Tripotassium Citrate: A Practical Overview
Historical Development
Chemists first started isolating citrates centuries ago, but it wasn’t until the 20th century that tripotassium citrate took on real significance outside the lab. Changes in how people understood kidney health and food preservation set the stage. As kidney stone rates climbed and processed foods became widespread, researchers and industry professionals looked for compounds that could tackle both nutrition and technical hurdles. I’ve seen scientific references from the 1930s detailing how potassium salts improved certain health markers, but modern production methods only took off when industrial chemists figured out scalable processes post-war. The emerging field of clinical nutrition also played a part, pushing tripotassium citrate toward a reputation as more than just a harmless filler—now, it’s a staple for doctors treating specific urinary tract issues, and food technologists who want potassium-rich, low-sodium solutions.
Product Overview
Tripotassium citrate is a white, odorless powder that’s become ubiquitous across different industries. It pops up in nutritional supplements, beverage mixes, and as an ingredient in functional foods. Someone flipping over a package of powdered sports drink will often find it on the label. It balances potassium content, supports a more alkaline pH in the final product, and in some cases, helps extend shelf life. Companies lean on its solubility and mild taste, and pharmaceutical producers add it to their toolkit for its urinary alkalinizing effect. As a raw material, it’s easy to transport and store, so manufacturers favor it when they need a bulk potassium source that won’t complicate logistics.
Physical & Chemical Properties
At room temperature, tripotassium citrate appears as a fine, crystalline powder that dissolves in water with little effort, but doesn’t mix at all with alcohol. Its chemical formula, C6H5K3O7, underlines the structure: three potassium atoms replacing three hydrogens from citric acid. The product melts at about 230°C and starts to lose water past that. In physical handling, it stays free-flowing unless it gets wet, so humidity control matters during storage. A dry warehouse makes all the difference in product performance, whether it’s destined for a clinical pharmacy or a beverage bottling plant.
Technical Specifications & Labeling
For industrial and medical use, specifying tripotassium citrate means checking for purity, loss on drying, and heavy metal content. Commercial grades carry certificates showing potassium content above 36%, moisture below 4.5%, and minimal lead, arsenic, or mercury. Pharmaceutical suppliers print precise potassium equivalents and batch numbers on every bag, keeping compliance officers and inspectors happy. Food-grade versions follow global standards, with E number E332(iii) marking each package for traceability. In practice, I’ve watched QA teams reject entire pallets for off-color or moisture that strayed half a percent above spec; getting these basics right is non-negotiable if you want regulatory approval.
Preparation Method
Production teams typically produce tripotassium citrate by neutralizing citric acid with potassium carbonate or potassium hydroxide. The reaction runs in large stainless steel vessels, with careful temperature and pH adjustment to maximize yield. Workers mix citric acid with water, then slowly add potassium carbonate, capturing released carbon dioxide. This step isn’t glamorous, but tight process control ensures full reaction and low residual citric acid. Filtration removes any solids, then controlled evaporation crystallizes the finished product, which is then dried further and milled down to the desired particle size. Modern plants recycle water from the process, aiming to cut operational waste.
Chemical Reactions & Modifications
Tripotassium citrate reacts with strong acids and forms potassium salts with different organic acids. In laboratory settings, heating it in air produces potassium carbonate, releasing water and breaking the citrate backbone. Adding calcium sources can form insoluble calcium citrate—a reaction used to scrub excess citrate from product streams in some factories. Chemists sometimes use tripotassium citrate as a buffer to stabilize other compounds that degrade in acids, and formulators pair it with other potassium salts to tailor electrolyte profiles. Its resilience in mildly acidic and neutral pH makes it a popular base for custom modifications.
Synonyms & Product Names
In global commerce and lab catalogs, tripotassium citrate appears under several names: potassium citrate tribasic, potassium citrate monohydrate, and the simple abbreviation K3C6H5O7. Makers and brokers use the E number E332(iii) for food labeling. Over-the-counter supplements in the United States call it potassium citrate and sometimes market it under trade names or proprietary blends for renal support. International shipments often include translations in major European, Asian, and Latin American languages, but the reference always circles back to potassium citrate, tribasic form.
