Tricalcium Phosphate: From History to Future Innovation
Historical Development
In the early days of studying minerals, scientists kept tripping over naturally occurring calcium phosphates in bones and rocks. By the 18th century, chemists like Carl Wilhelm Scheele closed in on ways to isolate and describe these compounds. Discovery of tricalcium phosphate’s role both in living things and the natural world set the foundation for its commercial production in the late 1800s, as agriculture and food fortification efforts ramped up. The story of tricalcium phosphate moved quickly after that — from feeding hungry soils with superphosphate fertilizers, to finding a place in toothpaste and tablets on modern store shelves. Each decade brought more curiosity and better methods to harness this white, chalky powder in forms people can trust for health and industry.
Product Overview
Tricalcium phosphate (sometimes listed as TCP, E341(iii), or tribasic calcium phosphate) falls into that rare category of materials trusted by health, food, and industry workers alike. Some call it “bone ash”, a throwback to its original source before synthetic production took off. Modern TCP appears in products labeled for dietary calcium, tablet binders, anti-caking agents, toothpaste abrasives, ceramics, and implant coatings. Factories crank out tons each year, relying on its stability and essential mineral content, and that long-standing record underwrites regulations covering everything from infant formula to chewable vitamins.
Physical & Chemical Properties
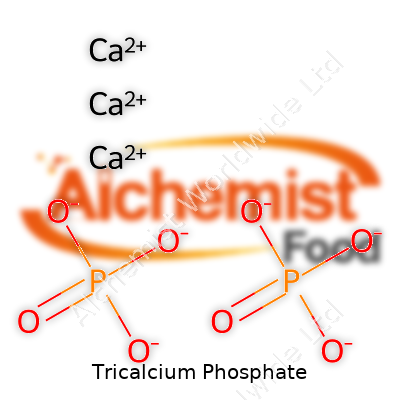
As a fine white powder, tricalcium phosphate feels much like soft chalk or silt between the fingers. It resists dissolving in water but reacts well with acids, breaking down and releasing calcium and phosphate ions. Its formula, Ca3(PO4)2, signals three calcium atoms bound to two phosphate groups. TCP holds up under high heat, so it gets used in ceramics and bone repair applications. The powder comes with a faint, earthy scent but no strong odor or taste. It doesn’t burn, doesn’t attract humidity, and stores easily away from acids or strong bases.
Technical Specifications & Labeling
In the United States, technical and food-grade tricalcium phosphate must meet set criteria for purity and heavy metal content. Labels on sacks or containers should feature batch numbers, country of origin, and manufacturer data to trace any issues. The Food Chemical Codex and European Food Safety Authority lay out benchmarks for loss on ignition, assay range limits (typically 34-40% calcium by weight), and acceptable levels for lead, arsenic, and fluoride—key for users in food, pharma, and biomed. In finished goods, ingredients panels often read “tricalcium phosphate,” sometimes with E341(iii) or a local designation.
Preparation Method
Synthetic tricalcium phosphate comes through wet or dry chemical processing, much improved from the days of bone burning. Most facilities start with pure phosphoric acid and quicklime (calcium oxide), blending them in water to yield a slurry. The reaction forms calcium phosphate, which then gets filtered, washed, and dried into the final powder. Factories keep a close eye on reaction temperature, pH, and washing cycles to keep impurities low. Alternative routes use sodium phosphate and calcium chloride, but the final filtration and drying steps always matter most for clinical or food use.
Chemical Reactions & Modifications
Mixing tricalcium phosphate with stronger acids (like hydrochloric) splits it apart, forming soluble calcium chloride and phosphoric acid, which chemists then redirect into fertilizers or nutrient solutions. In industry, roasting mixtures at over 1000°C can turn TCP into dicalcium phosphate or even hydroxyapatite, the latter matching mineral structures in human teeth and bone. To create specialized ceramics or implant coatings, TCP may be doped with trace metals or heated under carefully controlled atmospheres. All these modifications change solubility and bioactivity, tuning TCP’s usefulness for medicine or engineering.
Synonyms & Product Names
Shoppers may stumble onto names like tribasic calcium phosphate, bone phosphate of lime, or E341(iii) on packaging. Older texts call it BPL, especially within the fertilizer trade. In pharmaceuticals, ingredient decks show “calcium phosphate, tribasic.” Medical ceramics and devices may be labeled with alpha- or beta-TCP, signifying slightly different crystal forms. Most product certificates refer to its Chemical Abstracts Service (CAS) number, 7758-87-4, to clear up any confusion in global supply chains.
