Zinc Citrate Dihydrate: A Practical Look at a Key Compound
Historical Development
Zinc compounds have shaped medicine and industry for centuries. Zinc Citrate Dihydrate didn’t show up in labs overnight. Early pharmacists were on the hunt for new ways to deliver zinc, knowing its benefits in wound healing and fighting infections. Chemists in the 20th century began isolating zinc salts, and the citrate form stood out because of better absorption in the body and less disruption in taste. Companies rolled out dental products starring Zinc Citrate, betting on its ability to halt plaque growth and fight bacteria. Over the years, research around this form of zinc followed a steady uptick, with manufacturing processes tweaked for higher yields and cost control, driven by the needs of dietary supplement companies and oral care brands. Today, bulk suppliers in Europe, North America, and Asia invest in refining its purity and improving scalability for a wider range of products.
Product Overview
Zinc Citrate Dihydrate belongs in the corner of the lab where functional ingredients get things done. In its typical powder form, Zinc Citrate Dihydrate comes with a faint characteristic odor and a slightly tart taste. It works as a source of zinc in dietary supplements, oral care products, and certain fortified foods. The dihydrate tag signals two water molecules per formula unit, which brings slightly better solubility in water compared to the anhydrous kind. Demand for this specific salt hinges on its bioavailability and mild flavor, favored by toothpaste makers and supplement firms alike.
Physical & Chemical Properties
Zinc Citrate Dihydrate stands as a white to nearly white crystalline powder, with good flow properties, though it can pick up moisture on humid days. It carries the formula C12H10O14Zn3·2H2O and weighs in at about 610.3 g/mol with both hydrate water molecules included. The compound breaks down at higher temperatures, starting to lose water as the temperature crosses 100°C, then decomposing as temperatures climb. Its solubility in water lands between 0.29 g/L to 0.5 g/L at room temperature, enough for daily-use products but not so high as to cause rapid breakdown in moist environments. Acidic solutions raise its solubility—a reason it suits many oral health products. Zinc Citrate Dihydrate does not burn, so it poses no fire risk in manufacturing environments.
Technical Specifications & Labeling
Technical standards give buyers confidence in what they receive. Most reputable producers certify zinc content between 31% and 33%. Impurity limits, including lead, cadmium, and arsenic, follow global food and pharma regulations, with heavy metals measured down to a few parts per million. Particle size comes up in powder handling for food and tablets, typically ranging between 80 to 200 mesh, depending on the application. Labels ought to list the compound as “Zinc Citrate Dihydrate,” often referencing CAS number 14221-02-4 and stating additives, if any. Country of origin and manufacturing date feature on most containers to comply with local import rules. For supplements, clear labeling of elemental zinc content remains vital because overdosing on zinc brings health risks.
Preparation Method
The most common preparation route starts with zinc oxide or basic zinc carbonate, mixed with citric acid under controlled heating and stirring. Producers add water, ensuring slow pH adjustment to keep unwanted byproducts from popping up. After full reaction, the zinc citrate dihydrate forms as a slurry. Filtration removes unreacted ingredients and any insoluble waste, with several rinsing steps to achieve a high purity. The wet cake moves to a drying step at moderate temperatures, preserving the two water molecules for the dihydrate form but taking out excess moisture for product shelf stability. Advances in drying technologies have boosted energy efficiency and controlled batch-to-batch consistency, making it easier for manufacturers to scale production without running into quality headaches.
Chemical Reactions & Modifications
Zinc Citrate Dihydrate acts as a mild Lewis acid and can pair up with alkalis to create other zinc salts or citrates. In toothpaste labs, it gets paired with abrasives and fluorides. In acidic solutions, the citrate group shields the zinc ion, allowing slow and steady release, especially under oral conditions. Modifying the water of hydration or jumping to anhydrous zinc citrate changes its solubility and shelf stability—adapting to different foods, tablets, or powders. On the industrial side, it can assist as a cross-linker, binding agents in polymers or acting as a mild antimicrobial. Researchers keep tinkering with the citrate framework, searching for hybrid additives or chelates that outperform plain zinc salts in both nutrition and cleaning.
