Urea Phosphate: A Deep Dive into Its Role and Future
Historical Development
Urea phosphate entered the fertilizer world out of necessity, not accident. In the 1960s, as the Green Revolution spread, folks looked for tools that could increase output without exhausting soil. Traditional phosphate sources weren’t mixing well with high-analysis urea, especially in irrigated soils. Scientists spotted that combining urea and phosphoric acid resulted in a stable, water-soluble salt: urea phosphate. This compound resolved compatibility issues, which opened doors first for drip irrigation in Israel and later in California. Over time, the expansion outpaced the original vision. Today, the influence of those early developments echoes across high-efficiency agriculture in arid regions and greenhouse operations around the globe.
Product Overview
Urea phosphate appears as a white, crystalline powder. Farmers and greenhouse managers rely on its high nutrient concentration. Coming in with about 17% nitrogen and 44% phosphorus pentoxide (P2O5), this product addresses the basic needs of many crops in a single package. It dissolves fully in water, so it’s ready for fertigation systems, which demand nutrient sources that won’t leave residues or clog pipes. People often choose it because it pulls double duty: supplying both nitrogen and phosphorus efficiently.
Physical & Chemical Properties
Looking at its basic character, urea phosphate (CO(NH2)2·H3PO4) doesn’t just bring nutrients—it shapes the chemistry of the solution. It is mildly acidic, with a pH around 1.6 in a 10% solution, which makes it unique among common fertilizers. The acidity helps keep irrigation lines clean and can fend off unwanted precipitation of calcium and magnesium salts, particularly in regions that get their water from sources high in those minerals. Its powder form is stable at room temperature, easy to store, and doesn’t attract much moisture from the air. These traits give growers more control over their nutrient programs, especially in closed hydroponic or drip setups.
Technical Specifications & Labeling
Packaging on genuine urea phosphate often highlights its N and P2O5 content, acid equivalent, and the absence of potassium. Labels typically call out the product’s minimum solubility, sometimes nearing 1000 g/L at room temperature. Purity counts, and reputable manufacturers ensure low levels of unwanted heavy metals, as these can have long-term impact on soil health and food crops. Many countries now require clear hazard labels due to acidity. The acidity also draws attention in storage and handling instructions, so users don’t suffer burns or damage equipment.
Preparation Method
Urea phosphate production is pretty straightforward but demands careful control. Most processes start with good-quality urea and phosphoric acid. Mix them in precise ratios under gentle heat, and the reaction gives off urea phosphate almost exclusively. Some operations use wet-process phosphoric acid; others go for food-grade material, especially in greenhouse-grade product. Operators maintain pH, temperature, and timing to prevent decomposition of urea. After crystallization, centrifuges or filters remove the product, and a drying step finishes the batch so it meets transportation and storage standards. This method sidesteps the byproducts that show up in older triple superphosphate manufacturing, making urea phosphate relatively clean by comparison.
Chemical Reactions & Modifications
In solution, urea phosphate behaves as both an acid and a nutrient carrier. When it dissolves, it releases ammonium and phosphate ions, along with a small bump in solution acidity. This property allows it to reduce carbonate build-up in lines and on drippers. It can react with alkaline soil carbonates to free up locked-away phosphorus, giving struggling crops a better shot at uptake. Some companies experiment by blending it with potassium nitrate or calcium sources, aiming to hit all the major nutrient bases for hydroponic mixes. Careful solubility testing matters, since urea phosphate will react with high-calcium solutions to form insoluble calcium phosphate if not managed properly.
Synonyms & Product Names
This compound goes by several names. In the technical literature, “urea phosphate” stands firm. On commercial packaging, you sometimes see “ureaphos,” "urea phosphoric acid adduct," or “UP fertilizer.” International brands sometimes use proprietary labels, adding confusion to the market for inexperienced users. Chemically savvy folks zero in on the formula: CO(NH2)2·H3PO4. Despite the name game, the core material remains the same—a stable, highly soluble fertilizer targeting high-tech agriculture.
Safety & Operational Standards
Handling urea phosphate demands respect for its acidic nature. Direct skin or eye contact can cause irritation or burns, so protective gloves and goggles aren’t optional on the mixing floor. Storage rules call for sealed containers kept dry and away from direct sunlight or incompatible chemicals like bases and oxidizers. In the European Union and the United States, safety data sheets must spell out first-aid measures, environmental fate, and spill response. Concrete surfaces in mixing rooms often show early signs of acid damage, so facility managers look for tough chemical-resistant coatings to prolong equipment life. Workers learn from the training videos and follow procedures to make sure they don’t track residue home to their families.
