Zinc Lactate Trihydrate: A Practical Look from Discovery to Future Potential
Historical Development of Zinc Lactate Trihydrate
Zinc compounds have floated through the stories of human health for centuries. From ancient ointments meant to soothe rashes to industrial breakthroughs, zinc always finds its way into the conversation. Chemists first took a real interest in zinc salts in the 1800s, but Zinc Lactate Trihydrate came into broader recognition when nutrition research boomed in the twentieth century. Somewhere between the push for better food fortification and surging curiosity about bioavailable mineral forms, zinc lactate earned a spot in labs and product catalogs. I remember reading early nutrition journals stacked with trial data showing how this form does a better job entering the bloodstream, compared to some older zinc salts. Industry soon realized the potential for using the trihydrate for precise dosing and formulation, spurred on by these early findings.
Product Overview
This compound provides a source of both zinc and lactate, carried together with three water molecules per formula unit. It’s often found as a white to off-white crystalline powder, dissolving quickly in water and giving off a faintly tart flavor. Zinc Lactate Trihydrate isn’t a kitchen-table ingredient, but walk into supplement production, fortified beverage filling lines, or labs focused on food preservation and you’ll see bags of it stacked on pallets and carefully measured out for dosing. FDA regulations encourage looking for Generally Recognized As Safe (GRAS) status in these compounds, and most suppliers make a point of providing purity reports right alongside the product.
Physical & Chemical Properties
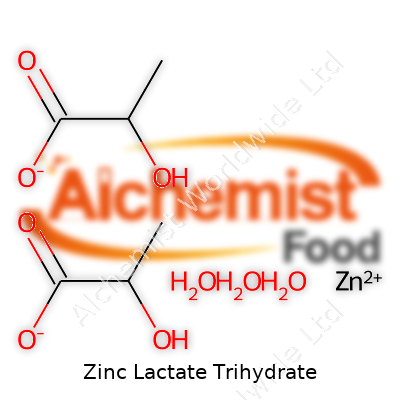
Holding the chemical formula C6H10O6Zn·3H2O, Zinc Lactate Trihydrate brings along a mildly acidic profile. The trihydrate form means stability wins over dry, anhydrous alternatives—water picks up some slack in maintaining its state even during rough storage or processing. The powder dissolves rapidly in water, breaking down into zinc ions and lactate ions without fuss, which makes it easy to incorporate into liquid formulations. Melting under high heat, decomposing instead of boiling, it steers clear of flammability and doesn’t give off toxic fumes in regular settings. Density sits around 2.1 g/cm³, and molecular weight hits 297.53 g/mol with the included trihydrate.
Technical Specifications & Labeling
Manufacturers usually demand high purity—often over 98%—with low levels of heavy metals. For markets in North America, product lots carry certifications referencing USP or FCC standards, detailing limits for lead, arsenic, and cadmium. On product packaging, you’ll find “Zinc Lactate Trihydrate,” its CAS number (6106-47-4), and the full structural formula. Regulations in the EU and US both want clear allergen labeling when a mineral salt comes from a natural fermentation route that might risk exposure to milk proteins or other allergens, though pure synthesis steers clear of this.
Preparation Method
Most zinc lactate trihydrate on the market comes from neutralizing lactic acid—often derived from sugar fermentation—using high-purity zinc oxide or zinc carbonate. Chemists add zinc oxide in small increments to lactic acid with stirring under controlled pH and temperature. The solution then forms a white precipitate as it cools. Filtration follows, then careful drying to keep the trihydrate’s water molecules intact. These steps demand close monitoring. Too much heat, and the trihydrate sheds water and becomes sticky or clumpy—most facilities use vacuum drying or gentle, low-heat tray drying to finish the product and preserve a free-flowing texture.
Chemical Reactions & Modifications
The chemistry opens paths for several modifications. In solution, the salt splits easily into free zinc ions and lactate. Reacting with strong acids, the compound releases lactic acid and soluble zinc salts, which can support secondary synthesis routes. Reagents with strong complexing abilities, such as EDTA, can yank the zinc ions out of solution. Food scientists sometimes react zinc lactate with phosphate sources to craft mixed salts for enhanced stability or improved taste in specialized applications. Pure zinc lactate itself rarely acts aggressively, but it sits at a useful crossroads—blending zinc nutrition with the preservative effects of lactic acid.