Synonyms & Product Names
On shipping documents and product labels, Zinc Citrate Dihydrate goes by several names. Common alternatives in industry include “Trizinc dicitrate dihydrate,” “Citrizinc,” or “Zinc 2-hydroxypropane-1,2,3-tricarboxylate.” For dietary supplements and food fortification, it usually appears as “zinc citrate” but for technical work, CAS 14221-02-4 provides certainty. Brand names sometimes play on its origin, like “Nature’s Zinc Citrate” in nutrition or “PlaqueGuard Zinc Citrate” in toothpaste ingredients. These multiple names can cause confusion if labels don’t match regulatory codes, so clear labeling and supplier documentation stay important for safety and quality control.
Safety & Operational Standards
Handling Zinc Citrate Dihydrate in a factory or lab doesn’t require extraordinary measures, but good personal hygiene remains crucial. Dust generation should be minimized; airborne particles can irritate mucus membranes. While not acutely toxic, chronic overexposure brings risks. Standard gloves, lab coats, and dust masks keep operators safe, and regular industrial hygiene audits check for airborne zinc. Facilities follow GMP for food and pharmaceutical use, ensuring equipment avoids cross-contamination and keeping microbial counts low. Transport follows guidelines for non-hazardous chemicals, with containers sealed against moisture and contamination. Warehouses commit to cool, dry storage. Training on Material Safety Data Sheets (MSDS) forms the backbone of health and safety programs around this ingredient.
Application Area
Zinc Citrate Dihydrate stands at the crossroads of nutrition and hygiene. Toothpaste manufacturers put it front and center, counting on its antimicrobial activity to help reduce plaque formation and improve gum health. Supplement brands highlight its better absorption in capsules and tablets, especially when compared to zinc sulfate or oxide. Food technologists find it useful in cereals and health drinks to raise zinc content without sharply changing taste. Animal feed producers turn to it for fortifying pet and livestock nutrition. Spray and lozenge products for throat health have added zinc citrate for an extra line of defense against daily infections. In industrial settings, it can serve as a cross-linker in some specialty coatings or composites where mild antimicrobial characteristics add value.
Research & Development
The past decade has generated more academic papers on zinc salts than many realize, and zinc citrate keeps showing up as a subject of interest. Research teams dig into its bioavailability, often comparing it with other zinc delivery forms and documenting higher uptake and lower GI irritation. Dentists lean on its antimicrobial data, with studies supporting its effect on oral bacteria like Streptococcus mutans, one of the main drivers behind dental caries. Biomedical labs have tried zinc citrate dihydrate in topical creams, hoping for new wound-healing applications. Meanwhile, formulation chemists search for ways to combine it with probiotics, aiming to amplify immune benefits in both children and older adults. Product development teams now eye customized granule sizes or sustained-release versions, thanks to evolving equipment and consumer demand for cleaner, more effective supplementation.
Toxicity Research
Every beneficial trace element has limits, and zinc sits no differently. Scientists know zinc as an essential micronutrient, yet excess intake triggers short-term GI symptoms or, over time, impacts copper uptake and weakens the immune system. Long-term studies place the tolerable upper intake at 40 mg elemental zinc per day for adults. Animal work suggests zinc citrate dihydrate’s oral toxicity stays low, with lethal dose values much higher than day-to-day use allows. Poor labeling, especially in the supplement space, still risks accidental overdosing. Inhalation studies show that regular industrial handling with dust control causes no significant lung changes in workers. Regulations keep evolving as new chronic exposure data rolls in, but basic precautions and accurate dosing protect both workers and consumers from harm.
