start showing weight loss, poor feed intake, and even kidney damage—a pattern repeated in trials across swine, poultry, and lab mammals. Research from the early 2000s through today argues for careful batching of zinc methionine sulfate in feed, with safety limits tailored by species and age. Food chain researchers track any residues in meat, eggs, or milk, since regulatory agencies zero in on consumer safety. In my own experiences working with feed labs, mistake-heavy dosing leads to flagged shipments, lost income, and even government shutdowns if toxicity isn’t managed. These issues make toxicity assessment a front-line priority.
Future Prospects
Feed mills, animal welfare groups, and environmental scientists all place strong expectations on chelated minerals over the next decade. Markets expect to see zinc methionine sulfate in “precision nutrition” blends, where farms dial back total mineral load but boost growth and health outcomes through smarter chemistry. Food traceability pushes product makers to offer more digital batch verification and transparent sourcing. Tech advances will likely pull in more custom particle sizes and extended-release delivery, and increasing overlap between feed and pharma spaces will boost demand for cleaner, more tightly audited raw materials. Sustainable agriculture programs in every major producing country point to chelated zinc sources as a better way to balance animal nutrition, food safety, and environmental stewardship—so the story of zinc methionine sulfate looks set to deepen as challenges in both rural and urban settings keep evolving.
What is Zinc Methionine Sulfate used for?
A Trace Mineral Most Folks Never Think About
Zinc has always carried weight, especially for anyone who pays attention to diets, immune health, or animal nutrition. Pop into a feed store or flip over the label of some vitamins, and zinc pops up right away. Zinc methionine sulfate, though, isn’t a name most people toss around over breakfast. Still, this specific compound plays an important, if unsung, role in farming and wellness.
Bridging Zinc Deficiency Where It Hits Hard
Farmers face continuous pressure to keep animals healthy and productive, whether it’s cattle, poultry, swine, or even pets. All of them need zinc — not just any zinc, but a form their bodies can actually take up and use. That’s where zinc methionine sulfate enters the barn.
Mixing sulfur, methionine (an amino acid), and zinc creates a package that animals absorb more efficiently than basic zinc oxide or zinc sulfate. This better absorption means improved growth rates, higher fertility, strong hooves and feathers, and more robust immune response. Commercial studies find that animals fed with zinc methionine sulfate maintain stronger coats, bounce back quicker from stress or disease, and pass that vitality on to the meat, dairy, or eggs produced.
Why Not Stick With the Old Standards?
Farmers used to load up feed with cheaper, less bioavailable zinc, hoping to cover gaps. Companies could check the “zinc” box on the feed bags, and everyone crossed their fingers. Trouble is, much of that zinc washed out in waste, passed into the environment, and never benefited the animals. Some studies report that over 80% of inorganic zinc gets flushed away, meaning money and resources go straight down the drain, along with increased environmental zinc runoff.
Switching to zinc methionine sulfate puts more of those essential minerals where they belong: in muscle, immune tissue, hooves, feathers, and fur. Farmers see results in real-world performance — not just numbers on lab reports but healthy, productive animals.
Does This Matter for People?
The main demand comes from livestock nutrition, but human supplements have taken notice. Nutritionists and supplement companies try to deliver minerals in forms our bodies actually use, rather than just pass through. Some nutritional supplements for people now include zinc methionine sulfate. The idea holds up in clinical work: more available zinc seems to translate into better immune defense, faster healing, improved taste and smell senses, and better skin.
Not everyone needs this form — most with balanced, diverse diets get their quota of zinc. Yet, for those with absorption problems, vegetarians with low dietary zinc, or older adults, these chelated forms offer a helping hand.
Real-world Solutions
What really counts isn’t which label gets slapped on a bag of feed or bottle of capsules. It’s about making sure the minerals reach their target. Zinc methionine sulfate eliminates waste, saves money, and protects water from mineral runoff. For both animals and people, it promises real value: nourishment you can actually use, not just claim on a marketing label.
Looking ahead, farmers, veterinarians, and nutritionists all need practical tools that make doing the right thing cost-effective. Improved mineral bioavailability could help meet growing demands for animal health, food production, and sustainability, all at once. No magic formulas here — just a small tweak that leads to bigger gains.
