Zinc Citrate: A Closer Look at Its Role, Development, and Future
Historical Development
Zinc citrate carved a space for itself after researchers discovered that combining zinc with citric acid produced a highly bioavailable supplement. Scientists in the 20th century realized that zinc, often overlooked as a trace element, played a bigger role in human health than most thought. Older civilizations didn’t know much about citrate complexes, but once food chemistry advanced, the importance of compounds like zinc citrate came to light. Over the years, manufacturers streamlined citric acid extraction and zinc purification, allowing for cleaner and more affordable production. This helped zinc citrate find a place in food fortification and pharmacy shelves all over the world.
Product Overview
Zinc citrate pops up most often as a dietary supplement, toothpaste additive, and food fortifier. It stands out because it dissolves well in water and offers up zinc in a way the body can use. People often prefer zinc citrate over other forms like zinc oxide or zinc sulfate, because it absorbs more efficiently, causing fewer stomach problems for sensitive users. Pharmaceutical companies gravitate toward it for its mild flavor, letting them slip it into tablets or chewables without much hassle.
Physical & Chemical Properties
Zinc citrate usually looks like a white powder or granule, slightly tart, with almost no smell. It does not clump much in storage, thanks to the nature of citrates. Water solubility isn’t the highest among zinc salts, but it still works well enough in aqueous mixtures, especially if the pH stays close to neutral. Chemically, it combines two zinc ions with three citrate ions, forming a stable and non-hygroscopic compound. Melting point sits high, which allows it to handle a range of processing scenarios. This makes it useful in tablets that may experience heat in manufacture.
Technical Specifications & Labeling
Most commercial zinc citrate claims a zinc content in the range of 31-35%. Absence of heavy metals, such as lead and cadmium, remains non-negotiable for pharmaceutical grade batches—rigorous screening and testing ensures this. Labels must state the net zinc content, use-by date, batch number, and any significant allergens or carriers used in preparation. Supplements in the United States generally follow FDA labeling requirements, and food additives need to comply with country-specific purity and content standards. A Certificate of Analysis often travels with every bulk shipment, giving details on purity, moisture, and appearance.
Preparation Method
Producing zinc citrate starts with combining purified zinc oxide or carbonate with citric acid in water, usually followed by slow mixing at moderate temperatures. The reaction generates zinc citrate and releases carbon dioxide if carbonate is used. After precipitation, filtration removes unwanted side products, then the slurry gets washed and dried to produce the final powder. Some producers use spray-drying for better control of particle size, helping the powder flow more easily for industrial applications. Maintaining good process control means the end product meets pharmaceutical or food-grade limits for contamination and microbial counts.
Chemical Reactions & Modifications
Zinc citrate stays stable through most of the pH range that foods or supplements might face, but it can break down if exposed to strong acids or bases for long periods. It won’t react violently with most common food additives, making it a safe bet in blends. Scientists sometimes tweak the basic recipe, substituting part of the citric acid with other organic acids to control solubility or taste. Converting zinc citrate into other zinc compounds—such as by adding strong acids or heating it in certain ways—doesn’t happen much outside research labs, as most consumers care more about gentle bioavailability than about industrial chemistry.
Synonyms & Product Names
On supply chain labels or regulatory documents, zinc citrate may appear as zinc(II) citrate, trizinc dicitrate, or using its chemical formula, Zn3(C6H5O7)2. Some supplement brands call it by proprietary trade names, though few stray far from “zinc citrate” to avoid confusing consumers. In multinational catalogs, you might spot names like “citrate of zinc” or “zincum citricum,” especially among ingredient suppliers catering to the pharmaceutical industry.
Safety & Operational Standards
Handling zinc citrate in large quantities seldom brings hazards, but dust might irritate eyes or the respiratory tract if workers ignore ventilation recommendations. Wearing gloves and dust masks during processing suffices for most workplace environments. The compound doesn’t build up in the body to dangerous levels under normal use—excess simply passes out through urine. Regulatory agencies like the FDA and EFSA set strict maximum limits for zinc content in supplements and food to guard against overconsumption, as chronic high intake can hurt copper absorption or gut health. GMP protocols and ISO standards guide manufacturers through production, labeling, and batch tracing, giving everyone in the supply chain better accountability.
