Zinc Carbonate: A Deep Dive into Its Role, Challenges, and Future
Historical Development of Zinc Carbonate
Interest in zinc carbonate emerged while miners combed through ore deposits centuries ago. The simple compound, sometimes discovered as smithsonite, provided a steady source of zinc long before metallurgists figured out pure zinc extraction. Knowledge about its chemical formula, ZnCO₃, expanded alongside the Industrial Revolution, as researchers looked for ways to refine zinc and boost industrial outputs. Traditional mining towns like those scattered across England and the American Midwest considered the greenish mineral treasure, since it helped galvanizers, chemists, and paint makers fuel their booming trades. The story of zinc carbonate goes hand in hand with a rising appreciation for fine chemical manipulation and precise product manufacturing.
Product Overview
Zinc carbonate appears as a white, powdery solid, sometimes with a faint greenish tinge due to iron impurities in natural samples. Laboratories and manufacturers produce this compound for its utility in paints, ceramics, cosmetics, agriculture, and pharmaceuticals. Each sector values the compound differently. Ceramics manufacturers count on zinc carbonate for its fluxing properties, while paint makers use it as a pigment and stabilizer. As regulatory scrutiny increases, industry has adjusted its packaging and documentation to provide clear guidance on use and handling, ensuring companies meet safety and environmental expectations.
Physical and Chemical Properties
This compound doesn’t dissolve easily in water, but reacts with strong acids to yield carbon dioxide and soluble zinc salts. At roughly 140°C, it begins to decompose, releasing CO₂ and forming zinc oxide—a transformation that turns up often in industrial processes. Handlers notice it feels soft and light, with minimal odor, and bulk containers move easily through most manufacturing environments. Zinc carbonate maintains stability at room temperature, resisting most mild chemical attacks, yet displays keen sensitivity to stronger acids and heat.
Technical Specifications and Labeling
Producers define product purity with figures above 97 percent, limiting substances like lead, cadmium, and arsenic because these heavy metals raise red flags for health and environment. Containers receive detailed labels listing batch number, production date, recommended storage conditions, and hazard data under global harmonization systems. Modern regulations demand clear transport labels and documentation, helping workers avoid accidental exposure or improper disposal. Handling guidelines and emergency procedures now appear front-and-center on packaging, a shift driven by rising expectations for worker safety and transparency.
Preparation Method
To create zinc carbonate in the lab or on a larger scale, chemists often react zinc salts like zinc sulfate or zinc chloride with sodium carbonate or sodium bicarbonate solutions. The resulting white precipitate settles, gets filtered, washed, and then dried under controlled conditions. This method offers steady yields and allows chemical engineers to adjust parameters for particle size or purity. Industrial setups recycle process solutions, cutting waste and minimizing costs, aligning with both economic sense and environmental stewardship.
Chemical Reactions and Modifications
One of the most straightforward reactions involves zinc carbonate’s decomposition when heated, producing zinc oxide and carbon dioxide gas. Acids break the carbonate down too, generating soluble zinc compounds useful for further synthesis or industrial application. Sometimes, researchers tweak the basic chemistry by introducing co-precipitation steps, inserting additives, or controlling the pH during reaction to fine-tune the product for ceramics, pharmaceutical, or paint applications. Advances in reaction engineering help reduce impurities and energy use, keeping processes efficient and environmentally sound.
Synonyms and Product Names
Smithsonite stands as the most recognized mineral form of natural zinc carbonate, named for the English geologist James Smithson. The compound surfaces under names like Zincous Carbonate or Calamine, although real calamine may also contain zinc silicate. On product labels, companies list Zinc Carbonate Basic, Zinc Monocarbonate, or simply ZnCO₃. These names allow buyers, regulators, and lab personnel to cross-check substances, avoiding mix-ups that could stall a project or disrupt a production run.
Safety and Operational Standards
Strict guidelines shape every step of zinc carbonate’s production and use. Workers must use gloves, dust masks, and protective goggles—standard personal protective equipment—since fine powders may irritate skin, eyes, or lungs. Regular training and workplace monitoring limit accidental exposure. Environmental agencies require firms to track waste streams, recycle where possible, and limit emissions of dust and carbon dioxide. Storage facilities follow rules on temperature, humidity, and chemical segregation to prevent reactions with strong acids or oxidizers. Updated standards focus on both worker safety and responsible stewardship of air, water, and soil.
