Zinc Acetate: A Deep Look Across History, Science, and Use
Historical Development of Zinc Acetate
The story of zinc acetate stretches back to the early years of experimentation with minerals and acids. Ancient physicians and metallurgists experimented with compounds containing zinc, often sourcing it from calamine or sphalerite, centuries before it took on a defined chemical role. Alchemists in the Middle Ages worked with zinc salts, and by the 18th century, clearer records described methods for preparing zinc acetate using vinegar and zinc oxide. Over time, the pharmaceutical world caught on to the usefulness of zinc salts, giving rise to industrial-scale production driven by consistent demand in medicine and textiles. By the twentieth century, zinc acetate featured in cough remedies, textile mordants, and chemical research across several countries.
Product Overview
Zinc acetate sits in the realm of simple inorganic compounds carrying a reputation among chemists, pharmacists, and manufacturers. It goes by the chemical formula Zn(CH3COO)2 and exists in both anhydrous and dihydrate forms. The substance often appears as a colorless or white crystalline solid with a faint acetic acid scent. In practical terms, it dissolves pretty well in water and, to a lesser extent, in alcohol. Its solid structure gives it a shelf life when kept dry, and manufacturers ship it in sturdy containers to avoid contamination and moisture uptake. Zinc acetate finds regular use in dietary supplements, lozenges, analytical chemistry, and chemical syntheses, making it a fixture in pharmacy cabinets and research labs.
Physical and Chemical Properties
This chemical demonstrates several traits that earn it a spot in both research and manufacturing. In its dihydrate form, zinc acetate shows a melting point around 237°C, and the anhydrous version melts near 237°C but decomposes before it can be liquefied under standard conditions. It dissolves in water, and in the process, the solution leaves a mildly acidic taste due to hydrolysis. Chemically, zinc ions in acetate can replace other positive ions in solution, opening the door to a slew of double displacement and complexation reactions. Pure zinc acetate appears as needle-like crystals and, if exposed to moist air, may absorb water and gradually cake or clump together.
Technical Specifications & Labeling
Regulated standards set benchmarks for product purity and composition. Labs and suppliers reference specifications such as assay percentages, heavy metal content, water content, and soluble impurities. Reputable zinc acetate brands provide a batch number, production date, expiration date, manufacturer’s address, and clear hazard and storage instructions. These details become crucial for traceability and health standards, particularly in food, supplement, or pharmaceutical applications. Several pharmacopoeias include zinc acetate; producers must meet those strict protocols to serve the medical sector.
Preparation Method
Synthesizing zinc acetate typically combines zinc oxide or metallic zinc with acetic acid using straightforward methods. Add zinc oxide directly to acetic acid diluted in water, stir the mixture, and allow it to react completely. The solution clarifies as zinc acetate forms, dissolving throughout. Excess water can be removed by evaporation, and crystallization begins as the solution cools. The resulting crystals are filtered, washed with cold water, and dried at modest temperatures to prevent decomposition. Industries chasing high purity repeat recrystallization steps for a cleaner end product. Some production lines use zinc carbonate as an alternative, offering similar yields through a comparable reaction path.
Chemical Reactions and Modifications
Zinc acetate works as a useful reactant in the chemistry lab. Mix it with hydrogen sulfide in water and it forms zinc sulfide, a key pigment in luminescent paints. It reacts with sodium hydroxide to produce zinc hydroxide, useful in certain catalysts. Scientists use it as a starting point for more exotic zinc compounds, and biochemists rely on it for modifying proteins through complex ion exchange. Zinc acetate, mixing with strong mineral acids, can revert to soluble salts or, in heated situations, transform into zinc oxide. The acetate component itself can undergo a swap with other carboxylic acids as part of esterification or transesterification processes.