Synonyms & Product Names
On paperwork, you might see “zinc bis(2-hydroxypropanoate) trihydrate,” “lactic acid zinc salt trihydrate,” or simply “zinc salt of lactic acid.” The world of supplements might list “zinc lactate” as the only name, though the trihydrate part usually matters a great deal for technical purity and label accuracy. I’ve seen ingredient decks in the EU reference E number E325, though this actually points to sodium lactate; mistakes happen, so checking technical sheets remains crucial.
Safety & Operational Standards
Handling practices for Zinc Lactate Trihydrate aim to reduce inhalation and accidental ingestion during production. The compound doesn’t pose the acute dangers of some more reactive zinc salts, but safe standards lay out gloves, masks, and ventilation during weighing and transferring. Dust can irritate the eyes or nose if handled carelessly in large volumes—especially in dry conditions. Food-grade production lines run with seals and controls that limit cross-contamination and batch mix-ups. Engineering controls such as dust extraction, air flow, and closed bag systems all rank higher in importance than personal protective gear alone. Routine training reminds staff about risks from long-term exposure and sets up good hygiene, reinforcing that all mineral salts deserve respect in the workplace.
Application Area
Look at the shelves in nutrition stores, and you’ll spot Zinc Lactate Trihydrate in child and adult multivitamins, often featured for better absorption. Beyond supplements, the food world uses it for fortifying juices, dairy products, breakfast bars, and even pasta. Personal care companies blend it into toothpaste, where zinc supports oral health, especially when combined with lactate for added flavor control and mild antibacterial effect. In animal feed, it serves livestock and domestic pets, picked for its digestibility compared to some tougher zinc compounds. The pharmaceutical sector sees it in mineral tablets and chewables, leaning toward its mild taste and dependable shelf-stability. My own use in the lab revolved around solution preparation for bioavailability tests, where the predictable dissolving quality saved both time and measurement headaches.
Research & Development
Modern research investigates how Zinc Lactate Trihydrate might influence immune response, especially given the growing interest in trace mineral supplementation to support overall health. Clinical studies dish out comparisons between various zinc sources, and the lactate form often shows promise in reducing gastric upset—a persistent problem when taking zinc sulfate or chloride. Food scientists continue to look at new beverage formulations, using zinc lactate for clarity and solubility in flavored drinks. Other projects dig into the use of lactate ions in oral care, where the pairing with zinc seems to dampen oral bacteria better than each alone. In animal studies, teams track absorption in different species, exploring whether the trihydrate form supports better zinc retention in the body.
Toxicity Research
Toxicology reports on Zinc Lactate Trihydrate consistently note that zinc remains both essential and potentially risky if overconsumed. Acute toxicity in humans usually requires large, repeated doses—levels not normally encountered in everyday fortified foods or supplements. Side effects from excessive intake include nausea, reduced copper absorption, and disruption of the gut lining. Regulatory bodies in Europe and North America set upper daily intake limits, reflecting broad data on chronic dosing. Animal models show similar trends, though younger animals and sensitive species develop symptoms quicker. Food safety panels stress proper dosing and labeling, particularly for kid-focused products. Inhalation or skin contact issues generally stay limited to bulk handling—hobbyists and household users rarely encounter real risks from exposure at home outside of accidental ingestion.
Future Prospects
Demand for Zinc Lactate Trihydrate rises alongside the broader push for trace mineral fortification in global food systems. As more markets ask for clean-label, well-absorbed zinc sources, the trihydrate’s position strengthens. Research keeps widening the base of uses, particularly as people seek immune support and improved oral and gut health. Controlled delivery forms—such as slow-release capsules or stable beverage blends—are already in the pipeline in response to consumer demand for convenience and taste masking. Advances in fermentation and purification could lower production costs, making the compound more accessible in developing regions. Across all these developments, the dialogue between safety, effectiveness, and practicality drives the most promising innovations, drawing from a wide base of old-school chemistry and new-school biological insight.
What are the main uses of Zinc Lactate Trihydrate?
Supporting Our Diet: Zinc in Food and Supplements
Walk down any pharmacy aisle, and you'll spot bottles promising to boost immunity and keep your body working its best. Many of those blends rely on zinc, and zinc lactate trihydrate often serves as the form tucked into those blends. This zinc compound comes with high bioavailability, meaning our bodies take up the mineral more easily. People who don’t get enough zinc through diet—think vegetarians, some pregnant women, or older adults—benefit from having it in supplement pills, tablets, or even in fortified foods like breakfast cereal and nutrition bars. Scientists have shown that zinc helps power the enzymes in our cells, shapes our immune response, and keeps skin and hair in good shape. Using zinc lactate trihydrate helps manufacturers meet daily nutritional requirements without wrestling with bitter flavors or gritty textures.