Safety & Operational Standards
Tripotassium citrate has a reassuring safety profile, but that doesn’t mean handling requires no caution. Workers exposed to high dust loads can get respiratory irritation, so production lines increasingly use sealed systems and supply good quality masks. Ingestion in approved doses shows low toxicity, but overuse bumps up blood potassium, which can be risky for people with kidney problems. Soliciting regular third-party lab analysis for heavy metals and microbial contamination is routine, as is monitoring water activity in storage rooms. US and EU agencies agree on its “generally recognized as safe” status, but batch traceability and allergen control never get less important.
Application Area
This one ingredient turns up in a surprisingly broad set of products. Food technologists use it in soft drinks, jams, jellies, and processed cheeses, replacing sodium in low-sodium foods and helping maintain pH balance. Hospitals rely on it for kidney stone patients, either to correct mild acidosis or keep stones from coming back. Sports nutrition blends need it for its potassium content, aiding muscle recovery. Manufacturers in the detergent sector once eyed citrate salts for chelating properties, but food and pharma applications drive most of today’s demand. Beverage makers and supplement brands lean on its mild flavor and simple regulatory path.
Research & Development
Academic labs and private companies continue to dig into new uses and performance tweaks. Scientists track the compound’s impact on urinary pH and potassium levels, tying intake to improved kidney stone outcomes. Formulators push for better dispersibility in powdered drinks, or look for blends with other mineral salts to fine-tune electrolyte absorption. Research teams experiment with granule size and coatings, aiming for slower release in the digestive system or longer shelf life in challenging climates. I’ve seen grant proposals calling for next-generation potassium supplements based on this simple molecule, as the basics still matter for athletes and patients alike.
Toxicity Research
Multiple studies place tripotassium citrate in the low-toxicity category for humans. Therapeutic supplements usually deliver below 100 mEq per day; lab studies show only mild gastrointestinal irritation at these levels. Reports indicate that significant overdose—usually only from intentional misuse or impaired kidney function—can elevate blood potassium enough to spark heart rhythm problems. Chronic toxicity doesn’t show up in rodents unless diet or water supplies are manipulated to push levels very high. Medical guidelines suggest regular monitoring of blood electrolytes for people who rely on it, especially in populations prone to kidney retention of potassium.
Future Prospects
Tripotassium citrate looks set to remain a workhorse for food, pharmaceutical, and supplement developers. An aging population and greater awareness of sodium reduction will keep pushing formulations toward alternative potassium salts. Researchers focus on less-processed product forms, cleaner sourcing, and potential roles in regulating gut health. As sustainable chemistry scales up, plants sourcing raw citric acid from fermentation offer greener credentials and reduced waste. Improved granulation and enhanced stability mean athletes and chronic disease patients can expect products that keep pace with today’s demands for purity and traceability.
What is Tripotassium Citrate used for?
Why Doctors Reach for Tripotassium Citrate
Tripotassium citrate pops up often in hospitals and clinics, especially among people who deal with kidney stones. Doctors see patients come in with pain, blood in their urine, or sometimes regular check-ups catch small stones before they start trouble. Most of the common stones are made of calcium, and acid in the urine helps them form. Adding tripotassium citrate makes the urine less acidic. I remember a neighbor who got kidney stones every two years — after starting a simple dose of tripotassium citrate, his stone count dropped way down, and his hospital visits almost stopped.
Hidden Benefits in Everyday Foods
The food industry relies on tripotassium citrate as a flavor stabilizer and acid regulator. Soft drinks, flavored waters, and gelatins often use it to keep tartness at the right level or balance the sweetness and sourness. Those sour gummy candies that kids love? Manufacturers add tripotassium citrate to punch up the flavor and keep it tangy. Nutrition labels list it for a reason — it helps food companies deliver the taste people expect each time.
How the Body Handles Potassium and Its Role
Potassium keeps our hearts beating steadily, nerves firing, and muscles moving. Some people need extra potassium because they lose it from certain diuretics, or because of illnesses that flush it out faster. Tripotassium citrate helps bump up potassium without adding a heavy salt load. Anyone who’s land in the emergency room for dehydration or low potassium knows how dangerous a shortage can become. Muscle cramps, weakness, irregular heartbeat — patients with these symptoms often improve once their potassium gets back on track.