Safety & Operational Standards
Workplaces handling industrial-grade tricalcium phosphate use dust control measures since long-term inhalation may irritate airways. It doesn’t show acute toxicity, though chronic overexposure in factories calls for open windows, dust masks, and good clean-up habits. Food-grade and pharmaceutical versions face stiffer purity rules to protect infants, patients, and anyone with kidney concerns. In line with Good Manufacturing Practice (GMP) and food safety codes worldwide, worker education also covers spill clean-up, eye protection, and storage away from acids.
Application Area
Tricalcium phosphate makes its way into all sorts of products people use or consume. In food, it works as a calcium fortifier and anti-caking aid in powdered mixes, salt shakers, and infant formulas. Hospitals and clinics see TCP in bone grafts, toothpastes, and tablet excipients. Farmers put it in animal feeds and phosphate-rich fertilizers. Biomedical engineers rely on TCP’s bioresorbable properties to make bone cements and coatings for replacement joints. It even pops up in ceramics, paints, and glass, underpinning sectors from construction to advanced manufacturing.
Research & Development
The science behind tricalcium phosphate keeps evolving. Universities and biotech firms test new forms that dissolve at different rates for more predictable outcomes in dental and surgical implants. Some labs embed antibiotics or growth factors in TCP ceramics, aiming for smarter healing in orthopedic surgeries. As nutritionists debate the best calcium forms for human absorption, comparative studies stack up for TCP versus citrate or carbonate. Green chemists innovate new production chains that cut down waste, look for alternative minerals, and explore low-energy synthesis routes.
Toxicity Research
Toxicologists factored in decades of animal and human data to clear tricalcium phosphate for food and supplement use. Overconsumption of phosphate—regardless of the source—raises concerns for kidney patients and those with impaired mineral handling, so care lies in diet balance rather than intrinsic TCP danger. Animal studies at extremely high doses show limited side effects, often tied to calcium imbalances more than toxic reactions. Occupational health studies give handling advice rather than strict restrictions. Even so, ongoing studies keep tabs on nano-scale TCP and potential effects still poorly understood.
Future Prospects
Looking ahead, tricalcium phosphate stands at the front of several growing sectors. Healthcare trends push for more biocompatible implant materials, where TCP’s close match to bone appeals to patients and surgeons. In food, populations seeking vegan, allergy-free, and mineral-fortified diets need stable calcium forms that dissolve well and stay safe across age groups. Agriculture continues to eye bioavailable mineral inputs as soils shift and regulatory pressures mount. Research points to new uses, from scaffolds for stem cell growth to greener fertilizers using less energy and raw material. Being both ancient and adaptable, tricalcium phosphate remains a staple science can count on, driving industries that feed, heal, and build.
What is Tricalcium Phosphate used for?
The Role of Tricalcium Phosphate in Everyday Life
I’ve seen tricalcium phosphate listed on food labels more times than I can count, usually tucked among ingredients that get far less attention than the headline flavors and calories. This mineral compound—composed of calcium and phosphate—serves an unspectacular but vital role in our kitchens and medicine cabinets. For anyone who remembers school science class, these are the same elements bones and teeth rely on for strength and structure.
Food Uses and Why It Matters
Bakers, food manufacturers, and even home cooks lean on tricalcium phosphate for more than just an extra dash of calcium. In powdered foods—think salt, spice blends, or powdered milk—this mineral acts as an anti-caking agent. My own pantry tends to collect clumpy salt when humidity rises, but with a little tricalcium phosphate added to the mix, everything stays smooth and pourable. That may sound minor, but anyone trying to shake out lumpy spices over a hot pan will agree: it saves time and frustration.
This stuff appears in breakfast cereals, flour, and baby formula, too. In these cases, it doesn’t only keep things from sticking together. It’s also a fortifier, raising calcium levels without needing folks to swallow a bitter pill. The bones and teeth story comes back here—milk alternatives and plant-based foods often include tricalcium phosphate so families swapping dairy don’t lose out on this fundamental nutrient. Research in the Journal of Food Science and Technology points out that calcium from tricalcium phosphate can help improve mineral intake, especially for people who keep dairy off their plates for health or ethical reasons.