Synonyms and Product Names
On chemical supply lists, zinc acetate shows up with terms like zinc ethanoate, acetic acid zinc salt, or diacetatozinc. Dihydrate forms may list as zinc acetate dihydrate or zinc monoacetate. Different global suppliers add trade names or product codes, especially for grades headed for labs or food use. Regulatory documents, like safety datasheets, may refer to CAS Number 557-34-6 (dihydrate) or 5970-45-6 (anhydrous) as shorthand for traceability.
Safety and Operational Standards
Handling zinc acetate calls for respect for industrial hygiene. The chemical can irritate eyes, skin, and the digestive system. Workers wear gloves, goggles, and sometimes dust masks in busy labs. Overexposure to zinc salts brings symptoms like nausea, stomach cramps, or headaches. Storage guidelines focus on keeping containers sealed, in dry, cool areas, and away from incompatible substances like strong oxidizers or acids. Local fire codes require clear labeling, spill procedures, and prompt cleanup with plenty of air circulation. Waste zinc acetate never hits the regular drain; it gets collected for proper disposal in chemical processing sites. Transport regulations line up with standards set by organizations such as OSHA, IATA, and the EU.
Application Area
Zinc acetate gets widespread use in several arenas. Pharmaceutical companies use it in supplements and lozenges, where its zinc content brings immune or healing benefits. It features as a mordant in textile dyeing, anchoring colors firmly to cotton or wool. Analytical chemists rely on zinc acetate when testing for sulfides, phosphates, or proteins. Veterinary medicine calls for zinc acetate powders in certain formulations for animal health. Researchers use it as a reagent in lab-scale synthesis, for producing quantum dots or advanced materials. In the electronics field, zinc acetate serves in the fabrication of thin films for solar cells or sensors thanks to its solubility and predictable reaction profile.
Research and Development
Recent research paints a promising picture for zinc acetate. Scientists explore its use in the synthesis of metal-organic frameworks (MOFs), where highly porous structures capture gases like carbon dioxide. Biochemists look to zinc acetate as a key additive for modifying enzyme structures, potentially opening new drug development channels. Environmental research includes zinc acetate coatings that slow down bacterial growth or reduce corrosion. Nanotechnology taps into zinc acetate as a precursor for zinc oxide nanoparticles, important for sunscreen formulations, ceramics, and even antimicrobial surfaces. Research teams across the globe keep probing for new processing techniques, from microwave heating to greener solvents, improving efficiency and reducing byproducts.
Toxicity Research
Scientists understand the double-edged nature of zinc acetate. On one side, zinc stands as an essential nutrient involved in hundreds of enzyme functions and immune responses. On the other, excess exposure poses health risks. Toxicity studies in animals and humans draw a line between safe supplement use and situations causing gastrointestinal upset or zinc toxicity, especially if the compound is ingested above recommended limits. Regulatory agencies review data on chronic exposure, cancer risk, and environmental persistence to set workplace and dietary guidelines. Researchers continue examining potential impacts of long-term intake and environmental runoff, especially in settings with direct disposal of zinc compounds.
Future Prospects
Zinc acetate stands poised for more breakthroughs. The growing need for advanced materials nudges research labs toward zinc acetate-derived nanoparticles and thin films. Medical studies examine its antiviral potential amid renewed interest in simple, shelf-stable health interventions. Environmental projects see zinc acetate chemistry as a useful tool for cleaner industrial processes or renewable energy solutions. Industrial chemists, eyeing the push for greener synthesis, develop streamlined, solvent-free methods that shrink the chemical’s environmental footprint. All roads lead to a future where this old compound continues to deliver new solutions, shaking off its status as a mere additive in favor of front-line technical and medical roles.
What is Zinc Acetate used for?
