Zinc Lactate: A Deep Dive Into Its Value and Development
Looking Back: The Story of Zinc Lactate
Tracing the roots of zinc lactate points to the intersection of nutritional science and industrial chemistry. The journey began as food experts explored fortifying products for better health. In the early 1900s, doctors recognized the toll of zinc deficiency on human growth and immunity. Chemical manufacturers, seeing a gap, reacted zinc oxide or carbonate with lactic acid, producing zinc lactate for the first time. By the late 20th century, with the rise of fortified foods, multinationals embraced it as an accessible, bioavailable source of zinc. Its presence grew in food, pharma, and animal nutrition circles, driven by research linking zinc to everything from immune support to healthy skin. Today, zinc lactate production fits seamlessly with methods perfected decades ago, yet reflects a century’s progress in health and technology.
Product Overview
Zinc lactate shows up as a fine white to off-white powder, easy to process and dissolve. It gives manufacturers a compound that combines lactic acid’s solubility with zinc’s importance for human and animal metabolism. Its taste runs mildly astringent, a modest presence compared to other zinc salts. Production experts favor it for its reliable purity, standardized quality, and compatibility with food and supplement applications. Many recognize its digestive gentleness compared to zinc sulfate or gluconate, which makes it a smarter pick for people sensitive to digestive upsets. In labs and warehouses, it stores well when kept dry and away from direct light, with little risk of caking or degradation under normal conditions.
Physical & Chemical Properties
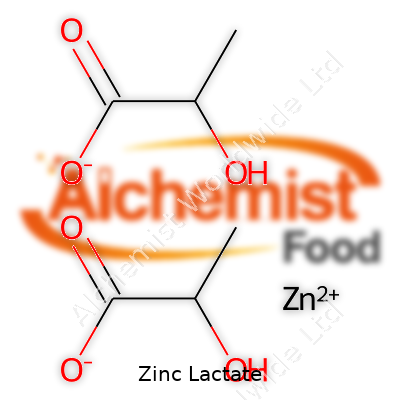
Chemically, zinc lactate takes the formula C6H10O6Zn and holds a molar mass just above 243 g/mol. It doesn’t carry a strong odor. This compound dissolves well in water, a trait that supports its use in liquids and easy absorption. At room temperature, the powder holds steady, free from clumping if protected from humidity. The pH of a 10% aqueous solution hovers near neutral to slightly acidic—usually between 6.0 and 8.0. It starts decomposing when heated past 200 degrees Celsius. Its zinc content averages 22–23% by mass, essential knowledge for food scientists and chemical engineers who rely on accurate dosing for product labeling and regulatory compliance.
Technical Specifications & Labeling
Ingredient buyers judge zinc lactate by benchmarks: zinc content minimum, purity, water solubility, and presence (or absence) of heavy metals. Pure food and pharmaceutical grades limit lead, arsenic, and cadmium to sub-ppm levels. In food and nutrition settings, companies label with precise zinc percentage, batch info, and regulatory codes such as E327, used in Europe. The FDA and EFSA scrutinize both content and labeling, demanding traceability and evidence of safety. Labeling laws require simple, clear declarations of zinc salt addition, allowing consumers and regulators to track dietary zinc intake. Certificates of analysis provide batch-specific proof of microbiological safety, chemical profile, and shelf life, often spanning two years in suitable packaging.
Preparation Method
Manufacturing plants create zinc lactate by reacting high-grade lactic acid with either zinc oxide or zinc carbonate. This neutralization process produces an aqueous slurry, which crystallizes as the solution cools. Operators filter and wash the resulting solid, then dry under gentle heat—protecting both purity and solubility. For food and pharmaceutical use, labs run additional purity checks, washing out trace impurities and filtering out insolubles. Some suppliers refine their drying process to produce a specific particle size, supporting fast dissolution in water and consistent handling during mixing. Modern plants automate controls for pH and temperature, dialing in purity and output with precision that handheld batch operations can't match.