Future Prospects
Market trends point to rising demand for plant-based and clean-label products, and zinc citrate dihydrate fits the bill with its gentle profile and mineral source. Analysts forecast continued growth in fortified foods, especially in parts of the world where zinc deficiency undermines health and child development. Supplement brands experiment with slow-release polymers and multi-mineral blends, hoping to carve out a niche with lower side effects and better compliance. R&D teams weigh enhanced zinc citrate blends for dental care to drive fresh claims against plaque and tartar. Emerging biotechnology firms are investigating combinations with synbiotics or antioxidants, targeting gut health and broader immune support. Green chemistry approaches push for more energy-efficient production routes, aiming to lower carbon footprints without sacrificing purity. As regulations sharpen around supplement labeling and purity, manufacturers putting transparency first will likely come out ahead, earning consumer trust in a crowded market.
What are the health benefits of Zinc Citrate Dihydrate?
The Value of Zinc for the Body
Zinc isn’t a headline grabber, but it deserves more credit. For years, I’ve watched how minor nutrient gaps can affect people’s everyday health. Zinc citrate dihydrate, chosen for supplements and foods because it dissolves well, backs up crucial tasks inside us—defending cells, healing skin, and steering the immune system on course.
Immunity and Everyday Wellness
At the start of cold season, shelves fill up with zinc products, and for good reason. Zinc fuels the creation and activation of T-cells, the watchdogs in our immune response. Studies show that even marginal zinc shortfalls open the door to more infections. In my own life, a couple of years working late and eating poorly hit my zinc intake hard, and I kept catching whatever bug was going around. After making changes that included zinc-rich foods (oysters, beef, nuts) and sometimes a supplement, I noticed fewer sick days and quicker recoveries.
Skin Repair and Growth
Cuts and scrapes heal better when zinc levels line up. Zinc citrate dihydrate supports enzymes that repair skin and form new tissue. Dermatologists often suggest zinc for conditions like acne or minor rashes, as it helps curb inflammation and regulates oil production. More than a decade in healthcare showed me that wounds and skin troubles last longer in those falling short on zinc. After patients increased their zinc through diet or gentle supplements, new skin formed faster and infection risk dropped.
Mental Sharpness and Mood Balance
A clear head and stable mood depend on many nutrients, with zinc playing a steady role. Zinc shapes how nerves talk to each other and helps regulate hormones driving our moods. In university, once exam stress kicked in, I saw classmates with the worst diets struggle more with focus and anxiety. Research backs this up—adults with adequate zinc keep their thinking sharp as they age, and studies in children and teens point to better attention and memory. Supplementing with zinc citrate dihydrate supports a healthy intake without harshness on the stomach.
Absorption and Practical Use
A key reason zinc citrate dihydrate lands in so many supplements boils down to digestibility. Some forms of zinc upset the gut or pass through unused, but this type dissolves smoothly and is gentle enough for sensitive stomachs. For people like vegetarians, older adults, or anyone with gut conditions, this matters. If the body can’t absorb what goes in, the benefits get lost.
Responsible Use and Solutions for Deficiency
Choosing foods high in zinc lays a strong base. Fast food, highly processed snacks, and restrictive diets chip away at mineral stores. When meals fall short, a quality supplement with zinc citrate dihydrate can patch the gap. Doctors recommend staying close to the daily value: 11 mg for men and 8 mg for women, according to the National Institutes of Health. Too much zinc over time brings its own issues, like lowering copper and harming immune balance, so more isn’t always better.
Recognizing early signs of zinc shortfall—slow wound healing, more infections, skin flares—can protect health before problems spiral. Checking labels for zinc citrate dihydrate means better absorption and fewer side effects, making it a trustworthy ally for a wide range of people. For anyone with doubts, a health professional can test and guide on the right options.
How should Zinc Citrate Dihydrate be taken or dosed?
Understanding the Basics
Zinc stands out as one of those minerals crucial for staying healthy. Zinc citrate dihydrate offers a common supplement form, often chosen because it’s easier on the stomach than some others. Most folks turn to it when looking to boost immune support, handle cases of mild zinc deficiency, or support skin health. Tablets, capsules, powders—all are widely found at local pharmacies and grocery stores.