Application Area
Fertigation is where urea phosphate shines. Its high solubility fits right into micro-irrigation and hydroponics, whether in Western greenhouses or Indian field nurseries. The compound often pops up in vegetable and orchard production, especially where growers face hard water or high soil pH. By delivering both phosphorus and nitrogen simultaneously, it streamlines supply schedules for cucumbers, tomatoes, berries, citrus, grapes, and lettuce. Some users experiment with foliar sprays, but this takes careful dilution because of the strong acidity—too much can damage tender leaves. Soil scientists note its potential for unlocking phosphorus in calcareous soils, while greenhouse growers appreciate its compatibility in stock tank mixes. Specialty crop advisors see it as an answer to micronutrient deficiencies that come from poor phosphate availability.
Research & Development
Recent research seeks to push urea phosphate past its basic role as a fertilizer. I’ve noticed a trend in tweaking the formulation to balance nutrient release rates when paired with slow-release nitrogen or micronutrient packages. Lab teams explore how mixing ratios affect plant uptake, particularly in closed-loop hydroponic systems where every kilogram of nutrient counts. Some research focuses on environmental fate, studying how it breaks down in different soils and climates. University labs in Israel and Spain evaluate its ability to dissolve stubborn calcium deposits that plague irrigation systems. Engineers keep dialing in crystal size to improve flow through metering pumps, hoping to minimize maintenance stops in large-scale operations. Every improvement on this front helps cut water use and drive up yields, which matters in a world with mounting pressure to produce more from less.
Toxicity Research
Concerns around toxicity mostly center on over-application and groundwater contamination. The low heavy metal content of well-manufactured urea phosphate gives an edge over older phosphate sources, which sometimes carry cadmium or arsenic. Direct ingestion or inhalation remains a hazard, particularly for children or untrained workers. Chronic exposure studies in rats have shown minimal risk when handling according to safety guidelines, but more research looks at accumulation in crops over successive seasons. Aquatic toxicity tests reveal modest impacts, but runoff from careless field application still risks downstream ecosystems. Regulators in the EU watch nitrate and phosphate levels in groundwater, so nutrient management plans must account for careful, measured use.
Future Prospects
Growing global demand for precision agriculture puts urea phosphate in a strong position. Water scarcity in Asia and Africa drives more growers to drip irrigation with soluble fertilizers, and urea phosphate’s unique blend of acidity and nutrient content fits perfectly. New production methods focus on reducing energy input and streamlining purity to meet strict regulatory standards. Expanded research efforts look at integrating urea phosphate within “smart” fertilizer blends, pairing it with polymers for controlled release or additives that improve micronutrient uptake. Manufacturers lean into the green chemistry movement, edging away from legacy acids with high environmental footprints. If industry and regulators work together, there’s little doubt that urea phosphate can help solve the food and water challenges facing agriculture. Keeping up with new research, maintaining transparent labeling, and strict adherence to safety standards will go a long way in making sure this compound delivers on its promise.
What is urea phosphate used for?
A Farmer’s Aid in Crop Nutrition
Walk into any major agricultural store, and you'll spot bags labeled "urea phosphate." I remember spending long hours on my grandfather’s small citrus farm, watching crops thrive better after switching to liquid feed blends containing urea phosphate. This stuff comes as a white, water-soluble powder or crystalline product, and has earned a reputation as a reliable fertilizer. Its main appeal isn’t hard to see—urea phosphate delivers both nitrogen and phosphorous, two nutrients crops grab onto for growth and fruit production.
Unlike old-school fertilizers that struggle in alkaline soils, urea phosphate dissolves easily and doesn’t leave sticky residues. Citrus groves, vineyards, and greenhouse growers use it to promote flowering and boost early yield. Its low salt index means root burn is far less likely. Commercial hydroponic systems use urea phosphate because it supplies nutrients without clogging drippers or leaving sediment in tanks—a major headache with traditional blends.
A study from Punjab Agricultural University highlighted how blending urea phosphate with irrigation water in paddy fields lifted yields by 10% compared to common phosphate fertilizers. That kind of margin makes a difference in communities where every kilo counts toward food security. Rural India, California’s Central Valley, and parts of Spain see huge benefit from precision feeding schedules using urea phosphate in drip irrigation. Farmers notice the healthier leaf color and sturdier stalks without complicated application schedules.