Oral Care: Cleaner Teeth, Fresher Breath
Flip your toothpaste box and the ingredient list might reveal a small but important addition: zinc lactate trihydrate. It’s a favorite in oral care for a good reason. This form of zinc kicks in to keep dental hygiene moving in the right direction. Research highlights how zinc in this form plays a role in neutralizing odors caused by bacteria, so you’re not just covering up bad breath—you’re weakening what causes it in the first place. It also works to reduce plaque buildup and helps slow down how quickly tartar forms on your teeth. Dental professionals care about what goes into care products, and zinc lactate trihydrate wins points for being gentle without skipping its punch against mouth bacteria.
Personal Care and Topical Relief
Think of lotions and skin creams promising to soothe irritation or boost skin recovery. Many turn to zinc as a key ingredient, but zinc lactate trihydrate stands out since it dissolves well, brings a mild feeling to formulas, and doesn’t leave a chalky film. It provides zinc in a form that skin can use, which proves crucial in calming minor irritations and supporting natural healing. Some deodorants and antiperspirants use it too, taking advantage of zinc's ability to calm sweat and neutralize odor. It’s impressive how one compound fits into so many bottles across the shelf—each product using zinc’s age-old benefits in new ways.
Preserving Products Safely
Anyone who remembers throwing out expensive lotions that turned sour in the cabinet knows how frustrating a short shelf life can be. Zinc lactate trihydrate also gets credit for helping stave off spoilage in some personal care and cosmetic products. Zinc has known antimicrobial properties, discouraging bacteria or fungi from growing in creams and solutions. This lets producers skip harsher preservatives that sometimes irritate sensitive skin. I’ve seen brands use this as a selling point, especially for customers who check ingredient lists for natural-sounding solutions.
Zinc's Role in Industry
Far outside health aisles and bathrooms, zinc lactate trihydrate crops up as a catalyst or stabilizer in chemical processes. Research labs working on greener technologies often experiment with zinc compounds to purify water or support sustainable reactions. Some companies add it as a source of zinc in animal nutrition, since livestock—like humans—can run low if feed isn’t fortified. The bottom line: wherever zinc’s unique traits matter, experts find creative ways to tap into what zinc lactate trihydrate offers, whether making health products, keeping skin safe, or building better industrial processes.
Is Zinc Lactate Trihydrate safe for human consumption?
Understanding Zinc Lactate Trihydrate
Most folks recognize zinc as an essential mineral that keeps the immune system running. Zinc lactate trihydrate sits in a quieter corner, working as a zinc supplement in food and medicine. It's made by binding zinc to lactic acid, which helps the body absorb it better than straight zinc oxide or zinc sulfate.
The Science Behind Safety
More research has ended up confirming that zinc itself is crucial for enzyme function, growth, and immune defense. A shortage brings real trouble: stunted growth, slow wound healing, and frequent illnesses. Food makers use zinc salts like zinc lactate trihydrate to fortify cereals, drinks, and lozenges. Past studies and regulatory checks suggest this form of zinc plays it safe, as long as intake stays under the adult upper limit of 40 milligrams of elemental zinc per day, as set by the U.S. National Institutes of Health.
Still, too much zinc triggers problems—nausea, stomach pain, and lowered copper absorption. Regulatory agencies like the European Food Safety Authority and U.S. Food and Drug Administration keep close watch, demanding evidence to back up any claim about supplements or additives. Those groups accept zinc lactate trihydrate as a source of zinc in foods, provided companies respect labeling laws and recommended dietary limits.
What Matters for Safety
Labels tell the story. Products listing zinc should reveal the amount in milligrams. For anyone facing a medical condition or popping multiple supplements, a check-in with a healthcare professional clears up any confusion. Zinc can interact with certain antibiotics and drugs for autoimmune diseases. Some doctors recommend taking zinc supplements a few hours before or after those meds.
The quality of supplements also matters. The supplement market sits under less regulatory scrutiny than prescription drugs. Quality varies between brands. Third-party certification seals from groups like NSF International or USP mean that a product contains what's listed and no more.
Transparency and Trust
One way to build consumer trust is making ingredient sources and lab testing public. Some supplement companies already step up by posting lab certificates on their sites. Reading ingredient lists, understanding recommended daily zinc intake, and knowing the upper safety limits go a long way in protecting health.