Application Area
Industry use of zinc citrate spreads across nutritional supplements, oral care products, and fortified foods. In toothpastes, it blocks the compounds that cause bad breath and helps slow plaque build-up. Food technologists add zinc citrate to breakfast cereals and dairy drinks to counteract deficiencies in populations that miss out on enough zinc from their diet. Chewable tablets and lozenges for cold remedies also depend on its profile, as many clinical trials point to a role for zinc in shortening or reducing the severity of upper respiratory infections. Its relative gentleness on the stomach compared to other forms helps manufacturers address complaints from customers who tried cheaper zinc options in the past.
Research & Development
Ongoing research continues to study how zinc citrate’s structure improves zinc uptake in the human gut. Scientists look for new ways to blend it into supplements that target groups most at risk for deficiency—pregnant women, elderly, and children—while minimizing aftertaste or gastrointestinal side effects. Advanced manufacturing teams experiment with encapsulation and granulation to stabilize the product, so it flows better in automated filling lines. There is also growing interest in its role for plant-based and vegan nutrition, since plant-based diets can fall low in bioavailable zinc, and zinc citrate offers a versatile, animal-free way to boost content.
Toxicity Research
Toxicity reviews back up zinc citrate’s safety at recommended doses, with animal studies rarely showing harm unless intakes reach far above normal ranges. Long-term use, just like any zinc source, can zap copper or iron absorption if users ignore upper intake levels set by health authorities. In toothpaste or oral care products, the ingredient does not reach harmful levels even with chronic use, according to clinical data. Poison control records hardly ever mention zinc citrate as a source of acute problems, and labeling rules for maximum daily exposures continue to prevent problems before they start.
Future Prospects
New trends in preventive health care and fortification strategies point to more places where zinc citrate will show up in everyday products. As knowledge spreads about marginal zinc deficiency’s impact on immune function, companies look for bioavailable, cost-effective forms of zinc with clean safety records and minimal side effects. Research may soon branch into targeted delivery technologies to make zinc citrate even more effective for groups with absorption issues. Food researchers also explore combinations of zinc citrate with other micronutrients to boost their collective benefit. The supplement industry grows each year, and ingredients like zinc citrate will likely ride that wave, provided regulatory bodies keep safety uppermost and manufacturers act responsibly.
What are the health benefits of Zinc Citrate?
Keeping the Immune System Ready
Growing up, I noticed that colds came around like a bad penny. Flus would sweep through my school each season, and my mother would stock up on orange juice and chicken soup. Only much later did I understand that those sniffles have a strong link to how much zinc you have in your system. Zinc citrate, a type of supplemental zinc that absorbs well and feels gentle on the stomach, helps keep immune function on alert. Modern research confirms this: lacking zinc can leave you at higher risk of infections, slow wound healing, and lengthen recovery time after minor illnesses. No fancy jargon here—your cells need zinc every day to build proteins, repair DNA, and direct white blood cells in their fight against bacteria and viruses.
Supporting the Senses: Taste, Smell, and Vision
Meals lose their flavor when zinc runs low. When I visited my grandmother at her assisted living home, she’d sometimes ask, “Why does food taste dull?” It turns out, zinc is a key helper for taste buds and sensory nerves. A shortfall leads to changes in appetite and a lost interest in eating. Zinc citrate helps restore proper taste and smell for older adults and people recovering from illness. It’s not just about appetite—strong evidence shows zinc plays a role in keeping your eyes healthy, too, particularly as you hit your 50s and 60s.
Smoother Digestion and Nutrient Use
Anyone with a fussy stomach learns quickly which supplements to trust. Zinc citrate dissolves more easily than other forms like zinc oxide, making it gentler for those with sensitive stomachs. From working with nutrition clients, I’ve seen people stick with zinc citrate over others because it doesn’t trigger nausea the way some multivitamins do. Better absorption means your body actually uses what you swallow. For those eating vegetarian or plant-based diets, this matters even more since plant foods have substances that block zinc absorption from food.
Hormonal Balance and Skin Healing
Teenagers in my community spent years trying to clear up acne, only to find out that topical creams weren’t always the answer. Zinc, especially in the citrate form, helps regulate hormones like testosterone, which reduces stubborn breakouts. Beyond acne, zinc helps skin rebuild after minor cuts or sunburn, helping wounds close and reducing the odds of infection. As an adult, I’ve reached for zinc during winter’s onslaught of dry skin and minor scrapes from hiking—skin seems to repair itself much more quickly with steady intake.