Pharmaceuticals and Toothpaste: Not Just About Food
The pharmacy brings out another set of uses. In tablets, this mineral helps bind powders so pills don’t crumble but also dissolve when needed. Its track record for safety means it still appears in many prescription blends. I remember years back, reading ingredient lists while taking a multivitamin and spotting tricalcium phosphate. At the time, I didn’t give it much thought. Now, I appreciate why drug manufacturers rely on it over flashier, untested options. The molecule’s stability and ease of handling mean both makers and patients can trust what they’re getting.
Dental products take it a step further. Toothpaste brands include tricalcium phosphate not just to fill space but to help repair enamel, working with fluoride to return minerals to areas where acid has eroded them. Dental health experts have said that this combination improves cavity resistance over time, especially in populations who don’t have easy access to regular care.
Challenges and Responsible Use
Even something with a solid record like tricalcium phosphate needs oversight. Too much phosphate in the diet can stress kidneys or affect bone health in rare conditions. Regulatory agencies including the FDA have set clear boundaries for how much can go into food. So, responsible product design plays a key part. People count on the trust they can place in store-bought staples, whether that means clear labeling or thoughtful use of additives like this one. As always, I pay closer attention to my food choices now that I understand more about what each ingredient actually does for me.
As the food industry continues growing, I’d like to see more transparency and better education for shoppers. A short, honest note about why tricalcium phosphate appears in a breakfast cereal or vitamin bottle would help people make smarter decisions, especially with more families managing allergies, dietary restrictions, or balancing nutrition for growing kids and aging parents. The goal: healthier, informed choices—without the confusion or hidden surprises.
Is Tricalcium Phosphate safe for consumption?
What Is Tricalcium Phosphate?
Tricalcium phosphate pops up on a lot of ingredient labels these days, especially in cereals, baked goods, dairy substitutes, and some vitamin supplements. It happens because tricalcium phosphate packs calcium that our bodies need, and food makers like it for its texture and calcium boost. Many wonder if eating foods with tricalcium phosphate actually raises any red flags for health, or if it just sounds more chemical than it really is.
Understanding Its Role and Source
On paper, tricalcium phosphate looks like a combo of calcium and phosphorus, two minerals that our bones rely on. The industry makes most batches from natural rock phosphate, then cleans them up for food use. Both calcium and phosphorus matter for bones, teeth, and hundreds of daily cellular processes. Tricalcium phosphate works almost like a two-in-one supplement, and for years, the Food and Drug Administration has put it on the Generally Recognized As Safe (GRAS) list at reasonable levels.
Research and Real-World Experience
Plenty of nutrition studies have put tricalcium phosphate under the microscope. Across typical serving sizes found in fortified foods or supplements, scientists haven’t found evidence that tricalcium phosphate triggers health troubles in healthy adults. High levels of calcium from lots of sources can eventually build in tissues, but most people don’t consume enough from additives alone to approach those zones. For children, parents of picky eaters sometimes rely on calcium-fortified food as a lifeline for strong bones.
I recall navigating the supplement aisle in college after a string of heavy-footed soccer injuries. Our local doctor always recommended checking for simple mineral blends like calcium phosphate over flashy, expensive combos. A balanced intake guided by dieticians usually went a long way toward bone recovery for classmates, despite plenty of skepticism about “chemicals” as food ingredients.
Digestive Comfort and Allergies
For folks with dairy allergies or lactose intolerance, tricalcium phosphate brings a form of calcium that doesn’t rely on milk, cheese, or yogurt. It beats out calcium carbonate and similar ingredients for gentleness on many stomachs, although taking huge doses of anything with calcium can clog up digestion. The biggest source of complaints around tricalcium phosphate often revolves around too much of it in single meals, which can briefly upset the gut.
Who Should Watch Their Intake?
People with chronic kidney issues should tread carefully, since their bodies struggle to remove phosphorus, and high phosphate levels can mess with bone and heart health. Anyone prescribed a restricted phosphorus or calcium diet should loop in a nutritionist before adding anything.
Food Label Transparency
Transparency in what goes into packaged food matters. Food labels in the United States must list tricalcium phosphate clearly, and those with rare sensitivities or on unique medical diets get a fair shake. Still, most families probably blend these foods into a larger eating pattern—milk, beans, leafy greens—so additive levels never add up much. A balanced shopping cart full of real fruits and vegetables makes the debate about single additives almost moot.