Let’s Talk About Dosage
Adults typically take between 15 to 30 milligrams of elemental zinc per day if they’re supplementing their diet. Most over-the-counter products fall within this range. Check the back of the bottle: the label usually explains how much elemental zinc comes in each dose. Zinc citrate contains a specific portion of elemental zinc, so reading and understanding the label saves you from accidentally overdoing it.
Many people take their supplement after eating, since it can sometimes make you queasy if taken on an empty stomach. This small adjustment makes a big difference, especially if your stomach’s sensitive. Swallow the tablet with a good-sized sip of water. Stick with food and water, since some juices, especially those that are high in calcium and iron, can get in the way of zinc absorption.
Who Needs to Be Careful?
Some groups should pay closer attention. Older adults, vegetarians, and folks dealing with absorption issues from the gut often pop up as people more likely to need extra zinc. These days, I notice that people following restrictive diets sometimes run low on minerals like zinc. Instead of guessing, it’s better to talk to a healthcare provider before diving into supplementation—blood levels, other medications, or health conditions could change things.
Too Much of a Good Thing
Getting more zinc than your body needs won’t always make you healthier. Taking high doses—say, more than 40 mg daily—can cause trouble with copper absorption, nausea, stomach cramps, or, over time, weaken your immune response. I once spoke with someone who figured doubling up on supplements was harmless, but after a few weeks started feeling worse, not better. That experience taught both of us it pays to take only what’s needed.
Quality and Safety
Choose reliable brands when buying supplements. Quality matters. The FDA doesn't screen supplements as tightly as prescription drugs, so trusted third-party seals can help. Keep an eye on expiration dates and store the bottle out of heat and dampness. If you ever notice the tablets changing color or texture, don’t use them. I've had my own share of bottles sitting in the back of a cabinet for far too long—freshness always counts.
Better Habits for Long-Term Health
Supplements tend to work best alongside balanced meals. Food like meat, dairy, beans, nuts, and whole grains already provide some zinc. Adding too much by supplementing skips past what your body naturally expects. I find it helps to focus on regular meals, double-check labels, ask for advice if unsure, and treat supplements as just one piece of the puzzle.
Simple Solutions
If swallowing tablets bothers you, try a different form. Powders or chewables taste better for some people. Sticking with a routine—taking your zinc at the same time every day—creates a habit that’s easy to remember and less likely to be missed.
A little care goes a long way. Checking in with a healthcare professional, reading labels, and sticking close to recommended doses make supplementing with zinc citrate dihydrate both safe and effective.
Are there any side effects associated with Zinc Citrate Dihydrate?
Zinc in Everyday Health
Zinc touches a lot of areas in daily life—boosting immune response, helping heal wounds, even influencing sense of taste and smell. Zinc citrate dihydrate ends up as a common choice in supplements. Some folks might grab a bottle off the pharmacy shelf after hearing about zinc’s role in fighting colds or supporting hair and skin. In my own family, a few took zinc after the pandemic hit, hoping for a stronger immune system. Conversations with pharmacists and friends revealed many people don’t pause long enough to ask, “Are there any downsides?”
Digestive Ups and Downs
It’s hard to ignore how our stomachs react when we introduce new minerals. Taking zinc citrate dihydrate on an empty stomach sometimes brings queasiness or stomach cramps. Some of my friends reported that gnawing, unsettled feeling after breakfast if they forgot to eat a good meal with their supplement. Nausea, vomiting, or diarrhea happen often enough when zinc doses go over what the body needs. These gut reactions often surprise people, since labels just say “dietary supplement.”
Taste, Mouth, and Headaches
Zinc can leave a metallic taste in the mouth, especially if chewed or taken in tablet form. This taste lingers and doesn’t make for a pleasant meal. Occasionally, I’ve heard complaints of mouth irritation or dryness. Sometimes, headaches follow a big dose. Healthcare guides mention these effects tend to pass if someone lowers their intake or stops for a few days.
Long-Term Concerns and Interactions
Over months, zinc citrate dihydrate—like many supplements—can cause bigger concerns. Consistently high doses make it tough for the body to absorb copper, another mineral we need for blood and nerve health. Low copper shows up as numb fingers or trouble keeping the immune response strong. One of my cousins ended up needing blood tests and extra copper after using zinc for a year without medical advice.
