Zinc Glycinate: Backbone of Trace Mineral Supplementation
Historical Development
Zinc deficiency carries a long and troubling history. Before scientists linked stunted growth and immune weakness to low zinc, societies muddled through unexplained health problems, missing the mineral’s central role. For most of the twentieth century, typical zinc supplements made use of salts like zinc sulfate or zinc oxide. Both release elemental zinc, but researchers and manufacturers noticed these forms often irritated the stomach and absorbed poorly. Nutrition science advanced and possibilities for improved bioavailability opened up. Feeding trials with chelated minerals—zinc bound to amino acids—showed absorption rates jumped and side effects dropped. Glycine, a simple amino acid already present in most food proteins, formed stable complexes with zinc. That kickstarted the use of zinc glycinate in nutritional supplements, animal feed, and food fortification, offering more absorbable zinc with fewer digestive troubles.
Product Overview
Zinc glycinate bridges the gap between chemistry and nutrition. It’s produced by joining elemental zinc to two molecules of glycine. This chelated structure protects the zinc as it travels through the stomach, so more zinc ends up getting absorbed into the bloodstream. Manufacturers offer zinc glycinate in powder or granule form, with the food-grade and pharmaceutical markets driving strict production standards. The product attracts people looking to boost zinc intake without the metallic aftertaste or queasiness that traditional zinc pills cause. Food technologists prefer it because it dissolves well and doesn’t spoil taste, giving them flexibility for breakfast cereals, multivitamins, and even energy drinks.
Physical & Chemical Properties
Pure zinc glycinate appears as a faintly off-white or almost colorless crystalline powder, free-flowing and easy to handle compared to sticky or clumping zinc salts. The chelate structure keeps it stable under ordinary conditions, so it won’t decompose if left in a drawer or added to a premix. Its solubility beats that of many inorganic zinc salts—especially in the pH range found in the human gut. Zinc glycinate boasts a stable, measured molecular weight and doesn’t throw off a strong odor. It dissolves in water, releasing bioavailable zinc and glycine as the body digests it. The chelation means the elemental zinc content per gram beats that seen in zinc gluconate or acetate, so serving sizes stay small and easy to formulate.
Technical Specifications & Labeling
Strict buyers look for consistent zinc content—usually 20% to 24% by mass—along with low levels of heavy metals and negligible insoluble residue. The labeling must comply with food and drug authorities, listing zinc glycinate by its proper name and showing the exact amount of elemental zinc per serving. Certifications for GMP or ISO quality give buyers confidence. Many suppliers voluntarily test for allergens and microbial contamination. The finished product gets packed in moisture-barrier packaging to prevent clumping and slow the risk of spoilage on shelves or in warehouses.
Preparation Method
The main route starts with purified zinc salts—most often zinc sulfate—dissolved in water. Add a slight excess of glycine and keep the reaction mixture at a gentle temperature. The zinc ions swap partners, leaving their original sulfate and bonding with glycine. After the reaction finishes, the solution gets filtered to remove any leftovers or solids. Bring the product out of solution by careful cooling and precipitation. Modern plants use centrifugation and drying under vacuum to turn the wet precipitate into the final powder. Steps for purification make sure residual chemicals drop below tight limits. The batch gets checked for zinc content, particle size, and flowability before packing. Every step must meet the purity, safety, and reproducibility standards of food or pharmaceutical manufacturing.
Chemical Reactions & Modifications
Chemists occasionally tinker with the zinc-to-glycine ratio, tweaking solubility or taste for different applications. Side chain substitutions on glycine have been tried for tailored bioactivity, though the standard bis-glycine complex dominates commercial production. Zinc glycinate resists breakdown under ordinary heating or neutral pH, though strong acids or bases can split the chelate—releasing zinc and glycine as separate, free forms. Other chemical treatments can help coat particles for special uses, like antioxidant blends, or combine the compound with vitamins for structured release in tablets and capsules.