Building Trust in Modern Food Ingredients
Beneath the surface anxieties about “processing” lies real power in straightforward ingredient education. Safe, affordable mineral additives keep fortified foods accessible and bridge gaps for people who struggle with regular calcium sources. In a world where processed snacks get a bad rap, tricalcium phosphate stands out as less about artificial fillers and more about meeting basic nutritional gaps for communities.
What are the side effects of Tricalcium Phosphate?
Looking Past the Label: Everyday Matters with Tricalcium Phosphate
Tricalcium phosphate pops up in a surprising number of foods and supplements. It keeps powdered drinks from clumping. It pumps up the calcium in your cereal. For anyone watching their nutrient intake, especially those low on calcium, that sounds like a win. But calcium isn’t the only thing that matters. The body cares about how much and in what way nutrients come in. Overdoing any supplement or additive never serves us well.
Digestive Unhappiness
The gut reacts the quickest to too much tricalcium phosphate. After a hefty dose, some people complain of bloating, constipation, or an upset stomach. Most of us have reached for a glass of water or gone on a walk to help move things along after eating too much calcium. I found out the hard way that my morning shake, jazzed up with extra powder, made me feel heavier than a Sunday roast dinner. Science backs this up, too—high doses of calcium, even from food additives, mess with regular bowel function.
Kidneys: On the Hook for Overuse
The kidneys play cleanup crew for excess calcium and phosphate. Over time, taking in too much tricalcium phosphate increases the risk of kidney stones. The odds go up if you’re already prone to stones or skimp on your daily water intake. Harvard Health reports that calcium supplements taken without food, or in mega doses, inch up the risk. Both calcium and phosphate can crystalize, especially if water intake lags behind and the minerals have nowhere else to go.
Long-Term Trouble: Bones and Beyond
The irony hits hard—too much phosphate throws calcium balance out of whack and actually weakens bones over the long run. This isn’t just theory. Research from the National Institutes of Health shows that an overload of phosphorus (including phosphate additives in food) leaves bones less dense. For anyone with kidney problems, the issue gets worse. Phosphate builds up, the body pulls more calcium from the bones, and bone health declines. Doctors call this secondary hyperparathyroidism, but really, it just means bones get robbed to keep blood chemistry in check.
The Food Label Trap
Foods labeled as ‘fortified’ lure shoppers who want to do right by their bodies. Most don’t realize how easy it is to pile up on phosphate just by mixing a few protein shakes, munching on breakfast bars, and reaching for processed snacks. The CDC points out that the push to fortify foods with calcium and phosphate has benefitted many, but not everyone needs more through additives. Most healthy adults, if they eat a varied diet, already get all they need.
Smart Ways Forward
The solution relies on balance. Read nutrition labels, especially on supplements and processed food. Doctors suggest getting most minerals from whole foods: dairy, leafy greens, and fish. For those with bone concerns, kidney troubles, or family history of stones, ask your health provider before boosting calcium with tricalcium phosphate. And trust how your gut feels after new foods or vitamins. That dull ache or sudden constipation—those are signs to slow down. Real health comes from listening to your body and not just the back of the box.
Is Tricalcium Phosphate vegan or vegetarian?
Understanding Tricalcium Phosphate
Anyone who checks the back of their oat milk or supplements has probably seen tricalcium phosphate on the ingredient list. It shows up all over—breakfast cereals, plant-based drinks, vitamins. Food companies use it for its calcium kick and its role as an anti-caking agent. So, does it fit the bill for vegetarians and vegans?
Where Tricalcium Phosphate Comes From
The story begins with the source. Most tricalcium phosphate on the market comes from mined minerals. Big players in the food industry process rock phosphate, which is mainly made up of calcium phosphate deposits—no animal bones involved. The process may include heat treatment and purification steps that match food safety standards set by agencies like the FDA and EFSA. No part of this mineral pipeline requires animal-derived materials, and the finished product does not come with animal-based additives mixed in.
How It’s Used in Food and Supplements
People often worry about animal ingredients when looking at additives. I’ve talked to folks who tossed out an entire brand of plant milk due to phosphate fillers, concerned about hidden surprises. Dig a little deeper, though, and the evidence is clear: tricalcium phosphate stands apart from things like gelatin and animal rennet. In processed foods, vegan and vegetarian labeling hinges on avoiding any by-products from animal slaughter. Major certifying organizations allow tricalcium phosphate in goods that carry their vegan or vegetarian logo.