Addressing Modern Gaps
Studies from the National Institutes of Health show that millions of Americans don’t meet daily zinc needs, mostly due to modern processed diets. Those who drink little milk and eat less meat—like many older adults or people following vegetarian diets—fall behind fastest. Zinc citrate offers a reliable solution. It partners well with a daily meal or snack, delivering what food sometimes misses.
Smart Supplement Use
Anything in excess creates new problems. High-dose zinc, above 40 mg per day for adults, can block copper absorption and upset digestion. Consulting with a registered dietitian or health provider helps you match the right dose to your needs. Bringing in more zinc-rich foods—oysters, pumpkin seeds, fortified cereals—along with a quality zinc citrate supplement, fills nutritional gaps that modern life creates.
Zinc citrate isn’t a magic ingredient, but it packs a quiet punch in daily routines, offering better immunity, faster healing, and a nudge toward balanced nutrition. It’s worth considering for those looking to stay on top of health, especially as nutritional needs shift with age, lifestyles, and changing dietary patterns.
How should I take Zinc Citrate supplements?
Why Consider Zinc Citrate?
Zinc shows up in headlines every year, usually around cold season. Diets low in seafood, red meat, or beans don't always stack up to what the body craves for zinc. Zinc citrate appeals because it absorbs easily and doesn’t upset the stomach like zinc sulfate can. Immune function, wound healing, and even your sense of taste rely on steady zinc levels.
How Zinc Citrate Fits in a Daily Routine
Mornings start busy in most households. Zinc citrate works best taken on an empty stomach—just a glass of water about an hour before breakfast. Food with lots of fiber or calcium, like cereal with milk, slows down absorption. I learned the hard way that coffee right after swallowing a supplement can ruin any benefit, so I wait for coffee until after breakfast if I take zinc.
Nausea sometimes sneaks up if you haven’t eaten for hours or if the dose runs high. On those days, swapping to after breakfast or pairing zinc citrate with a light snack like toast or fruit helps. From experience, dividing tablets between morning and evening if the label suggests larger amounts keeps things gentle on the stomach.
Getting the Amount Right
The Recommended Dietary Allowance (RDA) for zinc sits at 11 mg a day for most men and 8 mg for women. More isn’t always better. Going over 40 mg a day long-term tips the scale toward trouble—metallic taste in the mouth, nausea, and sometimes lowering copper too much. A reputable supplement labels the elemental zinc content clearly. Always check that number, not just the “zinc citrate” amount.
Do You Need a Supplement?
Many people meet their zinc needs through food, especially those eating meat, nuts, and dairy. Vegans, vegetarians, or people with digestive issues sometimes need a boost. Lab tests, like serum zinc levels, help pinpoint deficiencies. It pays to talk with a healthcare provider or registered dietitian before starting daily supplements, especially for kids, pregnant folks, or anyone with health conditions.
Choosing Quality
Supplement aisles overflow with options. Pick a brand that gets tested independently—look for ConsumerLab, NSF, or US Pharmacopeia seals. These tests catch contaminants and guarantee what’s on the label matches what’s in the pill. Store zinc citrate in a cool, dry spot away from sunlight. Bottles with clear instructions and batch numbers signal care in production.
Mixing with Other Supplements
Multi-mineral supplements, iron, calcium, and magnesium can compete for absorption. I keep zinc and iron at different times of day if I take both. Even multivitamins with everything packed into one dose can shortchange you on zinc’s actual benefit. Stand-alone zinc citrate works best for targeted support, not as a multi-mineral afterthought.
Potential Paths Forward
Access to high-quality, clearly dosed supplements shapes public trust. Clear regulations and transparent labeling help those looking for safe ways to fill nutritional gaps. Health educators and primary care teams play a part, too, flagging up-to-date guidance and helping people steer through choices that match their health goals and diets.
Are there any side effects of Zinc Citrate?
Looking at What Happens With Zinc Citrate
Zinc offers real benefits for our bodies, supporting the immune system, helping wounds heal, and contributing to taste and smell. Zinc citrate shows up in multivitamins, throat lozenges, and supplements at most pharmacies. Many folks reach for it hoping to stay healthy, but it pays to know the facts about its possible downsides.