Chemical Reactions & Modifications
Zinc lactate participates in mild reactions with both acids and bases, depending on the environment. It breaks down under strong heat, driving off lactic acid and leaving simple zinc compounds behind. That offers recycling options for process byproducts. Chemical labs sometimes tweak the lactate structure to tailor release properties in drugs, attach functional groups for research, or create novel zinc complexes for animal feed. Notably, it resists oxidation in storage and blends without unwanted side reactions, which means the zinc and lactic acid are both safely and reliably delivered. In testing with antioxidants or certain excipients, zinc lactate shows solid compatibility, making it a valuable ingredient across multiple industries.
Synonyms & Product Names
People find zinc lactate under names such as zinc(II) lactate, E327, or the systematic title zinc dilactate. Food technologists and suppliers also use “lactic acid zinc salt” or “lactate of zinc.” On food labels and in regulatory filings, the European E-number (E327) often appears, especially on packaged foods in the EU. Pharmacies and nutrition brands might market it simply as “zinc lactate” in vitamin blends. Catalogues from industrial suppliers sometimes list abbreviation ZL or CAS number 16039-53-5. This range of names sometimes leads to confusion in global trade, but each refers to the same key product.
Safety & Operational Standards
Zinc lactate earns high marks for safety in the recommended doses used in food, supplements, and pharmaceuticals. Regulatory agencies like the U.S. FDA and EFSA recognize it as GRAS (Generally Recognized As Safe), so long as production meets strict standards for contaminants. Production teams protect workers using gloves, goggles, and dust masks, as inhalation of fine powders poses irritation risk. Bulk handling areas often run ventilation systems to keep airborne dust low. In storage, sealed bags or drums keep the product dry and protected. Routine batch tests look for traces of heavy metals or microbial contamination. Staff also monitor zinc content to ensure nutritional claims line up with the published figures, sticking to local and international guidelines for food safety and occupational health.
Application Area
Nutritional supplements drive major demand for zinc lactate, reflecting the public’s hunger for better immunity, wound healing, and cognitive support. Food manufacturers add it to fortified cereals, infant formulas, beverages, and dairy products, leveraging both the zinc content and gentle flavor. In animal nutrition, feed companies use zinc lactate for piglets, poultry, and pets, especially where digestive sensitivity matters. Pharmaceutical companies draw on its solubility and mild taste to create chewable and effervescent tablets. In oral care, zinc lactate works as an antimicrobial, helping freshen breath and control bacteria. Certain cosmetics makers value it both for zinc’s skin-soothing properties and for the lactic acid component, which helps control skin pH. In specialty chemical circles, zinc lactate finds its way into buffer solutions, laboratory reagents, and topical creams where both zinc and lactic acid have recognized functional roles.
Research & Development
University labs and research firms continue digging into better ways to harness zinc lactate for wellness. Scientists compare its bioavailability to other zinc salts, finding that the lactic acid structure generally aids absorption. Innovation doesn’t stop there—chemists work to improve purity, extend shelf life, and pair zinc lactate with other trace minerals or vitamins. There’s growing interest in nano-encapsulation and micro-granule formulations, designed to slow release or protect the compound through harsh stomach conditions. Research teams constantly check for interactions in complex multivitamin blends and look for synergistic effects with other micronutrients. Some groups examine the gut microbiome, measuring whether zinc lactate supports healthy bacterial balance. The work continues around improved analytical techniques—better ways to measure zinc, lower detection limits for contaminants, and faster quality assurance methods for manufacturers facing tighter regulatory standards.