Is Zinc Methionine Sulfate safe for animals and humans?
Why Zinc Methionine Sulfate Draws Attention
Zinc often turns up in the conversation when health, nutrition, and growth become priorities. Methionine, an essential amino acid, also comes up for supporting protein synthesis. Zinc methionine sulfate stands out as a combination of these two, often found in supplements for livestock and sometimes for pets or humans.
In my years feeding animals on a small family farm, trace minerals make a big difference. You see it in the shine on a cow’s coat, eggshell strength, or steady weight gain in growing pigs. Nutrition research shows zinc delivered “chelated” to methionine often absorbs better than standard inorganic zinc. That’s because the amino acid helps the mineral pass through the gut wall more efficiently. You get higher bioavailability, which means you need less of it to do the same job, and you waste less, both in feed and eventually in the environment.
What Safety Looks Like on the Farm and Table
Overuse of any mineral causes problems. Too much zinc in feed blocks copper uptake and can damage gut lining. In humans, chronic high doses cause headaches, nausea, and even lower immunity. That said, animal studies in pigs, poultry, and cattle using zinc methionine sulfate in feeds show strong safety profiles when staying inside published guidelines. The U.S. Food and Drug Administration and European Food Safety Authority have cleared zinc methionine sulfate as an additive within regulated limits. Feed guidelines keep the total zinc well below toxic levels.
For people, most supplements using zinc chelates—like zinc methionine sulfate—aim to correct deficiency or support immunity. The National Institutes of Health says the safe upper intake for adult humans sits at about 40 mg per day of elemental zinc from all sources. Most multivitamins or zinc supplements stick well below that. Short-term studies in humans using chelated zinc haven’t shown any specific dangers beyond possible stomach upset if the dose is high.
Risks Worth Watching
One real risk: minerals don’t work alone. If diets miss out on copper, iron, or other nutrients, pushing any one element—even in a premium chelated form—can create new problems. Also, with so many zinc products on the market, quality and purity differ. Contaminants or inaccurate labeling can throw off diet balance in pets, livestock, or people.
On the production side, extra zinc in feeds, no matter the source, does make its way into manure, then soil. In regions with lots of intensive animal production, regulators monitor soil and water so zinc runoff does not pile up. Good practice means dosing only what’s needed—no “just to be safe” oversupplementing.
How to Improve Safety
Smart dosing based on actual need helps a lot. For farmers, regular feed and soil analysis avoids both deficiency and overload using zinc methionine sulfate. In homes, reading supplement labels and talking with a doctor or nutritionist keeps zinc intake safe and effective. Quality sourcing matters: buying from trusted suppliers with batch testing cuts contamination risks.
Feeding trials, field data, and years of real-world use support the safe use of zinc methionine sulfate for both animals and people—if used thoughtfully, with eyes open to both benefits and limits. Balancing good science, common-sense farm knowledge, and keeping an eye out for environmental impact give everyone the best chance at good health.
What is the recommended dosage of Zinc Methionine Sulfate?
Getting the Amount Right Matters
Zinc methionine sulfate brings together zinc and methionine, an amino acid, into a single supplement meant to boost absorption and, in turn, impact health. Anyone working in animal nutrition or concerned with mineral intake can’t ignore how dosage shapes real-world outcomes. Underdosing leads to little benefit; overdosing can risk harm. Getting that balance right takes knowledge, practical judgment, and zero guesswork.
Guidelines Make All the Difference
Regular zinc needs depend on species, age, and the stressors they face. For poultry, swine, and cattle, nutritionists draw from long-term studies and regulatory guidance. The National Research Council suggests daily zinc intake in the ballpark of 40-50 mg per kilogram of feed for poultry, 50-100 mg for swine, and up to 100 mg for cattle. Because organic zinc, like zinc methionine sulfate, tends to absorb more efficiently than inorganic options, suggested inclusion rates often drop by 30-50%. Most feed professionals land somewhere around 20-40 mg zinc from zinc methionine sulfate per kilogram of feed for livestock.
On a personal note, overseeing feed mixing for a local dairy operation showed me how tiny changes in mineral dosing ripple across productivity and health. Correcting a minor deficiency led to stronger hoof health and more robust immune response. On the flipside, a careless boost above guidelines didn’t speed up gains, but it raised costs and created regulatory flags around zinc runoff.