The Role in Medicine
People probably recognize zinc as a supplement, but zinc acetate steps up in medicine with some real, tangible uses. Doctors prescribe zinc acetate lozenges sometimes when folks come down with the common cold. Some clinical studies have shown that zinc ions can block rhinovirus replication, which may shave off a day or two from a sore throat. Not magic, but a cold that lasts one day less sure counts for something. More notably, zinc acetate stands out in treating Wilson's disease—a rare genetic disorder causing copper buildup in the body. Here, it works by encouraging the body to get rid of extra copper, preventing liver and neurological damage. This example underscores why scrutiny from regulatory bodies stays strong: patient safety depends on precision in how much zinc goes into each dose.
Daily Essentials: Food Fortification
Across the world, millions face zinc deficiency. Zinc acetate, thanks to its high solubility and stable profile, gets added to foods and animal feeds to make up for this shortfall. I remember looking at a box of cereal with a ‘fortified with zinc’ label and realizing how a sprinkle of this compound makes a difference for children not getting enough from their regular diet. Without enough zinc, immune function lags and wound healing drags—things that matter in places where diets lack variety.
Industry: More Than Just a Supplement
Shifting over to products outside the kitchen or pharmacy, zinc acetate finds work in manufacturing, particularly in the textile and chemical sectors. It acts as a catalyst in producing polyesters, helping stitch molecules together to create the fibers in clothing and packaging. Most people don’t pay attention to the origin of their clothes, but safe and efficient chemicals used early in the process end up influencing the quality of the shirts we wear or the materials that wrap our food. Plus, applications in adhesives and dyeing bring it close to other necessities. Here, strict quality controls make sure that only the required amount ends up in each product batch, keeping safety at the front of the line.
The Push for Greater Safety and Sustainability
With so many touchpoints through food, medicine, and industry, zinc acetate raises issues about balance. Too much zinc can cause toxicity; too little and deficiency creeps in. Regulatory oversight from agencies like the FDA prevents these products from drifting outside safe zones. Manufacturers rely on clear guidance, but consumers can influence outcomes as well—by staying aware, asking doctors about supplement safety, and supporting transparent labeling.
Sustainability comes into play, too. Zinc mining and processing require energy and clean technologies to minimize environmental harm. Some companies started exploring ways to reclaim and recycle zinc from industrial sources, trimming the environmental bill. In my experience with community recycling initiatives, folks often overlook metals, but new models could help bridge industry needs with environmental realities.
Practical Choices
At the end of the day, zinc acetate doesn't just sit on a chemistry shelf. It finds its way into health, nourishment, clothing, and packaging. Knowing its uses and playing a role in making smarter choices—whether through responsible consumption or watching for third-party certification—puts some control back in our hands. Zinc acetate reminds us that even something small and seemingly technical touches our lives in big ways.
Are there any side effects of using Zinc Acetate?
Why People Take Zinc Acetate
Zinc acetate usually pops up in conversations about boosting immunity or treating Wilson’s disease. Doctors prescribe it, and you’ll also see it in some lozenges at the drugstore. The idea is to replenish zinc levels when diet doesn’t deliver enough or lower copper absorption in the body. People feel hopeful about its benefits, especially because zinc stands out as an essential mineral our cells need to stay on track.
Not Without Its Rough Edges: Potential Side Effects
Using zinc acetate for more than a day or two sometimes brings some surprises. The most common issue is stomach upset. Nausea, queasiness, even vomiting—these problems come up more often than you’d expect, especially if someone tries it on an empty stomach. Zinc acetate can cause headaches or leave a strange metallic taste that hangs around much longer than anyone wants. Experience matters here: I remember helping an elderly neighbor who had to switch to a different form of zinc after days of feeling sour and uncomfortable because of these same stomach troubles.
Diarrhea isn’t rare. Over-the-counter tablets magnify this for people who already struggle with sensitive digestion. Too much zinc, whether in one noisy dose or steady over weeks, can throw off the copper levels in your body, setting the stage for anemia or weak bones down the line. I’ve seen people get handed a zinc supplement at the pharmacy with barely a quick word from staff. Not enough attention gets paid to these possible twists.