Application Area
Pharmaceutical companies use zinc carbonate to supply the zinc patients need, especially for treating zinc deficiency. In cosmetics, the compound finds its way into skin protectants and anti-dandruff formulations. Perspiring athletes and outdoor workers might find it in topical ointments or barrier creams. Agriculture relies on zinc carbonate to boost soil zinc levels, raising crop yields and improving plant health. Glass, ceramic, and paint makers run through vast quantities, exploiting its flux and pigment properties to produce everything from fine dinnerware to durable building materials. Each application calls for careful attention to purity, particle size, and regulatory guidelines.
Research and Development
Teams at universities, industry consortia, and government labs probe new ways to recycle zinc-containing wastes into high-purity zinc carbonate. Some groups experiment with nanostructured forms for use in advanced electronics and energy storage. Point-of-use purification processes and new chemical reactors help reduce waste and energy use when producing the compound. Collaborative projects aim to develop tailor-made grades for pharmaceuticals, food supplements, and cosmetics. The push for greener production drives interest in bio-based synthesis, exploring how certain microbes might turn out zinc carbonate under mild conditions.
Toxicity Research
Most studies agree that plain zinc carbonate, in limited quantities, poses low risk to humans. Problems arise with inhaling fine dust or accidental ingestion by children. Chronic overexposure to zinc causes symptoms like nausea, vomiting, and stomach cramps, although reaching these levels requires repeated, careless handling. Researchers track worker health and environmental release, publishing data on safe exposure levels and best practices in case of spills. Animal studies and workplace surveys help regulators set exposure limits and recommend safe working hours.
Future Prospects
Global interest in sustainable materials points toward greener zinc carbonate processes, especially those minimizing waste and energy. Engineers develop modular, closed-loop plants that offer both higher purity and reduced environmental footprints. Demand in electronics, as zinc finds new use in batteries and sensors, grows rapidly. With a renewed spotlight on soil health and nutrition, more countries turn to zinc carbonate fertilizers to prevent zinc deficiency in both people and crops. Research continues on advanced forms—nanoparticles, composites, and functional coatings—unlocking new electrical, optical, or catalytic properties. As regulatory pressure for transparency, safety, and sustainability increases, only companies able to document clean supply chains and clear labeling will thrive. Responsible manufacturing and careful stewardship will shape the future of zinc carbonate in every sector, from medicine and agriculture to industry and technology.
What are the main uses of Zinc Carbonate?
What’s Behind Its Everyday Importance?
People outside of manufacturing or science circles probably don’t think much about zinc carbonate. I sure didn’t, until one summer job at a small ceramics factory put me face-to-face with containers of the stuff, powdery and white, ready to get scooped into glazes. That experience stuck with me, showing how a basic-looking mineral can quietly shape everything from art to health.
Ceramics: Bringing Life to Pottery
Most folks may only think about pottery as either coffee cups or vases. Turns out, zinc carbonate transforms dull clay glazes into surfaces that reflect light and color in a distinct way. It’s more than just a touch-up. Ceramicists blend zinc carbonate to give certain pieces a crisp, glossy finish, and to add resistance against wear. You can hold a handmade mug, run your thumb over the surface, and never guess it owes some of its shine and strength to a mineral like this.
Personal Care and Sunscreens
Zinc carbonate helps in places you’d never guess, like inside toothpaste and sunscreen. Its low toxicity and gentle effects make it ideal in toothpaste as a mild abrasive and a way to control odors. Washing with a toothpaste that uses this mineral, people get extra cleaning power without harshness. It’s also used in some topical lotions and creams, making a difference for folks with sensitive skin. The zinc part does more than just polish—zinc ions help keep bacteria at bay, reducing minor infections and helping along the healing process for small skin irritations. I’ve met dermatologists quick to recommend creams where zinc carbonate plays a subtle but key role for everyday skin care.
Chemical Industry Foundations
So much of modern life spins on the wheels of the chemical industry. Zinc carbonate often serves as a starting point, where it’s processed into zinc oxide or used to help with the preparation of other zinc compounds. Industry uses these downstream materials for batteries, rubber, plastics, and paints. It’s easy to overlook how tubular batteries or sturdy tire rubber might lean, early in production, on a white mineral like this. Companies turn to zinc carbonate for its consistency and cost-effectiveness, helping manufacturers run large-scale operations smoothly.