Zinc Balance in Human Supplements
In human nutrition, zinc supplementation helps immune support and can correct deficiencies linked to dietary gaps. Zinc methionine sulfate offers higher bioavailability, which means people need less to get the same punch compared with zinc oxide or zinc sulfate. The typical adult recommendation sits around 8-11 mg elemental zinc per day. Specialist advice often tilts toward 15-30 mg for short bursts in cases of moderate deficiency, rarely more unless guided by physician oversight. Exceeding 40 mg per day for extended periods slides into territory known for triggering nausea and reducing copper absorption—a real risk in my experience coaching athletes who assumed “more is better.”
Why Getting Dose From a Professional Counts
A vet or doctor can read a blood test and spot mineral levels out of whack. They account for things like dietary intake, environmental loss, and breed-specific needs—a feat no label can manage solo. With experience in animal care, I’ve seen how easily copper deficiencies sneak up in herds dosed too high with zinc. Humans face similar risks, especially older adults on multi-mineral regimens without supervision.
Zinc methionine sulfate isn’t magic. Too little wastes time and resources. Too much undermines health in both subtle and obvious ways. Success means sticking with science-backed recommendations, talking regularly with professionals, and tracking outcomes instead of relying only on package instructions or trends.
Practical Steps to Safe Use
Read the feed or supplement label and spot the “elemental zinc” content, not just zinc methionine sulfate totals. Line up these numbers with official nutrition guidelines. Keep copper and other trace minerals in mind—balance matters more than a single number. Anyone increasing zinc above routine levels for disease prevention or response ought to set a time limit and schedule a health check-in, not just trust their gut.
Small changes in mineral management, whether for a farm or a household, add up—showing respect for research and real-world results always pays off.
Are there any side effects of Zinc Methionine Sulfate?
Why Consider Zinc Methionine Sulfate?
Zinc sits high on the list of minerals the body can’t ignore. Stress, lousy diets, and certain conditions can drag zinc levels down. Supplement companies have thrown lots of zinc products at the shelves, and one that keeps popping up is zinc methionine sulfate. Some folks claim this form helps the body use zinc better compared to older, basic blends. It's become common in animal feeds, and popping up in human supplements too.
Digestive Hiccups and Tummy Troubles
Anybody who’s messed with zinc pills probably remembers that nausea. Zinc methionine sulfate can bring the same queasiness, and some people get hit with diarrhea or a metallic taste in their mouth. Taking a dose right before breakfast never sat well for me—instead, I picked a meal with decent protein, and that usually cut out the worst stomach grumbles. More is not always better. The NIH sets the adult upper limit for zinc at 40 mg a day from supplements, not counting food. Stack up too much, and the harsh lesson is usually gut pain and running to the bathroom.
Zinc Overload: More Than Just an Upset Stomach
Heavy-handed doses of zinc methionine sulfate can knock other minerals out of balance. Copper, for example, stops getting absorbed well as zinc piles up. I’ve seen people taking extra zinc for their immune system and ending up with low copper status—leaving them just as tired and weak as before. Throwing one mineral sky-high often sets off a domino effect. Lab results from folks who overdo zinc sometimes show low HDL cholesterol or even anemia after a string of months.
Sensitivity and Allergic Response
I’ve bumped into rare stories where someone got a rash or hives from supplements with this zinc compound, usually mixed with methionine. Not everyone with a sulfur sensitivity reacts, but those who do know they have to check ingredient labels twice. Businesses sometimes cut corners by tossing out extra fillers, which could also spark a reaction.
Who Should Avoid Zinc Methionine Sulfate?
People with kidney issues don’t clear out minerals as fast as others. Any supplement can build up, including this one. Before adding it to a regimen, folks with kidney disease have to talk to a real professional, not just a clerk at a supplement shop. Pregnant or breastfeeding people—and those taking meds for autoimmune problems or blood pressure—should get the green light from their health care provider, since mixing zinc with certain drugs can block absorption or mess with the gut in odd ways.
Smart Sourcing and Smarter Dosing
Reading labels like a hawk pays off. Reputable supplement brands show third-party testing, share their certificate of analysis, and explain how much elemental zinc comes in each dose. People don’t need twelve different forms of zinc in one tablet, just one that fits the need without risking overload.