Room for Improvement and Oversight
Mislabeling, contamination, and unclear dosing instructions have surfaced across the global supplement industry. Better oversight, stricter manufacturing practices, and regular lab checks could fix a lot of these problems. Retailers can play their part as well, stocking products from brands committed to transparency.
Public health education creates a smarter consumer base. People who learn about mineral supplementation guidelines for their age and health status make safer choices. Doctors, nutritionists, and pharmacists should feel confident talking through supplement questions with patients.
Looking Ahead
Supplements like zinc lactate trihydrate can boost diets low in zinc. Choosing certified products, reading labels, and chatting with health professionals will clear up confusion and keep things safe.
What is the recommended dosage for Zinc Lactate Trihydrate?
Understanding Zinc Lactate Trihydrate
Zinc plays a big role in how the body works. From wound healing to supporting the immune system, the benefits stack up. Zinc lactate trihydrate is one form doctors and supplement makers use for its high solubility and how well the body absorbs it. Not all zinc sources go down smoothly, but in my experience seeing product breakdowns, this one stands out for absorption.
How Much Zinc Lactate Trihydrate Does the Body Require?
The daily zinc requirement for adults sits in the range of 8 mg for women and 11 mg for men, as established by the National Institutes of Health. Zinc lactate trihydrate is about 22% elemental zinc by weight. If you’re aiming for those daily numbers, each 45 mg serving of zinc lactate trihydrate provides around 10 mg of usable zinc. That calculation matters. Most supplement bottles miss this detail, leaving many surprised to find they’re taking less than they thought.
Many factors shape how much zinc a person should take: diet, age, health status, and other supplements all count. For example, vegetarians can need up to 50% more due to plant compounds interfering with how the body takes in zinc. Athletes push their bodies, sweating out minerals, so their needs may go up. There’s no straightforward one-size-fits-all number.
Risks Tied to Too Much Zinc Intake
Zinc can do harm in high doses. Take over 40 mg of elemental zinc a day for a stretch, and you may see side effects—nausea, digestive trouble, headache. Exceeding this for weeks can knock out your copper levels or weaken immune defenses, which feels like getting hit by what you’re trying to prevent. It’s easy to load up on hidden sources: multivitamins, fortified foods, cold remedies. A person really has to keep tabs on everything they take.
From what I’ve seen, many feel tempted to mega-dose at the first sign of a cold, chasing a promise of faster recovery. Research paints a murky picture here. Some trials suggest a mild benefit, especially if started early, but there’s a fine line before the risk outweighs any possible reward.
Best Practices for Taking Zinc Lactate Trihydrate
Doctors and nutritionists advise taking zinc with food if it upsets your stomach, though absorption goes up on an empty stomach. Citrus or fruit juice can help the body grab on to zinc more efficiently, while coffee or high-fiber foods slow it down.
Spreading zinc supplements out—say, two smaller doses instead of one big gulp—also curbs side effects and keeps things steady. It’s something those who’ve navigated sensitivity to minerals like magnesium will recognize. Reading ingredient labels matters. Checking all your supplements and foods for hidden zinc sources avoids stacking doses unintentionally.
Your best bet? Talk to a licensed healthcare provider before adding zinc lactate trihydrate, especially for kids, pregnant people, or those who manage chronic health concerns. A bit of blood work or a nutrition check-in can often shine a light on whether extra zinc will help—or if you’re already set.
Looking Ahead
Finding a healthy, balanced way to use zinc comes down to knowing the numbers, respecting safe limits, and taking a careful look at the bigger nutritional picture. Science still studies the long-term use of zinc supplements, and the right dose is one that pays attention to personal health history and ongoing needs.
Are there any side effects associated with Zinc Lactate Trihydrate?
A Look Beyond the Label
Zinc sits among the essential minerals every human body counts on. Plenty of people turn to zinc supplements, sometimes on a doctor’s advice and sometimes out of pure curiosity about boosting health. Among the different zinc compounds showing up in supplements, zinc lactate trihydrate stands out for how easily your body absorbs it. This molecule pairs zinc with lactic acid, drawing attention for its gentler impact on the stomach compared with other forms.
Side Effects You Might Notice
If you’ve ever taken a multivitamin with zinc, you’ve probably run into the warnings printed on the bottle. Stomach discomfort, queasiness, or a metallic taste in your mouth top the list of complaints. In my experience, taking any zinc tablet on an empty stomach practically guarantees a bout of nausea. Zinc lactate trihydrate causes fewer stomach issues than zinc sulfate or gluconate, but it’s no magic bullet against digestive upset for everyone.