Safety and Dosing: What People Should Know
Doctors carefully weigh the benefits before recommending tripotassium citrate. Taking too much potassium climbs up fast in the body, and can turn safe use into an emergency, especially for people with kidney trouble. The kidneys handle getting rid of extra potassium, so anyone taking this compound needs their kidney health checked first. For those on heart or blood pressure medicines, the risk rises, as some drugs push potassium higher. Blood tests keep people safe, adjusting the dose or stopping the medicine if things look risky.
Potential Solutions and Reducing Risks
Clear communication between patients and their health team goes a long way. Someone starting this supplement should know warning signs like tingling hands, muscle weakness, or a pounding heart. Pharmacists play a key role, too, double-checking other medicines before filling a new prescription. If a person picks up sports drinks or processed food at the market, paying attention to the label helps avoid adding up too much potassium over the day. Health educators can spread the word so those who benefit most from tripotassium citrate — people fighting kidney stones or potassium loss — get the help they need without landing in trouble.
Is Tripotassium Citrate safe to consume?
Understanding What Tripotassium Citrate Is
Tripotassium citrate shows up on food ingredient lists, supplement bottles, and sometimes even in medications. Manufacturers use it to help control acidity, keep ingredients stable, and sometimes to help manage kidney health. Some people worry about the unpronounceable names in their food, but tripotassium citrate has a straightforward makeup: it’s a potassium salt of citric acid. Potassium exists naturally in foods like bananas, potatoes, and leafy green vegetables, and citric acid comes from citrus fruits. The FDA recognizes tripotassium citrate as “Generally Recognized as Safe” for its common uses.
Potassium: Vital, But Not in Excess
Most people get potassium from fruit, vegetables, and beans. This mineral supports the muscles, nerves, and helps balance salt and water in the body. Low potassium can be risky, but too much is also a problem, especially for people with kidney disease or folks taking certain blood pressure medicines. If the kidneys don’t filter potassium out well enough, high potassium shows up in blood tests and can affect heart rhythm. The amounts in food additives like tripotassium citrate are usually small, but concentrated supplements taken without medical advice aren’t always safe.
Why Do Food Makers Use Tripotassium Citrate?
Manufacturers add tripotassium citrate to create the right taste, stop spoilage, or help supplements dissolve. In food, this could be in a fruit drink, a flavored gel, or processed cheese. In supplements, people with kidney stones sometimes get prescription-strength citrate to stop calcium stones from forming. Citrate helps by making urine less acidic, which slows stone formation. In these scenarios, medical guidance matters.
Safety Evidence From Research
Researchers and regulatory agencies have looked into the safety of tripotassium citrate for decades. Studies on animals and humans do not link it to cancer or toxic effects at typical food and supplement levels. When scientists reviewed its presence in processed foods, they found the daily intake for most people would not approach amounts that could cause harm, as long as the person is healthy and not on a potassium-restricted diet.
Who Should Watch Their Intake?
People managing kidney disease, Addison’s disease, or on certain medications for high blood pressure need to be very cautious about extra potassium. Some older adults and folks with major health issues miss out on kidney function checks, and tripotassium citrate adds invisible potassium to their overall diet tally. Regular bloodwork can catch potassium changes early — most doctors check it often in at-risk groups for exactly this reason.
Ideas for Consumers and Policy Makers
Food labels aren’t always clear about how much added potassium comes from tripotassium citrate. Clearer labeling would help people with medical reasons to watch potassium. For others, a balanced diet focused on fruits and vegetables delivers natural potassium without added concerns. Companies could provide phone numbers or websites for people seeking details about potassium sources in processed food. Staying informed, checking in with healthcare providers, and paying attention to labels go a long way toward keeping potassium in a healthy range.
What are the side effects of Tripotassium Citrate?
What Users Should Know
Tripotassium citrate often appears on prescription labels for people struggling with kidney stones or certain acid imbalances. Doctors recommend it because it helps the body get rid of extra acid and prevents some types of kidney stones from forming. More potassium sounds like a good thing, but taking it in a concentrated form isn’t without risk.
Common Issues That Crop Up
Anyone who has dealt with potassium supplements knows they don’t always sit easily with the stomach. Nausea pops up for a lot of folks just getting started. Some people mention a stomach that feels unsettled or borderline queasy after they take their daily dose. Others talk about cramps that linger through the day or appear out of nowhere. Vomiting makes the list, too — not ideal by any means, especially for those already feeling rough from kidney problems.