Synonyms & Product Names
Zinc diglycinate, bis-glycinato zinc, and zinc bisglycinate all point to the same chelate. Sometimes product brochures call it zinc amino acid chelate or just “chelated zinc”—a loose label that covers several other compounds, so sellers clarify by using the more precise chemical name. Food and supplement companies market it under catchy brand names, but the International Union of Pure and Applied Chemistry recognizes only its formal designation: zinc bis(glycinate).
Safety & Operational Standards
Efforts to boost trace mineral nutrition never overlook safety. Zinc glycinate meets tough standards for purity and contaminant levels, as heavy metals and solvents must hit near-zero targets in the edible product. Production plants take batch samples for analysis and rely on hazard assessments to keep cross-contaminants out. Handling guidelines limit worker exposure to fine powders; manufacturers use dust extractors or closed systems to minimize inhalation. Safety reviews rely on years of toxicology and human clinical studies that confirm zinc glycinate’s tolerability at standard supplementation dosages. Regulators set maximum zinc intake limits to prevent adverse effects, including copper deficiency from over-enthusiastic supplementation. Packaging gets labeled with best-before dates, zinc dosage, and any necessary usage warnings in line with consumer protection rules.
Application Area
Dietary supplement makers find zinc glycinate especially useful when formulating products for those with sensitive stomachs or special nutrient needs. Pediatric formulas, prenatal vitamins, and senior supplements all draw on this chelated form to raise zinc intake efficiently. Food manufacturers fortify beverages, cereals, and energy bars without worrying about bitter flavors or poor mixability. Veterinary nutritionists choose it for livestock feeds to promote animal health, fertility, and weight gain. It even finds a home in cosmetic applications—skincare brands use zinc glycinate as a calming agent in lotions and sunscreens, taking advantage of glycine’s gentle touch and zinc’s skin barrier support.
Research & Development
Scientists continue digging into the benefits and mechanics of chelated zinc. University groups test the supplement in athletic populations to see if it shortens illness or speeds up muscle recovery. Other teams pursue bioavailability trials in children with nutrient absorption disorders, comparing blood zinc levels after different formulations. Feed scientists perform long-term health tracking in livestock, exploring gains in fertility, disease resilience, and growth by swapping out inorganic zinc salts for glycinate chelates. A flavor chemist’s toolkit benefits from chelated minerals, opening doors to zinc supplementation in everything from yogurts to plant-based meat analogues. Safety and tolerability research never slows down, since changing regulations demand fresh test results and clear data.
Toxicity Research
All zinc sources risk toxicity at high doses, causing nausea, vomiting, and—over longer spells—disruptions in copper status or immune response. Research confirms that zinc glycinate avoids the gastric side effects often seen with zinc sulfate, but toxic doses still show up if guidelines are ignored. Toxicological reviews center on animal and cell studies, then translate these numbers into safe intake limits for humans. Clinical nutrition circles stress the value of balancing supplementation, not just loading up on zinc but watching iron and copper interactions as well. Surveys from supplement users rarely flag severe issues at standard doses. Still, oversight remains tight, with new studies always probing high-dose effects, especially among vulnerable populations.
Future Prospects
As synthetic nutrition grows, manufacturers look for ways to boost zinc glycinate’s solubility and flavor compatibility so it can pair with plant-based foods or functional beverages. Researchers seek nano-encapsulation and time-release tweaks, hoping to smooth out absorption and further cut any minor side effects. With more attention focused on sustainability, green chemistry approaches for large-scale synthesis gather momentum—less waste, fewer reactive chemicals, and eco-friendly solvents could reshape the production landscape. In animal and clinical trials, ongoing work tries to reveal whether the benefits in bioavailability lead to better health outcomes—faster immune responses, boosted gut integrity, or improvements in chronic disease markers. As regulatory science evolves, clearer guidance on claims and labeling supports transparency for both consumers and professionals. Zinc glycinate stands at the intersection of biochemistry, food science, and public health, with fresh uses and scientific questions emerging each year.
What are the benefits of taking Zinc Glycinate?