Medical reports, including reviews published by the National Institutes of Health, count these as common short-term side effects:
- Nausea and stomach ache
- Vomiting
- Diarrhea
- Headache
- Metallic taste in the mouth
If you stick to suggested doses, these side effects stay mild or pass quickly. Overdoing any zinc supplement lays out different risks. A friend of mine, convinced zinc would block every cold, kept popping tablets above the daily limit and paid for it with days of upset stomach and brain fog. Doctors say taking too much zinc (more than the recommended upper limit of 40 mg for adults) can interfere with how your body absorbs copper. That can drag down your immune system over time, leaving you open to more sickness, not less.
Who Should Pause Before Taking Zinc Lactate Trihydrate?
Some groups do better steering clear or at least talking things over with their healthcare provider before starting any zinc source. People with kidney disease, pregnant women, and those on certain antibiotics need to think carefully about potential interactions. There’s evidence zinc can cut the strength of some prescription drugs—especially certain antibiotics and blood pressure medications. That kind of clash might sneak up if you’re not paying attention.
Hands-On Ways to Lower the Risk
You don’t always have to face these side effects. Taking zinc lactate trihydrate alongside food tones down stomach problems for most people. Chasing your supplement down with a hearty snack or meal makes a big difference. Sticking close to the recommended daily allowance (about 8 mg for women, 11 mg for men according to the NIH) also gives you most of the benefits without the headaches.
Reading supplement labels and checking for zinc content in other products—like cold remedies or fortified cereals—helps avoid doubling up by accident. If supplements aren’t your thing, there’s always the old-fashioned way: oysters, beef, chickpeas, and seeds pack zinc and rarely lead to trouble unless you eat them by the pound.
Why This Conversation Matters
Health trends change fast, and the shelves stay packed with new forms of the same mineral. Zinc deserves respect—too little can leave you dragging through the day, but too much brings its own set of problems. Knowledge and a good relationship with a healthcare provider go farther than jumping on the latest supplement fads. Personal experience tells me the best results come from moderation, good food, and careful reading, not quick fixes.
How should Zinc Lactate Trihydrate be stored?
Why Storage Matters for Sensitive Compounds
Zinc Lactate Trihydrate shows up in labs and production spaces where purity and effectiveness shape the whole reason for stocking it. Keep it under the right conditions, and it’ll last, doing what it’s designed for. Let it sit in poor environments, and problems creep in—degraded material, clumps, or even a product that’s suddenly no longer safe or effective to use.
Essential Environment: Clean, Cool, and Dry
From my time working with supplements and specialty chemicals, it’s clear that the biggest enemy is moisture. In my lab days, a little extra humidity always meant more fuss during quality checks. Even in a storage closet, humidity finds a way inside containers that aren’t properly sealed. Zinc Lactate Trihydrate, with its trihydrate crystals, can take up room air and change its own make-up. Humidity brings the risk of it clumping together, and sometimes even starting to break down.
Keep it in a space where temperature avoids strong swings. Room temperature—somewhere between 20–25°C (68-77°F)—tends to work. Avoiding sunlight preserves color and stops further breakdown. Even a little sunlight in the wrong spot can kick off strange reactions. Most packaging for this compound comes with opaque or amber bottles to shut out the light; I’ve always been strict about returning the container to its proper place right after use.
Best Containers and Labeling
Once, I saw a batch go bad because someone left a container slightly open for “just an hour.” It only takes a short time for air and light to cause trouble. Resealable, airtight containers, made of glass or HDPE plastic, hold up the longest. After each use, the lid should go back on right away and the bottle should be put far from sources of heat or vibration. More than once, I’ve had to point out to new technicians that chemicals don’t belong near radiators or windows.
Easy-to-read labels save hassle too. Write down the name, date received, and lot number—this way, anything out of place can be traced. In larger operations, digital tracking picks up the slack, but handwritten labels in smaller spaces keep mistakes from happening. Nobody likes tracking down the source of a contaminated sample during an audit.
Shelf Life and Regular Inspections
Fresh quality can’t be guaranteed forever. Even with good storage, time chips away at potency. Most suppliers give an expiration date or period of guaranteed quality. Set up a schedule—every couple of months, check on the appearance. Any sign of lumps, yellowing, or strange smells begs for a replacement. Too many labs hold onto products “just in case” and end up risking results or safety.