Loose stools can catch people off guard. Potassium in this form sometimes moves things through the digestive tract a little too smoothly. If you notice more trips to the bathroom, it usually traces to the supplement. Some experience a drastic shift in bowel habits, with diarrhea sticking around until the body adjusts or the dosage changes.
Risks That Deserve Careful Attention
High potassium in the blood (hyperkalemia) gets far more serious. The chances go up for people with kidney issues because their bodies already struggle to process out extra potassium. Too much in the blood can cause muscle weakness or irregular heartbeat. In rare cases, dangerously high potassium can trigger cardiac arrest. These are the sorts of warning signs no one can ignore: muscle paralysis, numbness, chest tightness, or feeling like the heart is out of rhythm. Someone with any of these should skip waiting and get checked right away.
Who’s More Vulnerable?
Folks managing chronic kidney disease swim in deeper water. Their kidneys aren’t clearing potassium like they’re supposed to. Those taking other medications that raise potassium, such as certain blood pressure pills (ACE inhibitors, ARBs, and spironolactone), face a double whammy. Age also plays a part. As people get older, kidney function often drops, so their margin for error shrinks. This isn’t about scare tactics — it’s about knowing your body’s limits and speaking up before small glitches become emergencies.
My Take: Staying Out of Trouble
Long-term, sticking to blood tests really makes a difference. Doctors watch for high potassium and adjust dosages when numbers climb out of safe range. I’ve watched patients improve with honest reporting — flagging the subtle stuff like palpitations or muscle tingling before it graduates into something critical. Staying hydrated helps, unless your provider suggests cutting back because of other medical concerns.
Potassium-rich foods like bananas and potatoes don’t pose the same risks as concentrated medication, but on a supplement, it’s worth reading nutrition labels and keeping servings in check. If vomiting or diarrhea won’t quit, reach out. Changing medication or managing the dose often solves the stomach issues.
Steps for Safer Use
Reading up on the prescription always helps, but it won’t beat a quick check-in with a doctor or pharmacist. Taking the supplement with food can soften some GI side effects. A little planning goes a long way: never mix this with other potassium meds unless a doctor knows about it, and don’t miss routine bloodwork. Reports from the CDC show medication errors as a persistent problem nationwide. Vigilance on both sides — patient and provider — lowers the odds of a bad outcome.
If you end up worrying about tripotassium citrate, trust your hunch and talk it through with someone who can sort through your prescriptions. Potassium plays a vital role, but only in the right amount for each person. Listening to your body stays more valuable than any pamphlet or product insert.
How should Tripotassium Citrate be taken or dosed?
Understanding the Basics
Tripotassium citrate shows up in prescriptions for a reason—kidney stones and certain metabolic issues can really turn life upside down. Like a lot of things we use for our health, it’s easy to think of it as just another medicine. But that isn’t the whole story. This compound helps adjust the acid balance in urine and supports the kind of potassium levels the body depends on. So, the way it’s taken deserves careful attention.
Dosing Is Not One-Size-Fits-All
Getting the dose right isn’t about following the advice you hear from someone else. Doctors take blood tests, urine tests, and sometimes even a person’s diet into consideration. For adults dealing with kidney stones, doses often fall between 5 to 15 grams per day, divided into smaller amounts, but only after a professional gives the go-ahead. Nobody should decide quantity without a doctor. The body’s ability to handle potassium changes with age, medical conditions, and what other medicines someone might already use, especially blood pressure drugs or diuretics.
Timing and Taking With Food
A lot of folks notice stomach upset if they swallow tripotassium citrate on an empty stomach. Taking it with meals or a snack usually helps. Spacing the doses throughout the day, rather than downing it all at once, protects the stomach and keeps potassium levels from swinging. Swallow the tablets whole—no crushing or chewing. The powder works just as well when stirred into a glass of water and sipped slowly. Water matters here, as dehydration can make kidney issues worse.
Listening to Your Body — and the Numbers
Potassium helps nerves and muscles work, especially the heart. Too much, though, can be risky. Fatigue, muscle weakness, or irregular heartbeats should never be ignored, and regular blood tests help spot changes before they become a problem. From my own experience with long-term medication, staying alert to small changes in how you feel keeps you safer than trying to ride out side effects. One close friend ignored muscle cramps for a week while on a similar supplement—he ended up at urgent care.