Cleaner Industrial Processes
I once visited a water treatment plant that handled large poultry-processing runoff. Urea phosphate was being added to process water, and technicians explained its benefits. In industrial settings, it functions as a scale inhibitor and cleaner. Phosphate ions keep minerals dissolved, stopping them from clumping up and creating scale inside pipes or on machinery. Maintenance teams save both downtime and repair costs. Water with high calcium content can be difficult to treat, and that’s where urea phosphate offers a distinct advantage—unlike some acids, it breaks down mineral deposits without releasing harmful fumes.
Glass manufacturers and dairy processing plants pick urea phosphate for acid cleaning and for its ability to remove mineral deposits. It’s neither too harsh to handle nor dangerous for on-site staff. Industrial facilities appreciate the precise control: strong enough to dissolve limescale, gentle enough not to corrode equipment or leave toxic residue behind. Food-grade versions meet strict safety rules, so there’s little risk of cross-contamination when cleaning surfaces or tanks related to food preparation.
Safety and Sustainability Issues
I always worry when a chemical starts to pop up everywhere—convenience often leads to complacency. Urea phosphate contains both urea and phosphoric acid. Mishandling in storage or mixing with incompatible chemicals can cause dangerous fumes or burns. I’ve seen small businesses skimp on training to save money, which exposes workers and the environment to real risks. Overusing any fertilizer contributes to runoff and water pollution. The challenge—raising healthy crops without damaging local rivers or lakes—remains a stubborn problem in regions with heavy fertilizer application.
Responsible use works best. Farmers and industrial users turn to soil and water testing to measure actual nutrient levels before application. By tracking how much phosphate and nitrogen crops truly need, fields get just enough—no more, no less. Regulatory agencies continue to monitor urea phosphate production to ensure quality and minimize waste. Research into slow-release formulas and blending with organic matter offers a path forward for reduced runoff.
Final Thoughts From the Field
Anyone calling urea phosphate a miracle fix hasn’t seen its best or worst days. Used wisely, it supports food production, cleaner processing, and more efficient factory maintenance. Lax training, careless handling, and overuse carry consequences that show up in groundwater, lost yields, or workplace injuries. Urea phosphate deserves its place on the shelf, just not a free pass. Practical experience, honest testing, and a willingness to adapt keep its benefits in reach—without paying a steep price down the line.
How is urea phosphate applied as a fertilizer?
A Close Look at Crop Nutrition
Standing in a young cornfield, it’s easy to see how healthy growth links back to the right nutrients. One tool that keeps appearing on progressive farms is urea phosphate. This fertilizer brings both nitrogen and phosphorus to the soil—a duo many crops crave, especially in the early stages. In my years talking with farmers and seeing fields up close, I’ve noticed the results that this combo delivers. Balanced nutrition in the soil often shows itself in the leaves and yields.
Application in the Real World
Urea phosphate usually appears as a white, water-soluble crystal or powder. Most growers mix it into irrigation systems, turning drip lines into nutrition highways. It dissolves easily, and plants seem to respond quickly because both nitrogen and phosphorus become available at the roots, right where they're needed. This ease makes fertigation—adding fertilizer through irrigation—straightforward, especially in greenhouses or sandy soils prone to nutrient leaching.
Direct soil application also finds a place on many farms. In orchards and vineyards, a grower may spread it by hand or use a spreader for larger areas before a rain or scheduled irrigation. Mixing with water and applying as a solution works well for small plots and specialty crops that react badly to dry fertilizer sitting on the surface. The aim is always to get those nutrients to the roots with as little waste as possible.
Benefits for Farmers
Urea phosphate stands out by offering pure nutrients without extra salts. Farmers like knowing what they’re putting in the ground. The acidity of this fertilizer can lower the pH around the root zone, which comes as a relief in alkaline soils where phosphorus often gets “locked up” and unavailable to plants. I remember one greenhouse operator in California who saw immediate improvement after switching from standard phosphates to urea phosphate; blossom end rot and tip burn rates dropped, because plants finally accessed the phosphorus locked in the soil.
Concerns and Smart Practices
Of course, fertilizer isn’t something to pour thoughtlessly onto the land. Misuse means runoff, wasted money, and environmental headaches, like algae blooms downstream. Testing the soil first shows a farmer the actual situation, providing a baseline for how much and how often to apply. Overapplication only creates problems. Most agricultural experts recommend small, regular doses that match crop demand over time. In my experience, the most successful growers use sensor and lab data to guide these choices.