Common Side Effects People Notice
Gastrointestinal discomfort ranks at the top of the list. If you've ever taken zinc citrate on an empty stomach, the queasy feeling can hit quickly. Some people feel nauseated or might even vomit. Diarrhea or stomach cramps come up in reports, too. It's not rare—a 2021 study published in Nutrients mentioned digestive complaints in about 10% of participants using zinc supplements over a few weeks. Grocery store supplements don’t always mention these effects in bold print, but folks discover them on their own.
Taste changes show up on occasion. A metallic taste or loss of taste can sneak up on regular users, especially with higher doses. Chewable tablets or lozenges seem to bring this on more than pills you swallow. The issue usually fades after stopping the supplement, but it catches some people off guard.
What Happens With Too Much Zinc?
Chasing good health with zinc can backfire if you go overboard. Consistent use above the recommended daily limit—about 40 milligrams for most adults—can reduce copper levels in the body. Copper is important for nerve and immune health, and too little causes fatigue and problems with balance over time. Some folks taking high-dose zinc to fight colds have run into copper deficiency, even ending up with numb or tingling hands and feet.
Immune suppression becomes a real risk if zinc piles up week after week. Ironically, that can make you more likely to catch what you were trying to dodge. Chronic high intake also links to lower good cholesterol, which can strain the heart over years. A meta-analysis in the American Journal of Clinical Nutrition tracked studies showing lower HDL levels after months of high-dose supplementation.
Risks for Certain Groups
Pregnant and breastfeeding women must stick to recommended amounts. Their zinc needs are higher, but too much does more harm than good for mom and baby. People with kidney or liver troubles process zinc differently, raising the chance of side effects. Children face higher risk with adult-sized doses; they've ended up with vomiting and headaches from sweetened lozenges they grabbed like candy.
Drug interactions also enter the picture. Zinc citrate binds with some antibiotics and arthritis drugs, making the medicine less effective. Throwing supplements and prescriptions into the mix without asking a pharmacist can cause more frustration than relief.
Smart Solutions and Safe Use
Taking zinc citrate with food usually helps the stomach settle. Dividing up the daily dose keeps side effects milder. Checking supplement labels matters since some products pack in far more zinc than advertised. Sticking close to the recommended daily value (8 mg for women, 11 mg for men) avoids problems for most healthy people.
Doctors and registered dietitians provide the best advice if you consider regular use. Regular bloodwork shows whether zinc levels look healthy or not. Health is built on balance—swinging too far in any direction, even with good intentions, often brings unintended problems.
Can I take Zinc Citrate with other medications?
Sorting Out the Confusion Around Zinc Citrate Supplements
Walk down the vitamin aisle, and a person spots row after row of mineral supplements. Zinc citrate often appears on the label, promising immune support. A lot of us think, “If it's sold over the counter, it must play nicely with what’s in my medicine cabinet.” Not always. Some questions need answers before mixing supplements like zinc citrate with prescription or over-the-counter drugs.
Real Talk: What Does Zinc Do In the Body?
Zinc helps keep over 300 enzymes working in the body. It helps the immune system, heals skin, and keeps senses like taste sharp. Zinc citrate dissolves better than plain zinc and goes into the bloodstream more quickly. That makes it an appealing choice for people looking to boost their health, and for those with mild zinc deficiency. Yet, popping a zinc citrate pill isn’t as straightforward as grabbing a multivitamin.
Possible Drug Interactions
Mixing zinc citrate with some meds can set off trouble. For example, people taking antibiotics like tetracyclines or quinolones can end up blocking absorption of both the drug and the zinc. It works a bit like two cars trying to squeeze through a one-lane tunnel at the same time. Doctors typically recommend taking zinc or these antibiotics at least two hours apart.
Medicine for bones, like bisphosphonates that treat osteoporosis, can also get tangled up with zinc. Zinc in the gut may cut down how much of that bone medicine makes it into the body. The same goes for certain water pills, often prescribed for high blood pressure. Some “thiazide” diuretics actually make the kidneys get rid of more zinc. Taking high-dose zinc alongside them could tip the balance and, over time, cause low copper or iron.