Why This Form of Zinc Matters
People talk a lot about minerals, but zinc deserves extra attention. Zinc does a whole lot in our bodies, from helping the immune system do its job to keeping our skin healthy. Foods like meat, beans, and seeds deliver zinc, but some people turn to supplements, and not all zinc is equal. Zinc glycinate stands out because the body can use it easily. Glycine, the amino acid bound to the zinc, helps make absorption smoother. In my own trial with zinc, I felt a difference in energy levels during high-stress times, possibly because my body could finally get enough when regular zinc gluconate did not agree with my stomach.
Supporting the Immune System and Recovery
Zinc helps immune cells act fast. One winter, after a string of colds, I dug into why some folks bounce back quicker than others. Zinc kept coming up in research, especially around stopping viruses from multiplying inside the body. Several studies from peer-reviewed journals back up the link between zinc and shorter cold durations. A 2020 review in the journal "Nutrients" showed zinc can reduce how long a cold drags on. Zinc glycinate, being gentle on digestion, lets more people keep up daily doses, which matters when aiming for consistent immune support.
Helping with Acne, Skin, and Wound Healing
Acne doesn’t just hit teenagers. During stressful periods in college and again during stressful adult years, breakouts flared up. Derms pointed to zinc. Zinc influences inflammation, which plays a role in redness and swelling. A clinical paper published in "Dermatologic Therapy" noted that zinc can lessen the severity of acne, especially for people who don't tolerate other treatments. Zinc glycinate, with its better absorption, seemed to make a difference for my skin compared to basic drugstore zinc pills that upset my stomach.
Brain, Mood, and Focus
Zinc connects to brain chemistry, too. Low zinc links with trouble focusing and low moods. Medical research from the "American Journal of Clinical Nutrition" suggests people who supplement zinc have sharper focus and steadier moods. On days where work stress threatened to derail focus, taking zinc glycinate in the morning made a noticeable shift—not a magic fix, but a small, reliable support for mental clarity.
Addressing Dietary Gaps Safely
Many people eat less red meat, and vegans or vegetarians often wind up with low zinc status. My vegetarian friends have struggled with feeling run-down until they paid attention to minerals like zinc. Plant-based zinc sources carry phytates that block absorption. Taking zinc glycinate bridges the gap since the glycine chelate form escapes most of those absorption barriers. Still, it’s smart to talk with a qualified nutrition professional before adding any supplement, especially since too much zinc can throw off copper levels.
Potential Steps for Better Health
Small changes add up. Including more zinc-rich foods and considering zinc glycinate if signs of deficiency show up can go a long way. Testing zinc status through a healthcare provider and watching for stomach upset or taste changes helps catch problems early. For a lot of people, including myself, adding zinc glycinate proved easier on the gut and brought better results than grocery-store tablets. It isn’t a miracle solution, but it fills a genuine gap for many, especially during times of stress, high physical activity, or restricted diets.
How is Zinc Glycinate different from other forms of zinc?
A Closer Look at Zinc Sources on the Market
Most folks looking for a zinc supplement see plenty of options: zinc gluconate, zinc sulfate, zinc picolinate, zinc oxide, and, lately, zinc glycinate. Each form promises support for immunity, skin, and metabolic function. Not all forms deliver these benefits the same way. Zinc glycinate turns a lot of heads thanks to its unique structure, and plenty of people, myself included, have wondered if it's worth the extra attention in the nutrition aisle.
Bioavailability: It Matters More Than You Think
Zinc glycinate pairs zinc with glycine, an amino acid, forming what’s called a chelated compound. That chelation means your body notices it, takes it in efficiently, and hangs onto it longer during the digestive process. By comparison, zinc oxide, found in many multivitamins, doesn’t absorb nearly as well, with the digestive tract letting much of it pass through untouched. The National Institutes of Health points out that absorption can range dramatically between types; oxide falls short, while chelated forms like glycinate come out ahead.