Solutions for Safer Use
Doctors and registered dietitians don’t just toss supplements out based on guesses—they run labs, and recommend zinc only if there’s a real shortfall. A food-first attitude pays off: meats, seeds, and beans are steady sources of zinc with less risk of things going sideways. Using supplements for a short window, cutting back as soon as symptoms improve and keeping regular tabs on lab results, helps head off side effects that creep up over time.
How should Zinc Methionine Sulfate be stored?
Safe Storage Starts With Reliable Conditions
Storing zinc methionine sulfate seems simple—keep it dry, keep it cool. Still, anyone who's dealt with ingredients in agriculture or supplements knows it’s rarely that straightforward. Moisture comes out of nowhere. Dang humidity creeps into warehouses. I've seen entire batches clump together just because someone left the storeroom window cracked open. Once the moisture gets in, you get caking, lost potency, and sometimes ruined feed blends.
Best practice comes down to consistency. Most technical guidelines for storage recommend a cool, well-ventilated space, ideally between 2°C and 25°C. That’s not just a technicality. Higher temperatures mean faster breakdown, especially if the product sits in hot climates or direct sunlight. Even well-packaged micronutrient blends like zinc methionine sulfate can react over time if exposed to sunlight or heat. I remember an operator once left pallets next to a sun-facing bay door. A week later, the outer layers showed visible product deteriorations. That costs money and trust.
Packing and Prevention
All sorts of packaging options show up on the warehouse floor—multi-layered bags, lined drums, sealed containers. The real trick is picking a container that’s not just “moisture-proof,” but one that actually holds up when handled repeatedly. Rough loading, stacking and shifting, even forklift scrapes: every weak seam is an open invitation for humidity. In my experience, once you switch to bags with proper vapor barriers, you cut out half your storage headaches.
Labeling each batch ensures nobody’s guessing what's inside, especially if the container ever gets separated from the pallet or shipping documents. Simple, clear labeling with product name, lot number, and expiry date prevents accidental mixing or dispensing. Even though zinc methionine sulfate keeps pretty well, shelf-life matters. Most suppliers suggest using the product within two years of delivery. Products past expiration might show changes in texture or lose effectiveness, which undercuts the point of trace minerals in nutrition in the first place.
Cleanliness Matters—A Lot More Than People Think
It’s easy to overlook storage cleanliness, but contamination is real. Dust, old feed bits, chemical spills, even pest droppings: every contaminant is a problem waiting to happen. A dirty warehouse breeds more issues than it solves. One overlooked corner turns into a magnet for rodents or bugs, which can chew through packaging or deposit filth. Diluted or contaminated product undermines the reasons for supplementing in the first place.
I’ve seen warehouses that log cleaning and check for signs of mold or pests at least every month—those facilities always fare better long-term. Even quick sweeps and pallet rotations push older stock forward so you’re not left with forgotten product wasting away in the back.
Quick Response to Spills and Exposure
Accidents happen, but quick cleanup stops trouble from spreading. Zinc methionine sulfate powder isn’t toxic the way pesticides are, but inhaling dust isn’t good for anyone. Workers wearing gloves and sometimes masks when sweeping up spills avoid chronic exposure. Floor spills can leach into warehouse concrete and leave stains, or worse, attract water and start clumping.
In my years of managing storerooms, the best setups have spill kits within reach, staff trained to use them, and a written procedure taped near the door—with operational checklists that actually get checked. Staff know that fast response means less risk of batch rejection or product recalls. It's not just about ticking regulatory boxes—it protects bottom lines and reputations.