Zinc also interacts with certain antibiotics, iron, and medicines for autoimmune diseases. Those who juggle several prescriptions have to watch for changes in side effects. It’s best to share all supplements with a doctor or a pharmacist, even if it feels like small talk.
Who Needs to Watch Their Zinc?
Children need much smaller doses. Pregnant or breastfeeding women face stricter limits, since their bodies respond differently. Kidneys play the biggest role in removing excess zinc, so those with kidney issues must stay cautious. Seniors, who may take multiple supplements or medications, have higher risks. A community clinic I volunteered in often saw older adults with digestive discomfort or abnormal bloodwork who never suspected their daily vitamins played a role.
Guidance from Science and Practice
Taking the minimum amount that meets daily needs works for most folks. The National Institutes of Health flags 40 mg a day as the upper limit for adults. For many, less is better. Regular bloodwork and talking to a healthcare professional offer real insight before problems show up. Supplements can help fill gaps but can’t replace steady diet changes or a physician’s advice. A key lesson from years of conversations: Taking more never guarantees better health—and sometimes less protects us from feeling worse.
Is Zinc Citrate Dihydrate suitable for vegetarians and vegans?
Looking at Zinc Sources in Supplements
Zinc matters for wellness. The body relies on it to support the immune system, skin repair, and metabolism. Some people choose to rely on supplements for zinc because many plant-based foods don’t deliver enough, especially for those on vegan or vegetarian diets. Zinc citrate dihydrate lands in many supplements because it absorbs well and is gentle on the stomach.
Understanding What Makes a Product Vegetarian or Vegan
Living as a vegetarian or vegan means checking ingredients and how products get made. Few things break trust with a lifestyle choice more than finding animal byproducts where one expects safety. People ask about zinc citrate dihydrate for this reason. It isn’t just about the zinc itself—it’s also about what happens before the tablets hit shelves.
How Zinc Citrate Dihydrate Gets Produced
Making zinc citrate dihydrate happens by blending zinc (often as zinc oxide or zinc carbonate) with citric acid. Citric acid, almost always produced from corn sugar through a fermentation process, does not rely on animals or animal byproducts under normal commercial processes. The zinc source itself comes from minerals, not animals. This process, in its basic form, lines up with both vegetarian and vegan principles.
Hidden Ingredients and Additives
Not every supplement on the market keeps things this clean. Some tablets and capsules use binders, colors, coatings, or even gelatin as part of the delivery system. Gelatin, made from animal collagen, shows up in some capsules and can catch even careful consumers by surprise. More companies offer plant-derived capsules now—often using cellulose instead of gelatin. Whether zinc citrate dihydrate is suitable for vegetarians and vegans often comes down to these extra ingredients.
Certifications and Trust
Trust matters in supplements. Products that earn vegetarian or vegan certification labels go through audits. The Vegan Society and similar third-party groups check ingredients, manufacturing, and even cross-contamination risks. A supplement holding one of these labels gives shoppers confidence.
Sometimes companies cut corners or change suppliers without flagging it on the label. A certificate or claim from 2022 can mean little if the recipe changes in 2024. It pays to check lot numbers, up-to-date certifications, or contact the company directly before trusting a new supplement batch.
Why This Matters in Real Life
Plenty of people don’t want to compromise ethics or health goals for nutrients. Vegan and vegetarian lifestyles are about choices that reach beyond the plate—right into medicine cabinets and daily routines. Younger consumers report spending more time researching supplements, tracking clean labels, and sharing this information with friends and family. Everyone benefits from a market where labels have meaning and where companies answer questions about sourcing and processing.
Practical Steps for Consumers
Anyone relying on a supplement for zinc should look for brands that clearly list ingredients and hold relevant certifications. Plant-based capsules cut out hidden animal products. Some brands will provide detailed batch testing results or ingredient sheets on request. In a crowded marketplace, products that can’t answer these questions give plenty of reason for consumers to choose a different option.