Medicine and Nutrition
Doctors have trusted zinc’s health benefits for over a century. Zinc carbonate sometimes finds its way into supplements, especially for people who need extra zinc in their diet. The body uses zinc for everything from healing wounds to supporting the immune system. Nutritionists point to deficiencies as culprits for all sorts of stubborn health problems, especially in developing regions. By offering a stable, mild form of zinc, this compound supports efforts to fight malnutrition and boost public health outcomes in communities that need it most.
Paths Toward Safer and Greener Use
Industrial and health-focused uses come with responsibilities. Environmental safety stands out—overuse or careless disposal can disrupt soil and water balance. Factories producing or handling zinc carbonate take steps to limit dust and manage waste properly. It’s on everyone in the supply chain, from mine to lab to factory, to swap short-term convenience for long-term care. Researchers keep looking into alternatives, too, aiming for mineral sources and processing methods that lower the environmental load while keeping the benefits strong.
Is Zinc Carbonate safe for human consumption?
Understanding Zinc Carbonate
Zinc pops up everywhere in the body. It keeps immune systems working, helps wounds heal, and supports healthy growth and development. Zinc carbonate shows up more often in chemistry labs and industry settings, not so much in nutrition or medicine. Over-the-counter zinc supplements stick to forms like zinc gluconate, zinc picolinate, or zinc citrate, because they break down easily and release zinc that the body uses well.
What Happens Inside the Body
If small amounts of zinc carbonate make it into the diet, the stomach acid breaks it up. The body pulls zinc from this breakdown, which then joins the natural pool of zinc needed for hundreds of jobs. But unlike the supplements made just for swallowing, zinc carbonate can feel clumsy. Powder often feels rough on the mouth, and it takes a lot of stomach acid to break everything down. Uncoated zinc carbonate, especially at high doses, can lead to nausea, cramps, and other problems. Many people, including myself, can remember taking supplements on an empty stomach and paying for it later.
Safety Review and Dosage Concerns
Getting enough zinc ranks as essential. Too little leads to immune trouble, skin rashes, and sluggish healing. Too much, on the other hand, can hurt the stomach, lower copper levels, and even dampen the immune response. The recommended dietary allowance for adults sits at about 8–11 mg daily. People rarely run into trouble with regular food sources like beans, nuts, or red meat. High doses from powders or tablets—more than 40 mg every day—carry a real risk for trouble.
With zinc carbonate, the question isn’t just whether it contains zinc but also whether the body absorbs it well and handles fillers or impurities. In medicine and food, purity matters. Zinc carbonate not made for human use might contain contaminants. Industrial-grade chemicals often do not get the same controls that supplement-quality products must meet. Swallowing chemicals straight from a lab bottle or online industrial source isn’t just risky; it can be dangerous.
Trusted Sources and Professional Guidance
Most doctors and nutritionists stick to better-studied sources for a reason. In the United States, the Food and Drug Administration does not list zinc carbonate as “Generally Recognized As Safe” for use as a food additive. Some countries let manufacturers add it to certain foods as a mineral supplement, but strict limitations apply. Pharmacy shelves offer better-formulated zinc supplements that avoid most problems found with industrial zinc carbonate.
Anyone thinking about adding zinc from new or unusual sources should talk with a healthcare provider. Testing for zinc levels, and reviewing diet and supplement use, gives a more complete picture. Supplements can help fill gaps, but food should always come first. Oysters, beef, lentils, and nuts all offer zinc in a form that the body loves to use.
Protecting Health and Finding Balance
Taking shortcuts with supplements or turning to products not made for people puts health on the line. Personal experience and research agree on this: the best choice is always a safe, quality-controlled product made for human consumption, not something straight from a chemical warehouse. Reading labels, sticking with reputable brands, and consulting with professionals protects health and lets zinc do its work naturally.
What is the chemical formula of Zinc Carbonate?