Blending with other fertilizers often happens on farms with complex rotation cycles. Tomatoes, peppers, and leafy greens often need calcium alongside nitrogen and phosphorus, so farmers mix products, timing each one to the particular crop and its growth stage. Watching the weather pays off, too; nobody wants fertilizer to wash away before roots can make use of it.
Looking Ahead
As more growers dial in their nutrient programs and care about both yield and river water quality, urea phosphate will stay in the toolkit. It’s not magic, just one more option that—if used with a little thought and regular testing—can help both the soil and the crop reach their potential. For the farmer walking the rows and watching costs, that’s what matters most.
Is urea phosphate safe for all crops?
What Farmers and Growers Experience
I’ve watched growers reach for urea phosphate to get young plants off to a quick, vigorous start. The product offers big boosts in both nitrogen and phosphorus, plus lowers soil pH. On paper, that sounds like a versatile tool. In practice, some fields change for better, while others never quite recover. Farmers have learned to share their stories about specific crops, instead of trusting one-size-fits-all promises.
How Urea Phosphate Impacts Different Tactics
In greenhouses or hydroponic systems, urea phosphate helps tomatoes and peppers build up strong root mass. This fertilizer dissolves quickly and keeps irrigation lines unclogged. High-value vegetables usually thrive, especially in setups with precise pH management. Growers can steer results with constant water monitoring.
On open fields, especially with alkaline soils, growers using urea phosphate see improved phosphorus uptake. Alfalfa or maize, for example, often struggles in high pH environments. Applying urea phosphate reduces the problem, at least for seasons where pH management matters. The difference shows up in greener leaves and better early growth.
Some orchardists have different opinions. Blueberries, for instance, tolerate acidic amendments—but not too much. Too much acid tricks roots into shutting down. Citrus trees get extra growth but develop yellow patches if the pH drops out of their comfort zone. Watching soil and leaf health is not optional.
What Research Reveals
Agronomists point out potential problems with frequent and heavy use. Urea phosphate can leach into groundwater, leading to environmental impacts if not managed responsibly. A study from Punjab Agricultural University found that continuous application without soil pH checks hit wheat and barley yields negatively. Nitrogen becomes volatile at lower pH, and phosphorus may become fixed again at the other end of the scale. It’s a lesson: nutrient availability swings with chemistry, not just supply.
Urea phosphate’s phosphorus content works fast, but not every crop handles a rush of phosphorus and acid the same way. Leafy greens, for example, can burn or suffer root stunting without careful dilution. Legumes, especially beans and peas, have bacteria in their roots that get disrupted by too low a soil pH. Damage here is invisible until harvest shows what got lost underground.
Practical Steps for Growers
Soil testing becomes more important with specialty fertilizers. A handful of dirt scooped up before planting tells more than any label or spreadsheet. Matching pH and crop needs, then adjusting as the season moves, is how experienced farmers outpace their neighbors’ trial-and-error programs.
Extension agents recommend blending urea phosphate with other inputs instead of applying alone. Foliar sprays, split applications, and variable-rate technology provide safer paths for each crop’s quirks. Organic and rotating cover crops help buffer against chemical surprises over time.
There’s no one solution that fits every farm or greenhouse. Year-round observation, local soil wisdom, and communication among growers do more for sustainability than any bag or bottle. Urea phosphate delivers when used with respect for plant limits and soil biology, but chasing yield by shortcutting safeguards risks more than a bad season. Listening to the land pays many times over—season after season.
What is the chemical composition of urea phosphate?
Breaking Down the Compound
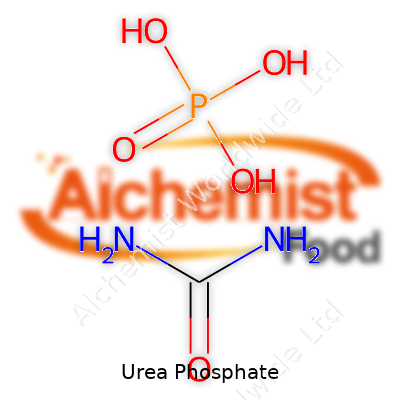
Urea phosphate goes by the formula CO(NH2)2·H3PO4. A look at that formula tells you the key parts: urea, which supplies nitrogen, and phosphoric acid, which brings in phosphorus. Chemists combine these two to form a single, solid, highly water-soluble salt. Unlike some chemical formulas, this one doesn’t hide any surprises—just straightforward nitrogen and phosphorus stuck together, making it a favorite in farming.