Some folks break out in a rash or feel extra tired after a stretch of zinc acetate. Those allergic to zinc or the ingredients in tablets might even face swelling or trouble breathing—symptoms that send people to urgent care. This isn’t everyone, of course, but numbers from the FDA’s side effect database back up the warning. If someone has other health problems, kidney trouble especially, the risks multiply. Zinc builds up instead of washing out like it usually does.
Supporting Claims with the Science
Zinc acetate works best under a doctor’s eye, and research from trusted medical journals shows why. A 2013 review found about 23% of adults reported stomach complaints when taking zinc supplements, even at recommended doses. The National Institutes of Health calls out the risk of copper deficiency if people take more than 40 mg of zinc each day for long periods. Hair loss, fatigue, and numb fingers sometimes link back to this misstep.
A lot of people think “over-the-counter” means “harmless,” but that’s not always true. Zinc acetate follows the same rules as any medicine—it can help or hurt depending on the person and the dose. Some folks lean in hard, believing more zinc gives better results. Instead, it usually just raises the chances of problems.
Practical Steps for Safer Use
One way to dodge most issues is to take zinc acetate with food. A solid meal takes the edge off those stomach problems. Never mix zinc tablets with dairy: the calcium in milk blocks zinc from getting absorbed. Set a timer, space zinc out from any other mineral or vitamin pills, and always check with a doctor about dosage. Blood tests help see if zinc’s doing its job or causing more trouble.
Pharmacists and healthcare workers should speak plainly with patients about what to watch for, especially with long-term prescriptions. Most folks aren’t experts in nutrition, and busy clinics sometimes overlook these small but important conversations.
Final Thoughts
Knowledge makes the real difference. Zinc acetate helps many people, but it has rough edges worth knowing. Listening to stories from people who’ve used it, double checking with trusted medical advice, and paying attention to your body help keep you safe.
How should Zinc Acetate be taken or administered?
Understanding Zinc Acetate Use
Taking any mineral supplement should never feel like a shot in the dark. Zinc acetate supports people fighting off frequent colds, or those with low zinc from different conditions. Most doctors talk about zinc supplements if diet alone doesn’t give enough. Experience taught me that following a healthcare provider’s advice always trumps guesswork with minerals, since too much zinc gets risky.
How to Take Zinc Acetate Tablets or Capsules
Zinc acetate usually shows up as a tablet or capsule. Swallowing the tablet whole with a glass of water on an empty stomach helps the body soak up more zinc. Some people feel nauseous from zinc on an empty stomach. If that happens, food helps settle things down, but not too much high-calcium dairy, because calcium keeps zinc from doing its job. Taking this at least one hour before or two hours after a meal often works well.
If using dissolvable lozenges, let them melt slowly in the mouth rather than chewing or swallowing quickly. Research suggests this method makes a bigger difference in shortening cold symptoms, as the zinc sticks around where viruses hit first.
Measuring the Right Dose
Getting the right amount makes all the difference. For adults, medical guidelines put recommended daily zinc at around 8-11 mg, but supplements used for treating deficiencies often come in higher doses. In treating Wilson’s disease or stubborn zinc deficiency, doctors might prescribe more, which means patients should never guess the dose themselves. Taking more than 40 mg a day for a long stretch causes trouble with copper absorption, digestive irritation, or even immune system blips.
Common Side Effects and What to Watch For
Zinc acetate can upset the gut. I’ve seen students complain of queasiness or stomach cramps after large doses. Usually, cutting down by half and splitting the dose can help. A metallic taste or dry mouth may show up but rarely lasts. Long-term, zinc overload hits harder: copper deficiency, poor cholesterol numbers, or trouble fighting infection—none of these should sneak up on someone who does regular labs with a doctor.
Interactions matter, too. Don’t take zinc with antibiotics like tetracycline or quinolones—zinc blocks these drugs from working well. Take them two hours apart at least. Zinc plus iron pills? Take them at different times, or risk the minerals tripping over each other.