Understanding Basics: Zinc, Carbon, and Oxygen in One Compound
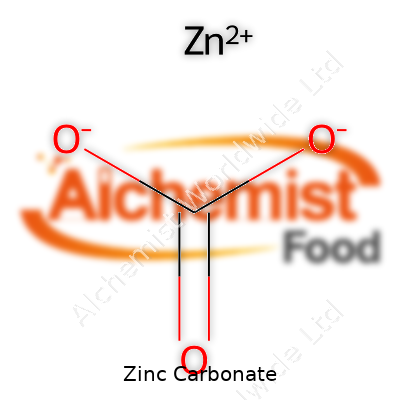
Zinc carbonate carries the formula ZnCO3. Seems simple at first glance, but there’s a story in those letters and numbers. Zinc, a shiny metal found in batteries, supplements, and even pennies, forms stable compounds with other elements. Here, it hooks up with one carbon atom and three oxygen atoms, creating a solid called zinc carbonate. White, powdery, and often overshadowed by more glamorous minerals, ZnCO3 still earns its place in a long list of materials with daily impact.
Zinc Carbonate in the Lab and in Daily Life
Lab techs appreciate zinc carbonate mostly for its reliability. Drop it in an acid, and you’ll see fizzing—the result of carbon dioxide bubbles escaping. That’s not just a science class demonstration; this reactivity helps chemists confirm the presence of carbonates almost anywhere. Artists and tinkerers know it as Smithsonite, a mineral form much loved by collectors for its range of colors. In medicine, ZnCO3 sometimes works as a mild astringent, helping with skin issues and filling out formulations for topical creams. You find it hiding out in ceramics, rubber industries, and even as a precursor in making zinc oxide, another chemical with a story of its own. The reach stretches wider than most expect from a name that pops up in high school chemistry.
Why Knowing the Formula Matters
Folks sometimes underestimate the value of chemical formulas. A formula isn’t just numbers and letters—it’s a roadmap. It tells manufacturers exactly how much of each raw material they need. Clean math equals clean production. In the pharmaceutical world, accuracy is non-negotiable. One missed atom and you have an entirely different compound, which spells trouble—especially in medication. In environmental work, chemists need to know what happens if zinc carbonate spills. Zinc can be both a nutrient and a pollutant. ZnCO3 helps soil sometimes, but too much upsets delicate ecological balances. Knowing the formula helps researchers track, contain, and recycle materials instead of dumping blind.
Potential Issues and Solutions
Disposal and environmental impact demand thought, especially as industrial waste grows. Too much zinc carbonate in water systems risks harming aquatic life, making regulation important. Waste treatment plans need strict checks so runoff doesn’t sneak past filters. Testing upstream and downstream from factories gives clearer data. On a small scale, chemistry teachers can lean on hands-on demos and conversations about safety. Discuss where that fizzing gas ends up, not just the experiment’s pretty visuals. That’s a step toward science literacy, something schools need to emphasize.
Stepping outside the lab, miners still pull zinc-rich ores from the earth. Processing those ores produces both products and byproducts, including zinc carbonate. Smelters face questions about air quality, worker safety, and community health. More sustainable practices make a dent: reusing waste, shifting to lower-emission methods, and supporting transparent supply chains. Regulators push for reporting, inspectors test soil and air, and community advocates demand answers. True progress depends on treating the formula not just as chemistry, but as a marker for change. ZnCO3 isn’t just science; it’s a signal about how we handle earth’s resources.
How should Zinc Carbonate be stored?
Understanding Why Storage Matters
Zinc carbonate sits on a shelf in many labs, factories, and even classrooms, and people often overlook just how hazardous mishandling can get. I’ve seen workplaces where chemicals fill every available space, containers bumped against pipes, and labels faded almost to the point of invisibility. You never think those small mistakes can spiral into serious headaches, but they do—and zinc carbonate fits right into that picture.
So, what makes zinc carbonate storage worth a second thought? Its powder form doesn’t help, since it can float up during spills and react when mixed with the wrong substances. It won’t catch fire, but improper storage opens up risks nobody wants, especially people working day in and out surrounded by chemicals.
Picking the Right Spot
You want zinc carbonate somewhere cool and dry—that much gets printed on every label, but not everyone notices why. Heat and moisture break down a good container fast, turning powder into rock-like lumps or triggering chemical changes nobody asked for. I once helped clean up a storage area after an unnoticed water leak soaked a shelf full of chemical jars. Hours wasted scraping gunky chemical masses from shelves, all because someone kept chemicals under a dripping condenser.