Elements that Make a Difference
Every bag of urea phosphate gives out two things plants crave: nitrogen (N) and phosphorus (P). Percentage-wise, this compound usually shows up as 17-44-0 in fertilizer language. What does that mean? Seventeen percent nitrogen, forty-four percent phosphorus (as P2O5), zero potassium. Farmers facing poor soil or battling shortages in either nitrogen or phosphorus trust this product to solve their problems because both elements are crucial for plant roots, growth, and overall yield.
The Chemistry in Practice
I’ve seen the difference on a small tomato plot in sandy, acidic soil. Ordinary fertilizer failed to bring much out of the ground. After shifting to urea phosphate, the plants started growing faster and set more fruit. The notable change comes from its chemistry: as soon as it dissolves, urea phosphate lowers the pH of the solution. In simple terms, it turns water more acidic. This property helps unlock nutrients in soils where alkalinity ties up phosphorus, so plants can finally use what’s there.
Beyond Plant Nutrition
The chemical composition goes beyond just farm fields. Because it releases no calcium or sodium, urea phosphate won’t flame up scaling or leave behind crusty layers in drip irrigation systems. Those who manage large greenhouses or spend time calibrating industrial fertilizers appreciate that aspect: reduced risk of clogged lines, fewer headaches, and reliable nutrient delivery to every plant.
Risks and What Needs Attention
It’s tempting to load up on this stuff, given its effectiveness. But balance matters. Too much nitrogen can burn roots, and phosphorus doesn’t just vanish; runoff can wash it into streams and lakes, feeding algal blooms and harming aquatic life. Farmers and gardeners who know their soil’s needs often combine regular testing with precise measurements. Some regions even set strict rules on phosphorus use for just this reason.
Looking for Solutions
People working the land have options to keep things safe and productive. Frequent soil tests show exactly what’s missing or abundant, guiding smarter applications. Blending urea phosphate with organic matter or slow-release fertilizers can also help maintain healthy growth without shocking plants or waterways. Education on best practices—aimed at both big producers and home gardeners—goes a long way, too. It’s one of those situations where chemistry in the lab turns into better choices out in the real world.
Credible Sources for Trust
The facts about urea phosphate’s composition and behavior come straight from scientific literature and guidelines published by trusted groups like the Food and Agriculture Organization (FAO) and the International Fertilizer Association. Universities with strong agricultural programs regularly study and share best application tips, which help keep environmental impact low while supporting better yields. Knowing what’s in your fertilizer and how it acts in real soil shapes more than plant health—it shapes the planet’s future, field by field.
How should urea phosphate be stored?
Understanding the Risks Behind the Product
Urea phosphate stands out as a solid fertilizer and cleaning agent. It mixes urea and phosphate into one crystal, dissolving readily in water. If you spend time in agriculture or horticulture, the stuff helps plants grow fast. This compound, like many chemicals, demands careful handling long before it even gets poured into a tank or hopper. A single mistake can lead to clumping, impurities, or worse, an accident.
Keeping Moisture Out
In my experience, moisture creates more trouble than almost anything else in a warehouse. Urea phosphate attracts water out of the air, hardening into lumpy blocks if storage isn’t sealed well. Even on a hot day, forgetting to seal a container lets humidity creep in. A leaky roof or sweaty concrete can turn that powder into rock. Store bags inside a dry building, off the floor. Wooden pallets work, but I’ve even seen old rubber mats do the trick to provide a buffer from damp ground. Tight drum lids and roll-down bag tops, firmly fastened, fend off nearly every moisture-related mishap.
Watch the Temperature
Steady temperature means less stress all around, especially in a chemical storeroom. Extreme heat kicks off slow degradation, while icy conditions just encourage condensation. In most climates, regular room temperature meets the mark. I tell friends in remote areas to avoid metal sheds under the sun—thin walls turn warehouses into ovens by mid-afternoon. Shade, good airflow, and insulation make a big difference, even if the storage is simple.
Quality of the Air Makes a Difference
Leaving bags open not only lets in moisture; it invites dust and airborne chemicals. Ever seen fertilizer powder turn discolored or clump after a few weeks near a spray room? That’s often due to ammonia or corrosive fumes floating nearby. Store urea phosphate away from open solvents, pesticides, lime, and acids. A separate room or sealed cabinet stops cross-contamination, which is a common headache.