Getting Zinc from Food vs. Supplements
Nutrition always comes first in my view. Foods like beef, lentils, cashews, and pumpkin seeds offer zinc with lower risk than bottles on the shelf. Most healthy eaters get enough, but strict vegetarians, pregnant women, those with digestive issues, or older adults may run low. People should talk with their healthcare provider before starting any supplement, not just zinc acetate.
Advice Goes a Long Way
Healthcare workers play a big part here. Pharmacists help spot risky interactions and guide the timing around other meds. Doctors adjust doses for kids, pregnant people, and folks with kidney or liver conditions. Self-medicating by following a friend’s routine always invites problems. Clear, simple instructions and real follow-up from a professional can save a lot of grief.
Zinc acetate works best with information, not instinct. With reliable guidance, people can avoid both the dangers of too little and the slip-ups of too much. Supplements should support, not sideline, good health—and that starts with careful use.
What are the storage requirements for Zinc Acetate?
The Human Element of Safe Storage
If you’ve walked into a supply room at a university or a small manufacturer, you’ve probably seen a few chemical jars on the shelf—a row of labels, sometimes a dusty top, maybe a splash of something from a past project. In that quiet, unassuming space, the smallest details about storage can define the safety of everyone who steps through the door. Zinc acetate sits among those jars. Reliable, versatile, but far from harmless; its storage shouldn’t fall on autopilot.
Straightforward Steps and Specifics
Zinc acetate, a compound used in everything from medicine to textile processing, reacts fairly quickly to moisture. Set the jar out on a humid day, and you’ll find the neatly packed crystals start to clump—a clear sign, the lid wasn’t tight enough, or the air drew in too much water. Best practice calls for a dry, cool location. Most folks seem to brim with confidence in their well-insulated labs or storerooms, but all it takes is one summer heatwave or faulty air conditioning unit to eat away at the shelf life of these chemicals. And once moisture gets in, the risk of caking grows fast, causing dosing errors or wasted material.
Having worked in older research labs, I’ve seen more than one bottle harder than concrete because humidity crept under a loose cap, making simple weighing impossible without breaking the glass with a hammer. The answer lies in vigilance. Desiccators—those sealed boxes with drying agents inside—turn out to be worth the trouble. They keep humidity out, just as an old thermos keeps lunch cool through the day. Some powdery chemicals, zinc acetate included, will seize any water they can from the air and hold onto it, so you fight back with sealed containers, well-fitted caps, and—where possible—a desiccator within easy reach.
Risks Go Beyond Clumping
It’s not just about texture or wasted supplies. Zinc acetate can irritate the eyes, skin, and even lungs if dust drifts free. Toss in an accidental spill near food or a drink, and now there’s a problem that goes far beyond chemical waste—think accidental poisoning or a trip to the emergency room if someone’s not paying attention. Label containers in bold, easy-to-read writing. Set them apart from anything resembling a consumable. Chemical safety isn’t just about ticking off boxes on a form; real lives depend on clear organization and attention to detail.
Shared Responsibility
It’s tempting to rely on the professionals—lab managers, supervisors, safety officers. But after seeing a summer intern reach for a cleaner that looked like sugar, I learned that everyone shares the duty of vigilance. Training all staff, even those who never run an experiment, can keep a single accident from spiraling out of control. Share best practices, hang up clear infographics in storerooms, and regularly double-check labels and tightness of jars. If a bottle looks suspect, don’t just leave it—report or replace it.
Small Changes, Big Rewards
I’ve never forgotten the time a leaky roof pooled water near a shelf lined with glass bottles. We caught it early, and moving those chemicals fast gave the team peace of mind for months. A bit of foresight—air control, waterproof storage bins under suspect rooflines, regular shelf checks—means a calm workplace and fewer sleepless nights. These small acts, done faithfully, help everyone walk through those chemical supply rooms with a clear head.