Easy on the Stomach Makes a Big Difference
Many folks, myself included, have felt queasy after taking regular zinc tablets. I once tried a common zinc sulfate pill after breakfast, only to spend the morning with a sour stomach. Zinc glycinate typically leads to fewer complaints like nausea and cramps. Glycine itself has a calming effect on the gut lining, which helps explain the easy ride.
The Taste Test Most Don’t Mention
Zinc gluconate and zinc sulfate come with a clear metallic taste. Some people gag when the powder touches their tongue or lingers afterward. Glycinate cuts that issue since glycine tastes mild and neutral. No aftertaste makes compliance easier, especially for those who struggle already with swallowing big pills or powders.
Digestive Interference—Or Lack of It
Supplements need to get absorbed, but they also need to stay out of the way of other nutrients. Some forms, like zinc sulfate or oxide, mess with copper and iron absorption. That back-and-forth can lead to deficiencies over time, especially if you’re using zinc for longer periods. Glycinate stays less reactive in the gut, since glycine helps shield the zinc, so it doesn’t grab at other minerals. Functional medicine practitioners often point out this advantage, recommending glycinate for folks already struggling with nutrient balance.
Cost vs. Value for the Body
Walk down a supplement aisle, and price drives picking for many of us. Zinc oxide is cheap and easy to find. Glycinate sits in the premium range, often double or triple the price per dose. The old saying “you get what you pay for” fits here—more of the higher-priced zinc reaches tissues, supporting immunity or skin health where it counts. If I’m paying for something to work, I’d rather invest a bit extra and avoid throwing half my dose away.
What to Watch for in Quality
Not all zinc glycinate comes from ethical sources. Clean manufacturing does matter—contamination or sloppy binding can rob any supplement of real impact. Reputable brands test batches for purity and make results public. Checking labels and third-party certifications offers peace of mind and supports proper use of science-backed supplements.
Looking Ahead on Choosing Zinc
Anyone looking for a zinc supplement has to weigh absorption, side effects, cost, and food interactions. Sometimes paying a little more up front saves trouble down the road—less stomach upset, better absorption, and a gentler effect on nutrient stores. With increasing research shining light on the differences, zinc glycinate has carved out a strong case among folks who want their money to go further in their daily nutrition.
What is the recommended dosage for Zinc Glycinate?
The Basics
Zinc Glycinate shows up on plenty of shelves now as a top choice for those looking to boost their zinc intake. This form binds zinc to glycine, aiming for better absorption and fewer stomach issues compared to older zinc supplements. But many folks have a hard time sorting out just how much they should take to see real benefits and avoid running into trouble.
Why the Right Dose Matters
I’ve crossed paths with people who buy a bottle of Zinc Glycinate, see big milligrams printed on the front, and figure more is always better. It’s easy to think loading up will quickly fix things like low energy, poor immunity, or thinning hair. Science doesn’t line up with this. Zinc is a trace mineral for a reason; your body needs a bit, but not mountains. Go too high and you can trip up copper levels, give yourself headaches, or bring on an upset stomach. Moderation always wins out with minerals.
Current Recommendations
The National Institutes of Health sets the recommended daily allowance for zinc at 11 mg for adult men and 8 mg for adult women. During pregnancy and while breastfeeding, the number nudges up to 11-12 mg. Most supplements, including zinc glycinate, list doses between 15-30 mg. This range covers average dietary gaps, as some folks eat less red meat or whole grains than others. Zinc from Glycinate usually absorbs better than zinc oxide or sulfate. Sticking close to the RDA matters. If a supplement offers 30 mg, that’s more than two days’ worth for most women and still well over the target for men. Taking this every day over months may edge you toward unwanted effects like nausea or lower immunity, quite the opposite of what you’re after.
Daily Life and Real-World Application
Tales from my own house line up with clinical studies — most people get a fair chunk of zinc from normal food. Lean beef, pumpkin seeds, chickpeas, and cashews show up often at my table. Counting servings for the week, I can see how a healthy eater lands in the safe zone for daily intake before touching a supplement. Short-term supplementation sometimes feels helpful during winter or under stress. Going above 40 mg a day, though, invites risk. That limit, the Tolerable Upper Intake, helps guard against long-term trouble.