Other Factors Most People Miss
Antacids, the chewable kind that help with heartburn, sometimes block zinc from being absorbed. Some people pop antacids daily, not realizing it could mess with mineral levels over months. This generally doesn’t affect people who only use those remedies once in a while.
Too much zinc itself can block copper uptake. Over years, this can lead to nerve problems or weakened blood cell production. Using zinc citrate for months without breaks adds risk, especially for people who don’t eat a lot of copper-rich foods.
Experience from the Pharmacy Counter
Years in the pharmacy world taught me a few patterns. Most people only mention their prescription meds, forgetting to list the vitamins and minerals. Shoppers rarely connect fatigue, gut issues, or taste changes to zinc or medication interactions until a problem builds up. Those on complex medication regimens or dealing with ongoing health issues run the biggest risk.
How to Stay Safe and Get What You Need
There’s no one-size-fits-all advice. Bringing a full list of pills, supplements, and even herbal teas to your health checkup works best. Pharmacists track these combinations every day, looking for sneaky interactions. Tools from the FDA and resources from the National Institutes of Health offer reliable info, but nothing beats asking a real person with experience. Health rarely works on autopilot — mixing medications and supplements should never be a guessing game.
Zinc citrate plays an important role, but smart timing and good communication with your care team keep things running smoothly. Everyone deserves to get the benefits without the mess that unwanted side effects can cause.
What is the recommended dosage of Zinc Citrate?
Zinc in the Real World
Zinc grabs a spotlight as an essential mineral, and it’s easy to overlook how much a body relies on tiny amounts of it. From supporting the immune system to helping wounds heal, zinc’s influence shows up in places most folks don't even notice until something’s missing. Zinc citrate, often found in supplements and some lozenges, stands out because the body can absorb it more readily than some other forms.
Guidance from Trusted Sources
Doctors and dietitians cite numbers based on both research and evidence gathered over decades. The National Institutes of Health recommends that healthy adults get about 8 mg of zinc per day for women and 11 mg for men, with amounts a little higher for people who are pregnant or breastfeeding. These numbers cover zinc from all sources—food, supplements, and even trace amounts from water. For zinc citrate specifically, manufacturers design most supplements to deliver close to these daily numbers, usually putting around 15-30 mg of elemental zinc in a tablet or capsule. That’s because not all the citrate compound is zinc itself—a 50 mg tablet of zinc citrate contains closer to 15 mg of actual zinc.
Supplements: Why More isn’t Better
For folks turning to zinc citrate to bolster everyday nutrition, one pill usually covers what’s missing from diet gaps. Some people believe taking more will speed up the benefits, especially if they feel under the weather. This idea doesn’t pan out in the long run. Zinc sticks around in the body, and too much of it gets in the way of copper and iron absorption, causing its own set of problems. Signs of excess—like stomach cramps, nausea, and headaches—show up when intakes climb past 40 mg a day through supplements over days or weeks. Most healthcare professionals caution against consistently going over the recommended amount without a clear medical reason.
Population Differences and Unique Needs
Personal history and daily habits shape someone’s true zinc requirement. Anyone with a diet relying heavily on grains and legumes but little meat or dairy finds absorption lower, since certain food compounds tie up zinc. Vegetarians may end up closer to borderline deficiency—so a supplement, like zinc citrate, sometimes fills those gaps. Age changes things too; older adults don’t absorb minerals as efficiently, and their taste or appetite may shift, so a doctor might recommend a low-dose supplement. The same goes for people with digestive conditions that interfere with nutrient absorption such as Crohn’s disease.
Quality Advice: Listen to Real Experts
The supplement aisle brims with choices and promises, but advice rooted in deep clinical experience beats catchy labels every time. Pharmacists and registered dietitians review real lab evidence and will weigh your history and other medicines before suggesting a dose. WebMD and the NIH both remind readers not to leap into high doses just because they read about it online. Blood work tells much more than guessing, so a healthcare provider will set a safe, effective amount based on real needs rather than vague “booster” claims.