Toxicity Research
Safe dosing stands as a top concern, especially in new foods or formulas marketed to children or pregnant women. Toxicologists have studied zinc lactate’s impact in high doses, both in lab animals and human volunteers. Most studies confirm its safety within recommended daily limits, usually between 8–15 mg zinc per day for adults. At very high levels, zinc can cause gastrointestinal upset, lower copper absorption, and alter immune response. Chronic excess, rare in balanced diets, sometimes damages kidneys or triggers anemia, so regulators urge avoid excess intake. Most animal studies find that zinc lactate compares favorably to other zinc forms, producing fewer digestive complaints and rare allergic reactions. The food and supplement industries rely on these studies to guide product development and consumer advice, especially for fortified foods or medical nutrition blends where doses run higher than diet alone.
Future Prospects
Zinc lactate’s role will continue expanding as consumers seek clean-label ingredients, and as demands grow for sustainable nutrition solutions. Researchers point to fortified foods and drinks as an enduring growth area, especially in countries fighting micronutrient deficiencies. Animal nutritionists see opportunities for more specialized formulas, using zinc lactate in blends adjusted for growth, reproduction, or disease resistance. Pharmaceutical innovators keep exploring new delivery forms—think dissolvable strips, transdermal patches, or gastric-protected capsules—each opening new frontiers for zinc supplementation. Ongoing regulatory tightening, especially on heavy metal impurities, will push manufacturers to refine their processes and develop new testing methods. The ingredient’s performance in new health and wellness trends—such as immune-boosting foods, cognitive support blends, or personalized nutrition—signals a promising road ahead, fueled by decades of safe use, strong evidence, and evolving consumer needs.
What is Zinc Lactate used for?
Zinc in Plain Sight
Some compounds never get much spotlight, and zinc lactate is one of those. Yet, behind the scenes, it shapes a surprising number of everyday products. Zinc lactate shows up as a useful source of zinc that dissolves well in water and doesn’t leave a metallic aftertaste. That may sound small, but this detail earns it a ticket to the ingredient lists of toothpaste, mouthwashes, food fortifiers, and even tablets sold over the counter.
Dental Care Gets a Boost
Zinc brings more to the table than most people expect, especially inside a tube of toothpaste or bottle of mouthwash. Researchers have found zinc ions can help fight the bacteria that cause bad breath and dental plaque. Zinc lactate works here because it doesn’t taste harsh or gritty, and it’s safe for gums and teeth. It actually makes the product feel and taste better. Brushing your teeth or using a mouth rinse with zinc lactate can make a real difference on mornings when coffee breath just won’t quit.
Food and Nutrition Applications
People do not always get enough zinc from their regular diets, and that’s a harder issue than it sounds. Zinc supports the immune system, helps wounds heal, and even plays a role in growth and development in children. Some breads and cereals claim added zinc, and often, it’s zinc lactate doing the heavy lifting. Since it dissolves well, it works in foods and supplements without changing flavor too much. For families on a tight budget, fortified foods can offer a practical safety net against zinc deficiency.
Why Zinc Lactate Matters in Supplements
Not every form of a mineral is easy for the body to absorb. Some zinc salts can upset the stomach or simply pass through without being used. Zinc lactate is gentler on digestion and widely absorbed in the gut, which is probably why health brands prefer it in chewables, lozenges, and children’s syrups. For people with dietary restrictions or absorption issues, a supplement using zinc lactate can tip the balance toward better general health.
Safety and Looking Ahead
Demand for zinc-fortified products rises during cold and flu season, and food safety agencies keep a close watch on manufacturers. Consuming too much zinc, in any form, causes problems, so companies stick to strict limits. The World Health Organization and national health authorities issue clear guidance on daily intake. It’s a balancing act, and that sets a standard for trust in products found online or at the pharmacy.
Better Consumer Awareness
Many people never look at a product label twice. A little information about zinc lactate can go a long way, especially in communities where dietary gaps threaten children’s health. Health care providers, consumer groups, and teachers can help by talking simply about the benefits of basics like zinc. Food banks, schools, and clinics can work together to make sure kids and seniors get zinc-rich meals, using science-backed advice.