Interactions and Special Considerations
Tripotassium citrate doesn’t exist in a vacuum. People who take heart or blood pressure drugs, those with kidney problems, or anyone with a history of ulcers need special planning. Even small changes in fluid or salt intake at home can affect how this supplement works. Sharing your full medicine list with the pharmacist helps avoid dangerous drug interactions. Even over-the-counter antacids or pain relievers can throw a wrench into things.
Smart Steps for Success
Doctors and pharmacists serve as the best guides. When starting out, write down the schedule and set phone reminders. Drink plenty of water. Check in for regular blood work, especially soon after changing the dose. Avoid “doubling up” if you miss a dose—instead, just resume the next one on schedule. I’ve seen how written logs and pill organizers make a world of difference for those new to long-term medications. They prevent missed doses and keep the guesswork to a minimum.
A Word on Reliable Information
There’s a lot of conflicting information out there, especially on forums and social media. Lean on guidance from board-certified physicians, pharmacists, and references like Mayo Clinic or the U.S. National Library of Medicine. Those sources update often based on real-world results and scientific evidence. Keeping information up to date helps patients avoid unnecessary risks.
Are there any drug interactions with Tripotassium Citrate?
Why Tripotassium Citrate Gets Prescribed
Tripotassium citrate usually appears on a prescription when someone has kidney stones or issues with too much acid in their urine. Doctors also reach for it to manage certain kidney problems. It helps balance pH by making urine less acidic, slowing the growth of stones and easing kidney strain. The body handles potassium in a tight range—too little causes muscle weakness and irregular heartbeat, too much makes nerves and muscles misfire, and even puts the heart at risk.
Common Medications That Raise Risk
Some medicines cause problems when taken with tripotassium citrate. One big group includes drugs that keep potassium in the body: ACE inhibitors such as lisinopril, angiotensin receptor blockers like losartan, and potassium-sparing diuretics (spironolactone, amiloride). Mixing these with tripotassium citrate can make potassium levels shoot up. The heart doesn't like this, and the chance of dangerous rhythm problems goes way up.
Over-the-counter painkillers, such as non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen, can also get people into trouble. These drugs slow how the kidneys get rid of potassium. If a person already has a kidney problem or is older, the added potassium load from tripotassium citrate may push things over the edge. High potassium is sneaky—it’s not easy to spot in the early stages. People often don’t notice anything until there’s muscle weakness, numbness, or an irregular heartbeat.
Using Tripotassium Citrate Safely
Doctors emphasize the need for blood tests while someone takes tripotassium citrate, especially at the start or after changing doses. Potassium can jump fast, so checking levels keeps everyone safe. People on medications known to raise potassium need even more careful watching.
Plenty of patients I’ve seen find themselves caught out: they think because something is prescribed it must be safe alongside everything else they take. The truth looks very different. One friend of mine had to stop his blood pressure pills for a stretch, just because he took tripotassium citrate and ended up with potassium numbers off the charts. He felt fine, which made it extra tricky—danger doesn’t always feel dramatic.
Practical Advice For Patients
Most people don’t memorize lists of drug interactions, and pharmacists catch plenty of these mixes. They help spot trouble before it starts. Always make sure every doctor knows about everything being taken—including vitamins and herbal supplements. Bananas, oranges, and salt substitutes carry a lot of potassium too, and doctors sometimes miss this detail during quick visits.
Those with kidney disease, heart failure, or those on certain blood pressure medicines walk a narrower tightrope. The best approach stands out: ask questions every time a new prescription comes home. Get labs drawn as directed, and don’t ignore pharmacies’ warnings or handouts about drug mixes.
Ways to Make Interactions Less Likely
Doctors and pharmacists both have a role in reducing mistakes. Electronic health records spot high-risk combinations, but checking in person at every visit digs up information software misses—updating every list, asking about symptoms, and checking heart and kidney function. Education helps, too. When patients know what signs point to trouble (weakness, tingling, palpitations), they’re in a much stronger position.
Tripotassium citrate works well in the right hands, but ignores drug interactions, and the story changes. Modern healthcare gets complicated quickly, but a team-based approach with checks, good communication, and updated medical records helps avoid most pitfalls.