How should Zinc Citrate Dihydrate be stored to maintain its quality?
Why Storage Matters for Zinc Citrate Dihydrate
Getting the storage conditions right for zinc citrate dihydrate matters more than some might realize. This mineral compound serves important roles in supplements and oral care products. Keeping its quality intact means less risk of product recalls and disappointed customers. I still remember a conversation with a supplement manufacturer who had to discard almost an entire batch because moisture crept into a container they thought was sealed. The financial hit made their team rethink every link in their supply chain.
Temperature and Humidity: The Foundation
Heat and moisture cause the real trouble for zinc citrate dihydrate. If temperatures rise too high, or the air gets damp, clumping and degradation follow. To avoid this, find a consistently cool, dry space. I’ve seen warehouses set their temperature limits at 25°C and use dehumidifiers to keep relative humidity below 50%. Anything higher than these figures, especially for long storage, invites problems. Too much heat or moisture breaks apart the delicate crystal structure, shortening shelf life and reducing effectiveness in the finished product.
Sealing and Air Exposure
Air exposure turns small mistakes into bigger issues. Once air and moisture seep inside, zinc citrate dihydrate may absorb water from the air and start to cake. I recommend using airtight containers. At the last company I worked with, we switched from simple bags to multi-layer, industrial-grade drums with tight lids. This change almost eliminated cases of product clumping. For producers or users who must keep containers open multiple times, consider working in a controlled environment or taking out only what’s needed for the day before sealing the rest back up tightly.
Light and Contaminant Control
Some overlook the effect of light exposure. Prolonged light, especially direct sunlight, accelerates degradation for many minerals, including zinc citrate dihydrate. Storing it away from windows and fluorescent lights helps protect the active compounds. At one facility, we relied on windowless storage rooms and only opened drums under dim lighting, which helped maintain product color and reactivity.
Avoiding contamination sounds obvious, but things sometimes slip through the cracks. Open containers around dust or other powders invite cross-contamination. I’ve worked with teams that required staff to use gloves and clean scoops each time they handled zinc citrate dihydrate. Investing in these extra precautions reduced the risk of foreign particles mixing in and ensured testing results stayed strong.
Rotation and Inspection
Letting old batches sit at the back of the shelf wastes time and money. “First in, first out” works best. Regular inspections help catch changes in texture, color, or any sign of moisture damage before problems make their way into finished goods. At companies with strict rules, I’ve seen staff check every drum against a logbook—not the most exciting job, but crucial for catching small issues before they grow.
Solutions for Smaller Operators
Not every operation has the budget for climate-controlled warehouses. Small businesses sometimes store ingredients in cramped corners or shared rooms. Even here, using sealable PET or HDPE containers, silica gel packets, and storing away from direct light can keep batches stable for months. For local producers or research groups, coordination—sharing good practices, bulk purchasing of quality containers, and setting shared standards—can go a long way.
Maintaining Long-Term Quality
The storage of zinc citrate dihydrate really comes down to respect for the material and the customer. Get the basics right—cool and dry conditions, airtight containers, protection from light, and a clean workspace. Whether working in a huge warehouse or a small lab, these habits help hold on to quality and keep trust with everyone in the supply chain.