Small Changes, Big Differences
Supporting science, asking smarter questions, and thinking about what goes into daily meals all play a part in public health. Looking out for ingredients like zinc lactate in foods and hygiene products can help families get a little extra support for their teeth, immune systems, and overall well-being—without much extra cost or effort.
Is Zinc Lactate safe for daily consumption?
What Drives Interest in Zinc Lactate?
Zinc has always meant something more than just a mineral on nutrition labels. For people fighting off frequent colds, zinc turns into that go-to supplement you stare at in the pharmacy, wondering if it’ll finally put a halt to the sniffles. Zinc lactate, a form of zinc bound to lactic acid, appears in more health foods, dental products, and dietary supplements every year. Folks ask: can I take it daily, and what’s the catch?
Understanding Zinc in Everyday Diets
Most of us pull zinc from foods like meat, beans, nuts, and dairy. Yet, studies point out that almost a third of the world’s population doesn’t get enough zinc. I remember mornings growing up when my grandmother would practically force-feed me fish, with the firm conviction that minerals like zinc kept bones and skin strong. Modern nutritionists back her up on zinc’s value for metabolism, immune support, and healing. Zinc lactate aims to fill the gaps where diet falls short.
Zinc Lactate’s Track Record on Safety
People often ask if combining zinc and lactic acid could cause harm. Regulatory agencies like the US Food and Drug Administration recognize zinc lactate as safe for use in foods. That says a lot, as the FDA sifts through piles of research before putting anything on that list. The real question comes down to dose, and whether you plan to get all your zinc from supplements or mix them with your regular meals.
Nutritionists recommend around 8-11 mg of zinc daily for adults. A standard serving of zinc lactate supplies this requirement without going over the tolerable upper limit (around 40 mg for adults per day). Clinical studies confirm that zinc lactate absorbs well and rarely causes upset stomachs, especially compared to other zinc forms. My own experience with daily multivitamins that included zinc lactate ended up being completely uneventful — no nausea, no odd taste, just a bit more peace of mind.
Risks and Misinformation
Problems crop up when people ignore labels and think more is always better. Too much zinc can block copper absorption, weaken immunity, or leave a metallic taste in the mouth. I once saw a friend take handfuls of zinc pills every time he felt a sore throat, convinced he was building a fortress. After weeks, he suffered stomach cramps and couldn’t figure out why until his doctor pointed out the overdose. Reliable sources like the NIH repeatedly warn about these self-made mistakes.
Making Informed Choices
No one needs a PhD to understand that moderation matters. Anyone with a medical history involving the liver, kidneys, or digestive system should double-check with a healthcare provider before changing their supplement game. Reading product labels goes a long way. Not every supplement matches what it says on the bottle, so looking for third-party testing seals can give more confidence.
Food remains the most sustainable way to get your zinc, but for those who fall short, zinc lactate stands as a dependable ally. Regular use means paying attention to both the total daily intake from food and supplements. I’ve watched close friends improve their energy and immune resilience by focusing on balance, not on megadoses.
Where to Go From Here
With so many fancy supplement names and micro-nutrient trends flooding the internet, zinc lactate can get lost in the noise. It quietly does its job, as it has for years. Sticking with safe doses, checking in with doctors, and treating supplements as helpers rather than miracle cures will keep zinc lactate a safe choice for many.
What are the benefits of Zinc Lactate supplementation?
Understanding Why Zinc Matters
Zinc impacts immune performance, skin strength, and even wound healing. Grab a bottle from the pharmacy shelf and you’ll see zinc paired with all kinds of compounds. Zinc lactate deserves a second look, playing a real role in easy absorption and gentle action on the stomach. My own journey juggling stress and diet gaps got easier with the right zinc compound, and dozens of studies agree it’s more than just a trace mineral.