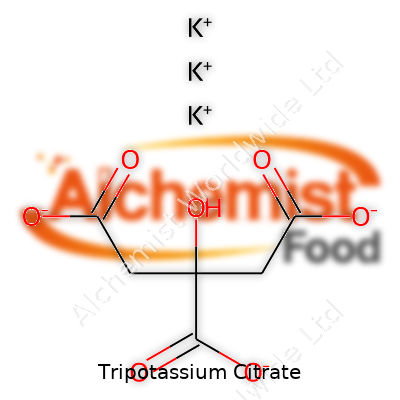
| Names | |
| Preferred IUPAC name | Tripotassium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Tripotassium salt of citric acid Potassium citrate tribasic Potassium citrate (3K) C6H5K3O7 Citric acid tripotassium salt |
| Pronunciation | /traɪ.pəˈtæsi.əm ˈsɪtreɪt/ |
| Preferred IUPAC name | potassium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Potassium citrate tribasic Tripotassium salt of citric acid Citrate de potassium Potassium citrate (3K) Potassium 2-hydroxypropane-1,2,3-tricarboxylate |
| Pronunciation | /traɪ.pəˈtæsi.əm ˈsɪ.treɪt/ |
| Identifiers | |
| CAS Number | 866-84-2 |
| Beilstein Reference | 1723079 |
| ChEBI | CHEBI:32599 |
| ChEMBL | CHEMBL1200984 |
| ChemSpider | 54609 |
| DrugBank | DB14527 |
| ECHA InfoCard | 100.246.860 |
| EC Number | 209-811-1 |
| Gmelin Reference | 12616 |
| KEGG | C14547 |
| MeSH | D017388 |
| PubChem CID | 167604 |
| RTECS number | TT2975000 |
| UNII | G2V6N2E1KX |
| UN number | UN3077 |
| CAS Number | 866-84-2 |
| Beilstein Reference | 17112 |
| ChEBI | CHEBI:11362 |
| ChEMBL | CHEMBL1201560 |
| ChemSpider | 22714 |
| DrugBank | DB14557 |
| ECHA InfoCard | '03b1a2d4-60f8-43df-9c98-8e6b89347568' |
| EC Number | 209-533-2 |
| Gmelin Reference | 160158 |
| KEGG | C01762 |
| MeSH | D017089 |
| PubChem CID | 167604 |
| RTECS number | TT2975000 |
| UNII | Z77F4QL6VS |
| UN number | UN3077 |
| Properties | |
| Chemical formula | K3C6H5O7 |
| Molar mass | 306.395 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | Density: 1.98 g/cm³ |
| Solubility in water | Very soluble |
| log P | -3.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa1 = 3.13; pKa2 = 4.76; pKa3 = 6.40 |
| Basicity (pKb) | pKb ≈ 11.6 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.49 |
| Dipole moment | 4.2 D |
| Chemical formula | K3C6H5O7 |
| Molar mass | 306.395 g/mol |
| Appearance | White, granular or crystalline powder |
| Odor | Odorless |
| Density | Density: 1.98 g/cm³ |
| Solubility in water | Very soluble |
| log P | -3.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa1 = 3.13, pKa2 = 4.76, pKa3 = 6.40 |
| Basicity (pKb) | pKb ≈ 3.13 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.50 |
| Dipole moment | 4.2 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 418.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2300.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3582 kJ/mol |
| Std molar entropy (S⦵298) | 465.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2294.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -373 kJ/mol |
| Pharmacology | |
| ATC code | A12BA03 |
| ATC code | A12BA03 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. IF ON SKIN: Wash with plenty of water. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 Oral Rat 2,000 mg/kg |
| LD50 (median dose) | 3900 mg/kg (Rat, oral) |
| NIOSH | RN:866-84-2 |
| PEL (Permissible) | 30 mg/m³ |
| REL (Recommended) | 300 mg/kg bw |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | > 220 °C |
| Autoignition temperature | > 470°C |
| Lethal dose or concentration | LD50 (oral, rat): 5400 mg/kg |
| LD50 (median dose) | 3900 mg/kg (rat, oral) |
| NIOSH | CAS No. 866-84-2 |
| PEL (Permissible) | 30 mg/m³ |
| REL (Recommended) | 340 mg/kg bw |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Monopotassium citrate Dipotassium citrate Trisodium citrate Citric acid |
| Related compounds |
Potassium citrate Monopotassium citrate Dipotassium citrate Sodium citrate Calcium citrate Magnesium citrate Citric acid |