Attention to Safety
Spills happen. Pouring out of ripped bags after the forklift grazes a stack is messier than most people imagine, and sharp granules can irritate skin or eyes. Clear labels and regular checks help avoid unmarked containers or accidental mixes. Safety data sheets should be handy and visible for anyone new to the storage area. Preparing a kit with eye wash, gloves, and dust masks takes only a minute, but that preparation matters the one day you need it most. Too many folks lean on luck—my view has always been to plan like someone new might handle the job tomorrow.
Why All This Effort Matters
Careful storage keeps product quality up, saves money, and most of all, protects the health of workers and neighbors. The stakes rise quickly if children, animals, or untrained staff wander near a storage area. Simple measures—covering stacks, blocking leaks, posting clear signs—spell the difference between quiet efficiency and disaster. Whether working on a massive farm or tending a small greenhouse, smart storage habits stick with you for life.
| Names | |
| Preferred IUPAC name | phosphoric acid; urea |
| Other names |
UP ureaphosphoric acid urea phosphate |
| Pronunciation | /juːˈriː.ə ˈfɒs.feɪt/ |
| Preferred IUPAC name | phosphonic diamide |
| Other names |
UP Urea Phosphoric Acid Urea Phosphate Monohydrate |
| Pronunciation | /ˈjʊəriə ˈfɒsfeɪt/ |
| Identifiers | |
| CAS Number | 4861-19-2 |
| Beilstein Reference | 3587937 |
| ChEBI | CHEBI:63034 |
| ChEMBL | CHEMBL1201630 |
| ChemSpider | 20584 |
| DrugBank | DB11324 |
| ECHA InfoCard | 100.027.735 |
| EC Number | 237-085-3 |
| Gmelin Reference | 57844 |
| KEGG | C13887 |
| MeSH | D014510 |
| PubChem CID | 198361 |
| RTECS number | YR8750000 |
| UNII | QH7WYX43SJ |
| UN number | UN1759 |
| CAS Number | 4861-19-2 |
| Beilstein Reference | 3564349 |
| ChEBI | CHEBI:33219 |
| ChEMBL | CHEMBL1201579 |
| ChemSpider | 10441 |
| DrugBank | DB11361 |
| ECHA InfoCard | ECHA InfoCard: 03-2119457016-46-0000 |
| EC Number | 14321-27-8 |
| Gmelin Reference | 8988 |
| KEGG | C18621 |
| MeSH | D014509 |
| PubChem CID | 13597 |
| RTECS number | YV9620000 |
| UNII | HB8S3R8QW6 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID1041119 |
| Properties | |
| Chemical formula | CO(NH2)2·H3PO4 |
| Molar mass | 158.07 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.8 g/cm³ |
| Solubility in water | Highly soluble |
| log P | -2.59 |
| Vapor pressure | Vapor pressure: Negligible |
| Acidity (pKa) | 0.18 |
| Basicity (pKb) | 2.0 |
| Refractive index (nD) | 1.433 |
| Dipole moment | 3.98 D |
| Chemical formula | CO(NH2)2·H3PO4 |
| Molar mass | 158.06 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.8 g/cm³ |
| Solubility in water | Highly soluble |
| log P | -2.59 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 0.2 |
| Basicity (pKb) | 2.14 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.483 |
| Dipole moment | 7.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 199 J mol⁻¹ K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -247.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −2066 kJ/mol |
| Std molar entropy (S⦵298) | S⦵298 = 181 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2050 kJ/mol |
| Pharmacology | |
| ATC code | A09AB53 |
| ATC code | V03CN01 |
| Hazards | |
| Main hazards | Causes skin and serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| Lethal dose or concentration | LD50 (Oral, Rat): 2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 11,200 mg/kg |
| NIOSH | SN 122 |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 42 kg/ha |
| IDLH (Immediate danger) | Not listed |
| Main hazards | Causes skin and eye irritation; harmful if swallowed; may cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H318: Causes serious eye damage. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| Lethal dose or concentration | LD50 (oral, rat): 11,200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 11,200 mg/kg |
| NIOSH | **WFJ86680** |
| PEL (Permissible) | Not established |
| REL (Recommended) | 36.0 |
| Related compounds | |
| Related compounds |
Carbamide Phosphoric acid Ammonium dihydrogen phosphate Monoammonium phosphate Diammonium phosphate |
| Related compounds |
Monopotassium phosphate Monoammonium phosphate Diammonium phosphate Urea Phosphoric acid |