Bioavailability and Digestive Friendliness
Absorption defines a supplement’s usefulness. Zinc lactate enters the bloodstream more efficiently than several other forms. The presence of lactic acid makes it soluble in water and easy for the gut to recognize. A properly absorbed dose avoids two big headaches: stomach irritation and wasted potential. Gastrointestinal discomfort drives some people away from zinc sulfate or gluconate, but zinc lactate sidesteps that complaint for most folks.
Supporting Immunity Through Real Science
There’s no denying zinc’s reputation in immune defense. The science backs its ability to reduce the length and intensity of colds, with many clinical trials pointing to zinc’s direct impact on T-cell production and function. Zinc lactate stands out in these studies thanks to its better absorption rate. The CDC and other recognized organizations highlight zinc as essential during infections, especially for children and older adults.
Benefits for Skin and Oral Health
Skin, being your body’s first protective barrier, draws heavy support from zinc. Topical creams for acne and rash often rely on zinc for a reason. Supplementing with zinc lactate has shown a beneficial effect in reducing outbreaks, and some dermatologists recommend it for stubborn eczema. In oral care, zinc lactate turns up in toothpastes and mouthwashes. It controls bad breath by disrupting the sulfur compounds produced by oral bacteria, and research shows it can reduce plaque formation.
Tackling Zinc Deficiency in Modern Diets
Fast food and restricted eating patterns can strip away trace minerals. The World Health Organization highlights zinc deficiency as a global concern, leading to sluggishness, poor wound healing, and increased infections. Vegetarian diets increase risk, since plant foods often contain phytates that limit zinc uptake. Supplementing with a highly bioavailable source like zinc lactate offers a practical solution, plugging nutritional holes for busy people who can’t always craft the perfect diet.
Potential Solutions to Zinc Supplementation Challenges
Choosing a supplement gets complicated fast—tablets, powders, chewables, all lined up next to each other. Many products overload on fillers or use cheaper forms that don’t dissolve well. Zinc lactate cuts down on these issues by dissolving quickly and blending cleanly into liquids, which helps parents and seniors who struggle to swallow traditional pills. More education from health professionals and clear labeling would help shoppers make smarter decisions. Digital health tools could remind people to check their nutrient status and spot common symptoms of low zinc before they escalate.
Improving Access and Consumer Understanding
Staying healthy means navigating shelves packed with obscure ingredient names. Zinc lactate offers a straightforward way to respond to modern nutrition gaps. Doctors, nutritionists, and product manufacturers need to share clear, science-backed information about why form matters as much as dose. Offering zinc in familiar products, not just supplements, will help close the gap for overlooked groups—teens, athletes, and anyone with chronic stress. Practical steps like community education and transparent product labeling improve consumer confidence and outcomes—something every household can benefit from.
Are there any side effects of using Zinc Lactate?
What Happens When You Take Zinc Lactate?
Zinc gets attention for its role in immune function, skin health, and metabolism. Zinc lactate, a form used in toothpaste, supplements, and some medicines, often gets touted as safe. You don’t see it causing trouble for most people at common doses. Still, it’s worth looking closely before adding any new mineral to your daily routine.
Digestive Upset: The Usual Suspect
Stomach problems show up most frequently. Nausea, stomach pain, and sometimes diarrhea hit first. Take zinc lactate on an empty stomach, and you’re more likely to feel queasy or even throw up. I learned this the hard way in college, chasing supposed immune boosts before big exams. Lesson quickly learned: food matters. Eating beforehand tames those rougher stomach reactions almost every time.
Copper Shortage: An Easy Miss
High zinc levels block copper absorption. That can lead to a copper deficiency, which brings its own set of headaches: everything from numbness and tingling to changes in blood counts. Most folks aim to boost immunity or help skin issues with zinc, not to create a new deficiency. It surprises many that these vital minerals affect each other. Overdoing zinc, whatever the form, shifts the body's balance. The National Institutes of Health points out that exceeding 40 mg of zinc a day risks this problem. I’ve seen friends develop strange fatigue or even nerve changes after months of high-dose supplements.