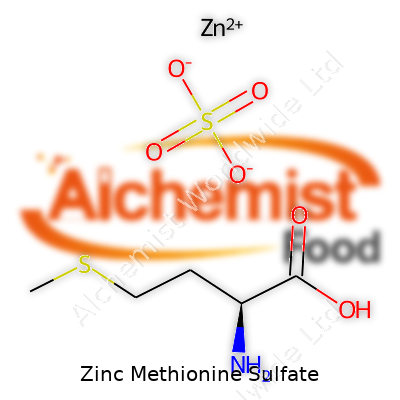
| Names | |
| Preferred IUPAC name | zinc;2-amino-4-(methylsulfanyl)butanoic acid;sulfuric acid |
| Other names |
Zinc bis(methionine) sulfate Zinc(II) methionine sulfate Zinc methionine chelate Zinc methionine complex Zinc methionine sulfate complex |
| Pronunciation | /ˈzɪŋk mɛˈθaɪəniːn ˈsʌl.feɪt/ |
| Preferred IUPAC name | zinc;2-amino-4-(methylsulfanyl)butanoate;sulfate |
| Other names |
Zinc bis(methionine) sulfate Zinc methionine sulphate Zinc methionyl sulfate Zinc methionine complex Zinc methionine chelate |
| Pronunciation | /ˈzɪŋk mɛˈθaɪəniːn ˈsʌlfeɪt/ |
| Identifiers | |
| CAS Number | 56329-42-1 |
| Beilstein Reference | 3921771 |
| ChEBI | CHEBI:131143 |
| ChEMBL | CHEMBL2104078 |
| ChemSpider | 22585773 |
| DrugBank | DB11445 |
| ECHA InfoCard | 14c12b43-435b-4313-87aa-823be87f5e02 |
| EC Number | 6.1.1 |
| Gmelin Reference | 786827 |
| KEGG | C17218 |
| MeSH | D000397 |
| PubChem CID | 101977660 |
| RTECS number | XN8572000 |
| UNII | Z2T19YZD7M |
| UN number | UN3077 |
| CAS Number | 56329-42-1 |
| Beilstein Reference | 4162722 |
| ChEBI | CHEBI:131142 |
| ChEMBL | CHEMBL2104057 |
| ChemSpider | 21541840 |
| DrugBank | DB14504 |
| ECHA InfoCard | 03d9f8a2-ecf5-435d-96e9-7e42407440ea |
| EC Number | 6.10.1 |
| Gmelin Reference | 841208 |
| KEGG | C22345 |
| MeSH | D013599 |
| PubChem CID | 16212014 |
| RTECS number | ZE5950000 |
| UNII | TCY6W365FV |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID5040156 |
| Properties | |
| Chemical formula | C10H22N2O8S2Zn |
| Molar mass | 337.66 g/mol |
| Appearance | White to off-white powder |
| Odor | Characteristic |
| Density | ~0.6 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.86 |
| Basicity (pKb) | 8.88 |
| Dipole moment | 2.82 D |
| Chemical formula | C10H22N2O8S2Zn |
| Molar mass | 337.66 g/mol |
| Appearance | White to off-white powder |
| Odor | faint odor |
| Density | Bulk density: 58 lb/ft³ (0.93 g/cm³) |
| Solubility in water | Soluble in water |
| log P | -2.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.1 |
| Basicity (pKb) | 8.20 |
| Dipole moment | 3.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 267.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1320.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | Std enthalpy of combustion (ΔcH⦵298) of Zinc Methionine Sulfate: "-2541 kJ/mol |
| Std molar entropy (S⦵298) | 356.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1326.7 kJ/mol |
| Pharmacology | |
| ATC code | A12CB05 |
| ATC code | QA07XC91 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, Exclamation mark |
| Pictograms | Acute Tox. 4, Eye Dam. 1, Aquatic Acute 1, Aquatic Chronic 1 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P270, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P332+P313, P337+P313, P362+P364, P403+P233, P405, P501. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 Oral Rat: > 5,000 mg/kg |
| LD50 (median dose) | > 2,000 mg/kg (rat, oral) |
| PEL (Permissible) | PEL (Permissible)": "Not established |
| REL (Recommended) | 21 mg |
| IDLH (Immediate danger) | Not established |
| Main hazards | Harmful if swallowed, causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid breathing dust. Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Lethal dose or concentration | LD50 Oral Rat: > 2,000 mg/kg |
| LD50 (median dose) | > 2,688 mg/kg (rat, oral) |
| PEL (Permissible) | 5 mg/m³ |
| REL (Recommended) | 30 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Zinc sulfate Methionine Zinc methionine Zinc gluconate Zinc oxide Zinc aspartate Zinc chelate Zinc monomethionine |
| Related compounds |
Zinc sulfate Methionine Zinc methionine Zinc gluconate Zinc oxide |