Is Zinc Acetate safe for children and pregnant women?
Why Parents and Expecting Mothers Take Notice
Walking through any pharmacy aisle, you’ll spot multiple vitamins, throat lozenges, and supplements boasting zinc as a key ingredient. Many people see zinc as a way to fight off colds more quickly or support immune health. Zinc acetate, a salt form of zinc, turns up in these products thanks to its ability to deliver zinc efficiently. Any parent or expecting mother might wonder if this same zinc acetate is really safe for their children—or themselves during pregnancy.
What Science Says About Zinc Acetate
The human body needs zinc. It helps in cell growth, immune support, and wound healing. Children building bones and bodies, and pregnant women supporting new life, rely on zinc. Zinc acetate, when used in recommended doses, offers a reliable, absorbable way to deliver this essential mineral. The U.S. Food and Drug Administration (FDA) lists zinc as generally recognized as safe (GRAS) for regular intake in supplements and fortified foods.
That said, medical professionals caution against overuse. Too much zinc leads to nausea, cramps, headaches, and in extreme cases, impairs the immune response instead of strengthening it. For small kids, toxic doses come easier because their bodies hold less zinc before side effects kick in. Pregnant women need more zinc than usual, but taking double or triple doses won’t boost health and may actually backfire, interfering with copper and iron absorption.
Zinc Acetate in Real Life: Medicines and Supplements
Experience shows most doctors only recommend zinc supplements for children diagnosed with a real deficiency, picky eaters, or those struggling with certain medical conditions that block absorption. Diet offers good sources—meat, whole grains, dairy. Only after a doctor checks dietary intake and symptoms would supplements come into play. For sore throats or colds, some parents reach for zinc acetate lozenges, but experts warn not to use these for small kids, due to a choking risk and the possibility of getting too much zinc. The National Institutes of Health sets safe upper daily limits: for children ages one to three, 7 mg; ages four to eight, 12 mg. Pregnant teens and adults need about 11-13 mg daily—easily hit with a smart diet.
Pregnancy Needs: Balancing Safety and Nutrition
During pregnancy, obstetricians check for iron and zinc in bloodwork. Supplements sometimes get prescribed, especially if diets lack meat or dairy. But pregnant women find plenty of zinc in regular food when eating varied meals. Swallowing extra zinc acetate capsules without clear need won’t make a stronger baby and could do harm. Some prenatal vitamins include zinc, but doses stay well within safe limits. Anyone thinking about extra supplements should run it by their healthcare provider.
Seeking Safe Solutions
For families thinking about zinc acetate, talking with a pediatrician or OB-GYN comes first. Checking supplement labels for zinc, knowing total intake from food plus pills, and steering clear of self-medicating at the first sign of sniffles help keep everyone safe. Education from pharmacists and family doctors on how much zinc the body actually needs prevents accidental overdoses. Sticking with whole foods and using supplements only under professional advice keeps health risks low—a rule that’s worked for generations.
References for Trustworthy Guidance
The National Institutes of Health, Centers for Disease Control, and FDA offer clear facts on zinc. Relying on these trusted sources and your family doctor makes decisions around zinc acetate straightforward, rather than filled with worry or guesswork.