Metallic Taste and Other Small Annoyances
Many users complain of a lingering metallic taste. It’s nothing dangerous, but it makes food less appealing. Sometimes zinc causes mouth irritation, especially in lozenges and toothpastes made for fighting bad breath. This mild burning or tingling rarely signals anything serious.
Long-Term Use: More Than You Bargained For
There’s always a temptation to stick with what “works.” Long-term use raises new issues, including changes to cholesterol or immune function. High zinc from supplements bumps down good HDL cholesterol over time. Some studies found overly high zinc may weaken immune defenses, not strengthen them. Pharmacy counters sell zinc in a lot of forms, but more is not always better.
Who Gets Into Trouble?
Kids and people with kidney or liver disease face higher risks even at routine doses. Pregnant women ask about zinc frequently. Medical experts recommend sticking close to standard dietary references. I remember a patient treating acne on a friend’s advice who soon suffered headaches and persistent nausea. Blood work showed zinc levels far above the norm.
Smarter Choices and What to Watch For
Products add zinc lactate for tooth health, body odor control, and as a dietary mineral. Choosing a trusted supplement gets overlooked. Quality varies hugely, and excessive doses aren’t always disclosed. Reports of allergic reactions stay rare but not unheard-of—redness, hives, or itching pop up now and then, most often in sensitive skin or after dental rinse use.
Safe Use Comes Down to a Few Simple Rules
Always check the label. Don’t stack zinc lactate on top of other high-zinc supplements. Stick to doses meant for daily needs, not wild immune hacks. If you feel sick, stop and ask a healthcare provider, especially if symptoms linger. Catching problems early keeps small side effects from turning into big ones down the line.
What is the recommended dosage for Zinc Lactate?
The Role of Zinc Lactate in Daily Health
Zinc plays an essential part in keeping the immune system strong and supporting cell repair. I’ve seen more people asking about mineral supplements lately, especially after the pandemic pushed conversations around immune health into the spotlight. Out of all the forms, zinc lactate has carved out a name because the body absorbs it more easily than some of the older, less bioavailable mineral salts. The thing people really want to know, though, is how much they should actually take.
What Science Says About Dosage
For a healthy adult, most reliable sources point toward a recommended dietary allowance for total zinc, not just zinc lactate, between 8 mg a day for women and 11 mg for men. Taking zinc above 40 mg daily for a long stretch might cause side effects—nausea, stomach cramps, or even problems with absorbing other important minerals like copper. This isn’t theoretical; I’ve spoken to patients complaining about stomach upset after zinc megadoses picked up on impulse from big-box stores.
Zinc lactate contains about 22% elemental zinc. In practical terms, 45 mg of zinc lactate gives you just under 10 mg of elemental zinc. Any supplement label that tells you 50 mg per tablet often means zinc lactate compound, not actual elemental zinc.
Who Should Consider Supplements
Most folks land on enough zinc through a diet filled with nuts, meats, beans, and whole grains. Someone who eats mostly processed foods, follows a vegetarian or vegan lifestyle, or faces digestive challenges (like Crohn’s disease) might need a closer look at supplementation. Pregnant people or kids with certain metabolic needs sometimes require extra monitoring, but dumping more zinc into your system “just in case” doesn't guarantee better health.
Risks That Come With High Doses
Too much zinc throws things out of balance. Over the years, I’ve seen people develop copper deficiency anemia or even lasting nerve issues from pushing their zinc intake too high for too long. The National Institutes of Health backs up that high-dose, long-term zinc isn’t a harmless experiment. Even at moderate doses, anyone with kidney disease or compromised digestion needs to check with a doctor before adding another pill to the mix.
Practical Tips for Supplement Use
Buying supplements off the shelf doesn’t guarantee quality or safety. Always look for a trusted name, preferably with third-party lab verification. Don’t just trust the internet or word of mouth. If you’re not sure about your status, a quick blood test can lay out the facts better than any self-diagnosis.