Humidity seeps through cardboard and even poorly sealed plastic. Put zinc carbonate away from sinks and windows. Metal shelves with a solid powder coat do well since they hold up if you wipe them regularly and can handle a stray splash or two. Sloppy storage doesn’t just hit your wallet—it messes with the safety of everyone sharing the space.
Containers Aren’t All Equal
A well-sealed, labeled jar lasts much longer. Glass jars with screw tops give extra peace of mind. Plastic jugs seem convenient until you find them bulging or cracked from slow reactions you can’t even see. Try to keep each container tightly sealed and always upright. Double-check labels before leaving anything on a shelf. Someone once left a batch of white powder in a repurposed coffee container—days later, nobody could say if it was calcium carbonate or zinc carbonate, and the whole batch landed in hazardous waste.
Take labeling seriously. Replace battered old stickers. If you use small containers for portioning, mark them right away with the full name, not just an abbreviation. This habit saves time and helps you keep each chemical separate.
Storing Beyond the Basics
Training plays a huge part in all of this. Some accidents happen because a new staff member or student grabs the wrong jar, thinking one powder looks much like another. Newcomers appreciate a simple, well-demarcated setup: acids in one section, alkalis somewhere else, and zinc carbonate nowhere near strong acids or ammonia-based products, which can trigger unwanted reactions.
Spills will happen. Store a clean-up kit nearby: disposable gloves, a dustpan—dedicated for chemical use—and sealable waste bags. People expect to clean up glass but forget fine powders. You don’t need fancy equipment, just reliable supplies and a plan.
Responsible storage often means revisiting habits, not just following a checklist. Keep it simple, keep it dry, and pay attention to what gets left on each shelf. Those small acts add up to fewer accidents, less wasted material, and safer work for everyone.
References
Centers for Disease Control and Prevention (CDC), “NIOSH Pocket Guide to Chemical Hazards: Zinc carbonate.”National Institutes of Health (NIH) PubChem, “Zinc carbonate compound summary.”Occupational Safety and Health Administration (OSHA), “Chemical hygiene plan requirements.”
Does Zinc Carbonate have any side effects or hazards?
Why Paying Attention to Zinc Carbonate Matters
Zinc carbonate gets into cosmetics, pharmaceutical products, and some industrial processes. Regular folks brushing their teeth or slathering on sunscreen might not recognize it on the ingredient label, but it plays a small role in everyday routines. Lab managers, chemistry teachers, and hobby crafters often handle powder or tablets containing this compound. Its presence isn’t always obvious, so knowing what it can do helps prevent trouble.
Direct Health Effects from Touch or Breathing
Direct contact with zinc carbonate powder can bring on skin and eye irritation. Itching and redness sometimes follow, especially with repeated exposure. Breathing dust can challenge lungs, leading to coughing or sneezing. People with respiratory sensitivities may notice discomfort quicker. The National Institute for Occupational Safety and Health (NIOSH) lists zinc compounds, including zinc carbonate, as substances that may irritate the lining of the lungs and nose if dust is inhaled.
Zinc is an essential mineral, but inhaling or swallowing too much is never good. Short-term symptoms can show up as a sour stomach, nausea, or vomiting. Extended or high-dose exposure leads to headaches or a metallic taste. Metal fume fever has cropped up in factory workers inhaling zinc fumes from molten forms, though zinc carbonate itself rarely reaches temperatures where this risk grows high. Still, grinding or mixing powders always stirs up particles that should not be inhaled.
Chronic Exposure, Accumulation, and Environmental Questions
Prolonged, heavy contact with zinc carbonate can add to total body zinc. Medical nutritionists and toxicologists point out that excessive zinc can lower copper and iron levels in the body, throwing off cell balance. Too much zinc brings immune function down and upsets gut bacteria. No huge pile of evidence singles out zinc carbonate as a regular cause, but health agencies like the US Environmental Protection Agency (EPA) and the European Chemicals Agency both note risks if workers spend years handling dusts with little protection.
Spilled powders can also make environmental impacts. Water runoff from waste piles or industrial accidents sometimes carry trace metals downstream, changing soil and water chemistry for fish and plants. Cleanup standards aim to keep these spills away from crops and rivers. For home users, keeping any powdered chemicals sealed and up high, away from pets or children, cuts risks way down.