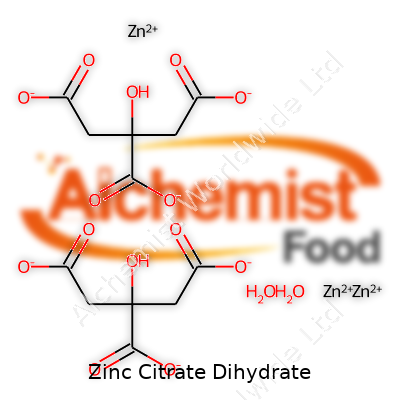
| Names | |
| Preferred IUPAC name | zinc 2-hydroxypropane-1,2,3-tricarboxylate dihydrate |
| Other names |
Zinc(II) citrate dihydrate Citrate of zinc dihydrate Zinc citrate hydrate Dihydrate zinc citrate Zinc(2+) citrate dihydrate |
| Pronunciation | /ˈzɪŋk ˈsɪtreɪt daɪˈhaɪdreɪt/ |
| Preferred IUPAC name | zinc bis(2-hydroxypropane-1,2,3-tricarboxylate) dihydrate |
| Other names |
Zinc citrate Zinc(II) citrate dihydrate Citrate of zinc dihydrate Zinc citrate (2:3) dihydrate |
| Pronunciation | /ˈzɪŋk ˈsɪtreɪt daɪˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 14221-61-3 |
| Beilstein Reference | 3623442 |
| ChEBI | CHEBI:86355 |
| ChEMBL | CHEMBL1201715 |
| ChemSpider | 22833109 |
| DrugBank | DB14545 |
| ECHA InfoCard | 3ac4a46d-6a10-495e-bb3a-aa304f9c8afd |
| EC Number | 231-913-4 |
| Gmelin Reference | 84258 |
| KEGG | C01836 |
| MeSH | D017676 |
| PubChem CID | 159232 |
| RTECS number | ZG1925000 |
| UNII | N9L79L018N |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DF5A2N4X5H |
| CAS Number | 14221-74-0 |
| Beilstein Reference | 3921986 |
| ChEBI | CHEBI:9156 |
| ChEMBL | CHEMBL1201743 |
| ChemSpider | 152221 |
| DrugBank | DB11092 |
| ECHA InfoCard | 03c65f0b-efd7-48e4-9baa-c60081e4e9d1 |
| EC Number | 231-943-8 |
| Gmelin Reference | 671793 |
| KEGG | C14746 |
| MeSH | D018111 |
| PubChem CID | 24893351 |
| RTECS number | ZN6475000 |
| UNII | U83D3Z8U5M |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C12H10O14Zn3·2H2O |
| Molar mass | 574.18 g/mol |
| Appearance | White to almost white powder |
| Odor | Odorless |
| Density | 1.8 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -1.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | Acidity (pKa): 3.13 (for citric acid) |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | -0.9×10⁻⁶ cm³/mol |
| Dipole moment | 6.05 D |
| Chemical formula | C12H14O14Zn3·2H2O |
| Molar mass | 574.25 g/mol |
| Appearance | White to almost white crystalline powder |
| Odor | Odorless |
| Density | 1.80 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -1.7 |
| Acidity (pKa) | 3.4 |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | '-0.9 × 10⁻⁶ cm³/mol' |
| Refractive index (nD) | 1.59 |
| Dipole moment | 2.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.96 J/(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | -1567.3 kJ/mol |
| Std molar entropy (S⦵298) | 302.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1587.1 kJ/mol |
| Pharmacology | |
| ATC code | A12CB05 |
| ATC code | A12CB02 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, Exclamation mark, Warning, H319: Causes serious eye irritation. |
| Pictograms | Corrosive, Health Hazard, Exclamation Mark |
| Signal word | Warning |
| Hazard statements | H410: Very toxic to aquatic life with long lasting effects. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Autoignition temperature | > 450 °C (842 °F) |
| Lethal dose or concentration | LD50 Oral Rat 2,840 mg/kg |
| LD50 (median dose) | LD50 Oral Rat: 2,846 mg/kg |
| NIOSH | ZT3500000 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 30 mg/day |
| IDLH (Immediate danger) | No IDLH established. |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H332 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 Oral Rat: 2,950 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Zinc Citrate Dihydrate is >2000 mg/kg (oral, rat) |
| NIOSH | ZT3500000 |
| PEL (Permissible) | 15 mg/m3 (total dust), 5 mg/m3 (respirable fraction) as Zn |
| REL (Recommended) | TRS 044 |
| Related compounds | |
| Related compounds |
Zinc citrate Zinc gluconate Zinc sulfate Zinc acetate Zinc oxide Citric acid |
| Related compounds |
Zinc citrate Zinc sulfate Zinc acetate Zinc gluconate Zinc chloride |