Why Careful Storage Builds Trust
Poorly kept ingredients make for unreliable work. Whether formulating a batch of supplements, preparing samples for analysis, or storing bulk ingredients in a warehouse, taking shortcuts catches up. The end user expects what’s written on the label to match what’s in the bottle. I’ve learned this lesson time and again: quality comes from the small habits day after day. Tidy shelves, labeled containers, and a habit of checking on stock—these simple actions mean the difference between a trusted final product and a return shipment or, worse, a failed regulatory inspection.
| Names | |
| Preferred IUPAC name | zinc bis(2-hydroxypropanoate) trihydrate |
| Other names |
Zinc dilactate trihydrate Zinc(II) lactate trihydrate Lactic acid zinc salt trihydrate |
| Pronunciation | /ˈzɪŋk ˈlæk.teɪt traɪˈhaɪdreɪt/ |
| Preferred IUPAC name | zinc bis(2-hydroxypropanoate) trihydrate |
| Other names |
Zinc lactate hydrate Zinc(II) lactate trihydrate Zinc(2+) 2-hydroxypropanoate trihydrate |
| Pronunciation | /ˈzɪŋk ˈlæk.teɪt traɪˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 16039-53-5 |
| Beilstein Reference | 94080 |
| ChEBI | CHEBI:91445 |
| ChEMBL | CHEMBL3304746 |
| ChemSpider | 21633603 |
| DrugBank | DB14530 |
| ECHA InfoCard | 200-682-9 |
| EC Number | 209-953-9 |
| Gmelin Reference | Gmelin Reference 54270 |
| KEGG | C14148 |
| MeSH | D014895 |
| PubChem CID | 159295 |
| RTECS number | OE9850000 |
| UNII | Z20R2PMN1P |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID7020186 |
| CAS Number | 16039-53-5 |
| Beilstein Reference | 3859161 |
| ChEBI | CHEBI:91514 |
| ChEMBL | CHEMBL3306549 |
| ChemSpider | 11699438 |
| DrugBank | DB14299 |
| ECHA InfoCard | 03aac6bc-e1a7-483b-8bbc-ff4b6162951a |
| EC Number | 209-170-2 |
| Gmelin Reference | 1661134 |
| KEGG | C15852 |
| MeSH | D015399 |
| PubChem CID | 16760761 |
| RTECS number | OJ8225000 |
| UNII | C517BQ838L |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | CompTox Dashboard (EPA) of product 'Zinc Lactate Trihydrate' is "DTXSID3054547 |
| Properties | |
| Chemical formula | C6H22O10Zn |
| Molar mass | 343.57 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | D=1.8 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.62 |
| Acidity (pKa) | 3.8 |
| Basicity (pKb) | 8.41 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.45 |
| Dipole moment | 1.87 D |
| Chemical formula | C6H10O6Zn·3H2O |
| Molar mass | 309.58 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.74 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.26 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.6 |
| Basicity (pKb) | 8.70 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 2.02 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 219.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1625.6 kJ/mol |
| Std molar entropy (S⦵298) | 253.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1676.58 kJ/mol |
| Pharmacology | |
| ATC code | A12CB06 |
| ATC code | A12CB05 |
| Hazards | |
| Main hazards | May cause irritation to eyes, skin, and respiratory tract. |
| GHS labelling | GHS07, Warning, H315, H319, H335 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: P261, P305+P351+P338, P280, P304+P340, P312 |
| Flash point | > 100 °C |
| Lethal dose or concentration | LD50 (oral, rat): 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 4,680 mg/kg (rat, oral) |
| NIOSH | Not Established |
| PEL (Permissible) | 5 mg/m³ |
| REL (Recommended) | 10 mg (as zinc) |
| IDLH (Immediate danger) | Not listed. |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, Warning, H319, P264, P280, P305+P351+P338, P337+P313 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid breathing dust. Use personal protective equipment as required. Wash thoroughly after handling. Do not eat, drink or smoke when using this product. |
| Lethal dose or concentration | LD50 (oral, rat): 1200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral >2000 mg/kg |
| NIOSH | Not Established |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 10-15 mg as Zn/day |
| IDLH (Immediate danger) | Not listed. |
| Related compounds | |
| Related compounds |
Zinc lactate Zinc gluconate Zinc sulfate Zinc acetate Zinc citrate |
| Related compounds |
Zinc lactate Zinc acetate Zinc chloride Zinc sulfate Magnesium lactate Calcium lactate |