Nutrition isn’t mechanic work—a good meal plan usually covers everything. I’d rather see my friends and patients focus on real food first, then talk about supplements only if those basics don’t cover it. Zinc lactate offers a bioavailable choice, but the safe path lies in sticking close to daily recommended amounts and talking to someone knowledgeable, like a doctor or registered dietitian, before making it a regular part of your routine.
| Names | |
| Preferred IUPAC name | zinc;2-hydroxypropanoate |
| Other names |
Zinc dilactate Zinc(II) lactate |
| Pronunciation | /ˈzɪŋk ˈlæk.teɪt/ |
| Preferred IUPAC name | zinc 2-hydroxypropanoate |
| Other names |
Zinc salt of lactic acid Zinc(II) lactate Lactic acid zinc salt Dilactatozinc Zinc dilactate |
| Pronunciation | /ˈzɪŋk ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 16039-53-5 |
| Beilstein Reference | 3639822 |
| ChEBI | CHEBI:9150 |
| ChEMBL | CHEMBL3303606 |
| ChemSpider | 159432 |
| DrugBank | DB11106 |
| ECHA InfoCard | 03b2e4c2-0e33-44da-99b0-41bde9a7bbf7 |
| EC Number | 231-946-9 |
| Gmelin Reference | 77855 |
| KEGG | C14298 |
| MeSH | D013599 |
| PubChem CID | 92678 |
| RTECS number | OU8225000 |
| UNII | 5483783P8D |
| UN number | UN3077 |
| CAS Number | 16039-53-5 |
| Beilstein Reference | 1723736 |
| ChEBI | CHEBI:86441 |
| ChEMBL | CHEMBL1201564 |
| ChemSpider | 24287569 |
| DrugBank | DB14540 |
| ECHA InfoCard | 18a24d69-1511-43eb-a8e6-d5081ceb93de |
| EC Number | 209-954-4 |
| Gmelin Reference | 87971 |
| KEGG | C14826 |
| MeSH | D013603 |
| PubChem CID | 5796 |
| RTECS number | OU8400000 |
| UNII | ZWA1B1B04M |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C6H10O6Zn |
| Molar mass | 243.53 g/mol |
| Appearance | White or almost white powder |
| Odor | Odorless |
| Density | D=1.861 g/cm³ |
| Solubility in water | Soluble |
| log P | -2.61 |
| Acidity (pKa) | pKa ~3.85 |
| Basicity (pKb) | 8.60 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.53 |
| Dipole moment | 2.2 D |
| Chemical formula | C6H10O6Zn |
| Molar mass | 243.57 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | D: 0.87 g/cm³ |
| Solubility in water | Soluble |
| log P | -2.5 |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | 8.40 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.6 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 221.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1463.9 kJ/mol |
| Std molar entropy (S⦵298) | 217.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1357.2 kJ/mol |
| Pharmacology | |
| ATC code | A12CB05 |
| ATC code | A12CB06 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory irritation. Harmful if swallowed. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P270, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-0-1 |
| Lethal dose or concentration | LD₅₀ Oral - Rat - 4,640 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Zinc Lactate: 2,950 mg/kg (oral, rat) |
| NIOSH | Not Established |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 10-20 mg/day |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: H319 Causes serious eye irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Lethal dose or concentration | LD₅₀ Oral - Rat: 2,945 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3,900 mg/kg (rat, oral) |
| NIOSH | Not established |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 10-15 mg Zn/day |
| IDLH (Immediate danger) | Not Listed |
| Related compounds | |
| Related compounds |
Lactic acid Calcium lactate Magnesium lactate Iron(II) lactate Zinc acetate Zinc gluconate |
| Related compounds |
Calcium Lactate Magnesium Lactate Ferrous Lactate Copper Lactate Zinc Gluconate Zinc Acetate Zinc Sulfate Zinc Citrate |