Making Informed Choices
Years of personal observation have shown me that chasing quick fixes with supplements rarely leads to lasting benefits. Real gains come from a diet rich in meat, seafood, beans, and nuts. For those who choose zinc citrate, sticking close to label directions and checking with a health expert forms a stronger defense for your body. Any supplement works best as a backstop for a balanced routine, not a replacement for good food and mindful living.
| Names | |
| Preferred IUPAC name | zinc 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Zinc dihydrogen citrate Zinc(II) citrate Citrate of zinc |
| Pronunciation | /ˈzɪŋk ˈsɪtreɪt/ |
| Preferred IUPAC name | zinc 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Trizinc dicitrate Zinc(II) citrate Zinc citrate tribasic |
| Pronunciation | /ˈzɪŋk ˈsɪtreɪt/ |
| Identifiers | |
| CAS Number | 546-46-3 |
| Beilstein Reference | 1911731 |
| ChEBI | CHEBI:3335 |
| ChEMBL | CHEMBL1201641 |
| ChemSpider | 12314 |
| DrugBank | DB14545 |
| ECHA InfoCard | 03b9d75d-5096-431d-b22d-8b306dd4b403 |
| EC Number | 263-896-0 |
| Gmelin Reference | Gmelin Reference: **Zn/Cit/1** |
| KEGG | C14336 |
| MeSH | Dental Agents |
| PubChem CID | 24598636 |
| RTECS number | ZH5200000 |
| UNII | S5SY353YP7 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | `DTXSID1020634` |
| CAS Number | 546-46-3 |
| Beilstein Reference | 3572497 |
| ChEBI | CHEBI:34542 |
| ChEMBL | CHEMBL1201781 |
| ChemSpider | 159348 |
| DrugBank | DB11092 |
| ECHA InfoCard | 03d1ea36-81a6-4097-b680-3a44c1098dad |
| EC Number | 200-014-9 |
| Gmelin Reference | Gmelin Reference: **1740** |
| KEGG | C11315 |
| MeSH | D017678 |
| PubChem CID | 24599422 |
| RTECS number | ZF7875000 |
| UNII | N4J4176ZSE |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID5020662 |
| Properties | |
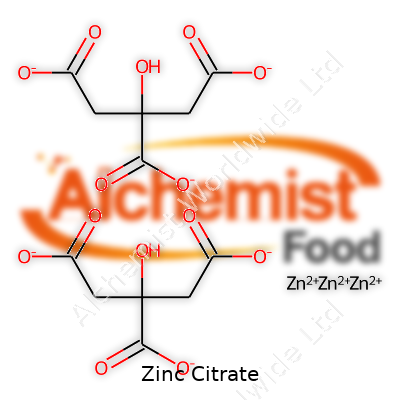
| Chemical formula | C12H10O14Zn3 |
| Molar mass | 574.3 g/mol |
| Appearance | White to almost white, crystalline powder |
| Odor | Odorless |
| Density | 1.74 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.1 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.4 |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | χ = -33.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.74 D |
| Chemical formula | Zn3(C6H5O7)2 |
| Molar mass | 574.3 g/mol |
| Appearance | White to almost white, crystalline powder |
| Odor | Odorless |
| Density | Density: 2.3 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -1.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.4 |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | -1.1×10⁻⁶ |
| Refractive index (nD) | 1.52 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 256.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1568.5 kJ/mol |
| Std molar entropy (S⦵298) | 344.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1544 kJ/mol |
| Pharmacology | |
| ATC code | A12CB06 |
| ATC code | A12CB05 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Wash hands thoroughly after handling. If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. |
| Lethal dose or concentration | LD₅₀ (oral, rat): 2,584 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2,843 mg/kg |
| NIOSH | WI2275000 |
| PEL (Permissible) | 15 mg |
| REL (Recommended) | 11 mg |
| IDLH (Immediate danger) | Not listed. |
| Main hazards | Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | **"GHS07, Warning, H319, Causes serious eye irritation, P264, P280, P305+P351+P338, P337+P313"** |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Lethal dose or concentration | LD₅₀ (oral, rat): 3,000 mg/kg |
| LD50 (median dose) | 291 mg/kg (rat, oral) |
| NIOSH | ZH4875000 |
| PEL (Permissible) | 5 mg/m³ |
| REL (Recommended) | 11 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Zinc gluconate Zinc sulfate Zinc acetate Zinc oxide Zinc chloride |
| Related compounds |
Citric acid Zinc gluconate Zinc sulfate Zinc acetate Zinc oxide Sodium citrate Calcium citrate |