Trustworthy Guidance and Safe Choices
Doctors and registered dietitians know the true signals for deficiency better than a web search or a sales pitch. Ongoing symptoms like poor wound healing, recurrent colds, or hair loss can point toward a real need for extra zinc. Blood tests bring clarity. A professional weighs diet, health status, and current medications before recommending a Zinc Glycinate dose. For anyone on chronic meds, especially drugs blocking mineral absorption like diuretics, a personal plan matters more than the serving size on the bottle.
Smart Supplement Habits
Read the supplement facts—don’t just pick the highest dose. Look for verification from independent labs so contamination doesn’t sneak into the picture. Watch for signs your body isn’t happy: nausea, unusual fatigue, or headaches could signal you need to cut back. Cycling off after a season or alternating days can see you through times of higher demand without overloading your system.
The Value of Moderation
Zinc Glycinate brings real potential: better absorption, mild on the gut, and solid backup for short-term health needs. Tuning in to your own needs—and not the size of the dose—will steer you closer to real health gains. As with much in nutrition, a daily handful isn’t better than a single, well-placed serving. If questions pop up, the best path leads straight to someone who knows your dietary needs best.
Are there any side effects or interactions with Zinc Glycinate?
Knowing Zinc Glycinate
Zinc glycinate steps into the supplement scene blending an essential mineral and an amino acid. The aim? Better absorption with less stomach upset. People look to it for immune support, skin health, or just patching up a gap in their daily nutrients. For many, it works as an easy fix. Still, just because it carries a “gentle” reputation does not mean it comes without caution signs.
Spotting Side Effects from Zinc Glycinate
Supplements promise benefits, but the body can react in different ways. I’ve spoken to folks who noticed queasiness or a funky taste in their mouth after a zinc pill. Digestive issues like stomach cramping, nausea, or even diarrhea show up in some people. Sometimes, upset stomach leads folks to bail on supplements altogether. A big reason: taking zinc on an empty stomach twists up the gut. Swallowing with food, as most nutritionists recommend, often keeps problems away.
In my own circle, one friend doubled her zinc supplements during a cold and ended up with an unpleasant metallic taste and some pretty regular bathroom trips. That overzealous approach? It sent her over the tolerable upper intake level, which experts peg at 40 mg per day for adults. The body needs some zinc to work well, but too much can end up causing copper deficiency, lowering good cholesterol, and even leading to suppressed immune function—the exact opposite of what most users want.
How Zinc Glycinate Interacts with Other Drugs
Mixing zinc glycinate with certain medications can trip up the body in hidden ways. Some folks may not realize that antibiotics like tetracycline and quinolone types (for example, ciprofloxacin and doxycycline) can get blocked by zinc. The mineral binds to these drugs in the gut, cutting their effectiveness. People who count on these antibiotics to fight infections could lose time and end up sicker if they unknowingly take zinc alongside.
Diuretics, commonly handed out for blood pressure or heart issues, crank up zinc loss in urine. Over time, regular use can leave someone running low. On the flip side, those using zinc to make up for a shortfall should chat with their doctor about timing and dose. Other problem areas include penicillamine (used for rheumatoid arthritis), and even some osteoporosis medications, which can act differently when zinc climbs into the mix.
Watching Out for Hidden Pitfalls
Supplements often land on store shelves as “natural helpers,” but the truth is the FDA treats them more like food than medicine. No strict rules force companies to prove safety or track every single side effect before people buy their products. That places the burden on shoppers to do detective work. Anyone starting zinc glycinate—especially folks with ongoing medical conditions—really should share their supplement habits with a healthcare provider.
Online, it’s easy to find stories of people trying to chase away long COVID, acne, or “just boost immunity” by piling on zinc. In clinic settings I’ve watched people bring in supplement lists longer than their prescription forms. The best outcomes tend to come from honesty about all pills in the routine, close guidance, and lab work checking for nutrient imbalances after months of extra zinc.