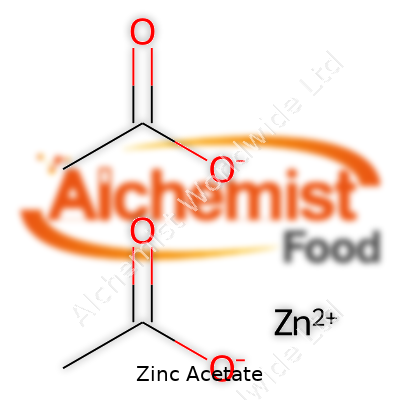
| Names | |
| Preferred IUPAC name | zinc diacetate |
| Other names |
Acetic acid, zinc salt Zinc diacetate Zinc ethanoate |
| Pronunciation | /ˈzɪŋk ˈæsɪteɪt/ |
| Preferred IUPAC name | zinc diacetate |
| Other names |
Acetic acid, zinc salt Zinc diacetate Zinc ethanoate |
| Pronunciation | /ˈzɪŋk ˈæs.ɪ.teɪt/ |
| Identifiers | |
| CAS Number | 557-34-6 |
| Beilstein Reference | 1900726 |
| ChEBI | CHEBI:35139 |
| ChEMBL | CHEMBL1200440 |
| ChemSpider | 10922 |
| DrugBank | DB14597 |
| ECHA InfoCard | 03d76eaf-d2c4-4e4e-b833-753e68301ac4 |
| EC Number | 2.7.1.50 |
| Gmelin Reference | 8787 |
| KEGG | C14822 |
| MeSH | D015927 |
| PubChem CID | 24621 |
| RTECS number | ZL3490000 |
| UNII | N9UOI1V6EC |
| UN number | UN3077 |
| CAS Number | 557-34-6 |
| Beilstein Reference | 7540 |
| ChEBI | CHEBI:35139 |
| ChEMBL | CHEMBL1200612 |
| ChemSpider | 53373 |
| DrugBank | DB14597 |
| ECHA InfoCard | ECHA InfoCard: 100.007.297 |
| EC Number | 2.7.1.50 |
| Gmelin Reference | 8470 |
| KEGG | C00694 |
| MeSH | D015275 |
| PubChem CID | 32051 |
| RTECS number | ZHVDU8075 |
| UNII | 9GXB180M2L |
| UN number | UN3077 |
| Properties | |
| Chemical formula | Zn(C₂H₃O₂)₂ |
| Molar mass | 183.48 g/mol |
| Appearance | White crystalline powder |
| Odor | slight acetic odor |
| Density | 1.84 g/cm³ |
| Solubility in water | Freely soluble in water |
| log P | -1.4 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.8 |
| Basicity (pKb) | 8.81 |
| Magnetic susceptibility (χ) | −0.000021 |
| Refractive index (nD) | 1.422 |
| Viscosity | Viscous liquid |
| Dipole moment | 6.49 D |
| Chemical formula | C4H6O4Zn |
| Molar mass | 183.48 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.735 g/cm³ |
| Solubility in water | soluble |
| log P | -1.4 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa 4.76 |
| Basicity (pKb) | 5.7 |
| Magnetic susceptibility (χ) | -1.2×10⁻⁵ |
| Refractive index (nD) | 1.582 |
| Viscosity | Viscous liquid |
| Dipole moment | 5.45 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 150.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -348.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1165.6 kJ/mol |
| Std molar entropy (S⦵298) | 155.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -348.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1165.6 kJ/mol |
| Pharmacology | |
| ATC code | A12CB01 |
| ATC code | A12CB01 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H332, H410 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-0-1 |
| Autoignition temperature | > 400 °C (752 °F; 673 K) |
| Lethal dose or concentration | LD50 Oral Rat 482 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1,462 mg/kg |
| NIOSH | ZN3500000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Zinc Acetate: "15 mg/m³ (total dust), 5 mg/m³ (respirable fraction) as zinc oxide (OSHA PEL) |
| REL (Recommended) | 30 mg daily |
| IDLH (Immediate danger) | 250 mg/m3 |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H319 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 Oral - Rat - 482 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 582 mg/kg |
| NIOSH | SR7170000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Zinc Acetate: 5 mg/m3 |
| REL (Recommended) | 30 mg daily |
| Related compounds | |
| Related compounds |
Zinc chloride Zinc sulfate Zinc oxide Acetic acid Magnesium acetate Copper(II) acetate |
| Related compounds |
Copper(II) acetate Iron(II) acetate Iron(III) acetate Manganese(II) acetate Nickel(II) acetate |