Practical Safety Steps
Reading the safety data sheet before working with a new jar or bag of zinc carbonate goes a long way. Gloves, goggles, and a dust mask give protection during short projects. Local exhaust ventilation, or working outdoors, keeps air cleaner. Washing hands after use sounds like common sense, though it’s worth mentioning for anyone teaching kids or working on group projects. Safe storage and proper labeling also prevent accidental misuse.
Rules for disposal usually come from local hazardous material guidelines. Flushing pure zinc carbonate down the drain sends it beyond where treatment plants can catch every particle, so sealed container drop-offs at a household hazardous waste site keep things above board. With smart handling and common sense, risks drop to nearly zero, though ignoring hazards invites unnecessary trouble down the road.
| Names | |
| Preferred IUPAC name | zinc(2+) carbonate |
| Other names |
Zinc Carbonate Basic Zinc(II) carbonate Smithsonite Calamine Zinc monocarbonate |
| Pronunciation | /ˌzɪŋk kɑːrˈbəʊ.neɪt/ |
| Preferred IUPAC name | zinc(2+) carbonate |
| Other names |
Zinc carbonate basic Zinc monocarbonate Smithsonite Calamine Zinc carbonate hydroxide |
| Pronunciation | /ˈzɪŋk kɑːrˈbəʊ.neɪt/ |
| Identifiers | |
| CAS Number | 3486-35-9 |
| Beilstein Reference | 3586811 |
| ChEBI | CHEBI:33344 |
| ChEMBL | CHEMBL1201251 |
| ChemSpider | 47911 |
| DrugBank | DB15797 |
| ECHA InfoCard | 100.032.066 |
| EC Number | 209-942-9 |
| Gmelin Reference | Gmelin Reference: **1948** |
| KEGG | C14674 |
| MeSH | D015212 |
| PubChem CID | 26022 |
| RTECS number | ZF8775000 |
| UNII | SOI2LOH54Z |
| UN number | UN3077 |
| CAS Number | 3486-35-9 |
| Beilstein Reference | 3203915 |
| ChEBI | CHEBI:33314 |
| ChEMBL | CHEMBL1201712 |
| ChemSpider | 30851 |
| DrugBank | DB11092 |
| ECHA InfoCard | echa-ec-209-956-9 |
| EC Number | 209-942-9 |
| Gmelin Reference | Gmelin Reference: 36938 |
| KEGG | C13511 |
| MeSH | D015744 |
| PubChem CID | 23666347 |
| RTECS number | ZF9810000 |
| UNII | J3P2JZ4Z95 |
| UN number | UN3077 |
| Properties | |
| Chemical formula | ZnCO3 |
| Molar mass | 125.38 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 4.4 g/cm³ |
| Solubility in water | Insoluble |
| log P | -6.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 6.2 |
| Magnetic susceptibility (χ) | +118.0e-6 cm³/mol |
| Refractive index (nD) | 1.7 |
| Dipole moment | 0 Debye |
| Chemical formula | ZnCO3 |
| Molar mass | 125.38 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 4.4 g/cm³ |
| Solubility in water | Insoluble |
| log P | -6.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 6.2 |
| Magnetic susceptibility (χ) | -25.1·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.7 |
| Dipole moment | 0 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 81.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -820.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -815.1 kJ/mol |
| Std molar entropy (S⦵298) | 81.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -822.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -427.8 kJ/mol |
| Pharmacology | |
| ATC code | A12CB05 |
| ATC code | A12CB05 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Harmful if swallowed. Causes skin and eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H410: Very toxic to aquatic life with long lasting effects. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P280, P302+P352, P304+P340, P312, P305+P351+P338, P337+P313, P332+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 8,400 mg/kg |
| NIOSH | Z3550 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 10 mg/kg |
| Main hazards | May cause respiratory irritation. Causes skin and eye irritation. Harmful if swallowed. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H410: Very toxic to aquatic life with long lasting effects. |
| Precautionary statements | P264, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): >5000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2,840 mg/kg |
| NIOSH | WT2450000 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 2500 mg/L |
| Related compounds | |
| Related compounds |
Cobalt(II) carbonate Copper(II) carbonate Iron(II) carbonate Magnesium carbonate Manganese(II) carbonate Nickel(II) carbonate Zinc oxide Zinc hydroxide |
| Related compounds |
Zinc oxide Zinc hydroxide Zinc acetate Zinc sulfate |