Moving Forward With Caution
Anyone weighing zinc glycinate should lean on reputable sources. Look for products checked by third-party labs to sidestep hidden doses or contaminants. Track all medications, check with pharmacists, and aim for balanced meals that give nutrients a natural route into the body. Supplements can bring benefits when gaps exist, but mindful choices stop short of swapping one problem for another.
Is Zinc Glycinate suitable for vegetarians and vegans?
What Goes Into Zinc Glycinate?
Zinc glycinate shows up on more supplement bottles today, pitched as a well-absorbed form of zinc. This compound binds zinc with glycine, an amino acid. Many people following plant-based diets run into zinc shortfalls since grains, beans, and nuts contain compounds that get in the way of zinc being absorbed. So, the question keeps coming up: can someone who swears off animal products count on zinc glycinate to fill that gap?
Simple Ingredients—But Origins Matter
Glycine makes up half the chelate. This is a simple amino acid found in both plants and animals. Glycine used in supplement manufacturing comes from two main sources: fermentation (using bacteria that digest sugars from plants) or hydrolysis of animal protein, like gelatin. Many companies don’t state the source on their labels.
Zinc, by itself, isn’t animal-derived—it’s a metal, and suppliers extract it from mineral ores dug out of the earth. Both vegans and vegetarians can safely take zinc, if it’s made without animal additives.
What the Label Does (and Doesn’t) Tell You
Most big brands in North America and Europe lean toward vegetarian ingredients because plant-based diets are growing in popularity. Some companies even stamp “vegan” or “vegetarian” on their bottles, often validated by third-party certifications. But some still use animal-based glycine. The supplement world runs light on strict regulations—unless you reach out to customer service or check the company’s website for transparency, you could simply be guessing.
Additives play another role. Many capsules use gelatin (from animal tissue), sneaking animal content into what looks like a harmless zinc supplement. Vegetarians might accept dairy byproducts, but vegans look for plant cellulose or starch capsules. Dyes and fillers can also spring from animal origins.
Why It Matters for Nutrition and Ethics
For anyone not eating meat, dairy, or eggs, getting zinc can be tough. Some plant foods—chickpeas, lentils, nuts—pack zinc, yet they also carry phytates, which make absorption less efficient in the gut. Without enough zinc, symptoms creep up: weaker immune system, slower wound healing, dull sense of taste. I’ve watched friends switching to plant-based eating run into fatigue or persistent colds until they sorted out their zinc intake.
Many doctors steer their patients toward chelated zinc like zinc glycinate, because studies show it absorbs better than plain zinc sulfate or oxide. Fewer gastric side effects also make it gentler. Yet if the glycine comes from animal hide, vegans run into the same ethical dilemma they’re trying to avoid. This is a meaningful issue—not only for nutrition, but also for staying true to one’s values.
Finding Plant-Based Zinc Glycinate
Several companies now step up with explicit statements: glycine in their zinc glycinate products comes from plant fermentation, and all co-ingredients are animal-free. Certifications like the Vegan Society’s mark or independent laboratory verification give shoppers peace of mind. Online reviews and consumer forums often dig into the details, surfacing brands that align with plant-based ethics.
If product packaging, websites, and customer support stay vague, consumers need to demand more. Some companies respond quickly to these requests, others lag. Those who care about animal welfare or environmental impact shouldn’t have to settle for uncertainty. Until regulations catch up, the clearest path to plant-based zinc glycinate lies with transparency and third-party endorsement. If you value both your health and your ethics, it helps to do a little digging before taking a supplement off the shelf.
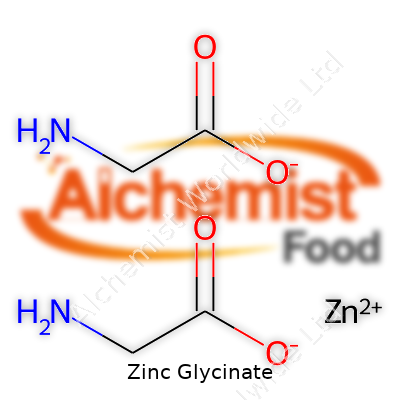
| Names | |
| Preferred IUPAC name | zinc;2-aminoacetate |
| Other names |
Bis(glycinato)zinc Zinc bisglycinate Zinc diglycinate Zinc amino acid chelate Glycine zinc chelate |
| Pronunciation | /ˈzɪŋk ɡlɪˈsɪneɪt/ |
| Preferred IUPAC name | zinc;2-aminoacetate |
| Other names |
Zinc Bisglycinate Zinc Diglycinate Zinc Glycine Complex Bisglycinatozinc Zinc (II) glycinate |
| Pronunciation | /ˈzɪŋk glaɪˈsɪneɪt/ |
| Identifiers | |
| CAS Number | 14281-83-5 |
| Beilstein Reference | 1694442 |
| ChEBI | CHEBI:131186 |
| ChEMBL | CHEMBL3724739 |
| ChemSpider | 178156 |
| DrugBank | DB14605 |
| ECHA InfoCard | 100.039.858 |
| EC Number | 70161-97-2 |
| Gmelin Reference | 120793 |
| KEGG | C02413 |
| MeSH | D015399 |
| PubChem CID | 16218689 |
| RTECS number | ZG0870000 |
| UNII | VJ0F3V4L6Q |
| UN number | UN3077 |
| CAS Number | 14281-83-5 |
| Beilstein Reference | 1420487 |
| ChEBI | CHEBI:131326 |
| ChEMBL | CHEMBL613310 |
| ChemSpider | 19816497 |
| DrugBank | DB14503 |
| ECHA InfoCard | 16aac3e7-8eb6-4149-b53c-0e42e6f225c8 |
| EC Number | 931-954-5 |
| Gmelin Reference | 641117 |
| KEGG | C01799 |
| MeSH | Dietary Supplements"[MeSH] |
| PubChem CID | 122195884 |
| RTECS number | MV8050000 |
| UNII | zn3r3n12x8 |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C4H8N2O4Zn |
| Molar mass | 179.48 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | Density: 2.39 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.97 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.08 |
| Basicity (pKb) | 8.06 |
| Magnetic susceptibility (χ) | 'Diamagnetic (-1.0 × 10⁻⁵)' |
| Refractive index (nD) | 1.56 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.32 D |
| Chemical formula | C4H8N2O4Zn |
| Molar mass | 179.48 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | Density: 0.5 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -1.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.4 |
| Basicity (pKb) | 8.1 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Dipole moment | 4.18 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 101.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -967.0 kJ/mol |
| Std molar entropy (S⦵298) | 220.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1276.6 kJ/mol |
| Pharmacology | |
| ATC code | A12CB06 |
| ATC code | A12CB06 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes skin irritation. May cause allergic skin reaction. Harmful if swallowed. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid breathing dust. Wear appropriate protective equipment. Wash thoroughly after handling. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| Lethal dose or concentration | LD₅₀ Oral - Rat: 3,384 mg/kg |
| LD50 (median dose) | 2,000 mg/kg (rat, oral) |
| NIOSH | Not Listed |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 30 mg daily |
| Main hazards | May cause respiratory irritation. May cause eye, skin, and respiratory tract irritation. Harmful if swallowed. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Lethal dose or concentration | LD₅₀ Oral – Rat: > 2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Zinc Glycinate: > 2000 mg/kg (rat, oral) |
| NIOSH | Not Listed |
| PEL (Permissible) | 5 mg/m³ |
| REL (Recommended) | 15 mg per day |
| IDLH (Immediate danger) | No IDLH established |
| Related compounds | |
| Related compounds |
Zinc sulfate Zinc gluconate Zinc picolinate Zinc citrate Zinc oxide Zinc acetate Magnesium glycinate Copper glycinate Iron glycinate |
| Related compounds |
Zinc sulfate Zinc gluconate Zinc picolinate Zinc acetate Zinc oxide |