What About the Exceptions?
Phosphate can come from animal bone ash, but that belongs to the past or occasional medical and historical contexts. Large-scale tricalcium phosphate for today’s food world does not trace back to bones. Companies dedicated to vegan products vet their ingredient suppliers and track sourcing documentation, staving off contamination from animal sources.
Quality, Health, and Transparency
Shoppers put their trust in manufacturers who openly share their supply chain information. Labels that show both the ingredient and a vegan or vegetarian stamp give shoppers the green light. Regulatory agencies step in if labeling turns out to be misleading. Tricalcium phosphate passes those honesty checks. No fishy or meaty loopholes here. And with mineral-based calcium on the rise, especially for people avoiding dairy, this ingredient stands out as an accessible source for body health.
Potential Solutions to Ingredient Confusion
Ingredient anxiety is a real challenge. Simplifying food labeling could solve half the stress. Clear “plant-based calcium source” language on packaging saves a lot of head-scratching in the grocery aisle. Education matters, too—school nutrition programs and plant-based advocacy groups can spread the word on the most common additives safe for vegans and vegetarians.
Final Thoughts
After years spent poring over ingredient lists at the supermarket, and conversations with dietitians and food chemists, it’s obvious that tricalcium phosphate fits comfortably into both vegan and vegetarian diets. Sourcing almost always stays on the mineral track, untouched by animal by-products. As more folks look for plant-forward options, tricalcium phosphate delivers on both nutrition and peace of mind.
How is Tricalcium Phosphate different from other calcium supplements?
What Makes Tricalcium Phosphate Stand Out?
These days, picking a calcium supplement can feel like staring down a pharmacy aisle packed with too many choices. Tricalcium phosphate finds its way into protein drinks, cereals, and even kids’ gummy vitamins, standing shoulder-to-shoulder with the giants like calcium carbonate and calcium citrate. The scientific name might not sound inviting, but this compound has some traits that set it apart.
Understanding Absorption and Bioavailability
Calcium doesn’t travel alone; it needs a good partner for absorption. Some forms, like calcium carbonate, want an acidic stomach and hit a roadblock for folks on antacid therapy or for older adults with declining stomach acid. Calcium citrate doesn’t fuss as much about stomach condition but can cost more and comes in bulkier pills.
Tricalcium phosphate lands somewhere in the middle. It dissolves moderately well and doesn’t need intense stomach acid for your body to make use of it. Still, since it contains phosphate, there’s a catch—phosphate and calcium can mix in the gut and form particles your body struggles to absorb if you overdo it. That’s a factor if someone already takes in loads of phosphate from processed food and soda.
More Than a Bone Builder
Most people eye calcium for bones and teeth. That’s a big part of the story, but it misses why tricalcium phosphate even shows up in supplements and fortified food in the first place. Plenty of common supplements source pure calcium from limestone or oyster shells. Tricalcium phosphate pulls its calcium from minerals with phosphate included, which helps fill in for those who don’t get enough phosphorus—and phosphorus counts for energy production and even DNA repair.
Not every calcium pill covers both minerals. Some diets shortchange people on phosphorus, especially if they cut back on meat and dairy. Tricalcium phosphate chips in here, letting users top up their stores of two critical minerals at once.
Digestibility and Dosing
Large, chalky calcium carbonate pills sometimes make people uncomfortable. Calcium citrate softens the blow, but it’s bulky in chewable forms. Tricalcium phosphate flows easily, blends into powders, and works for food fortification, making it better for mixing into smoothies or protein shakes.
From my own experience in nutrition counseling, I’ve seen athletes and older adults gravitate toward tricalcium phosphate because they want options beyond giant pills. Some people find it easier on their stomachs, though others worry about phosphate intake if they eat a lot of fast food or processed cheese, which already carry a load of phosphates.
Concerns and the Bigger Health Picture
Epidemiological data in the United States points to adults getting more phosphorus than they need, mostly from processed foods. Excessive phosphorus can tilt the calcium-phosphorus ratio in blood and potentially strain kidneys. For healthy folks with a balanced diet, tricalcium phosphate brings a practical, steady-release option for topping up both minerals. For people with kidney disease or those told by their doctor to cut phosphate, better to tread carefully.
Education plays a role, not just about which supplement to buy, but learning how diet habits affect mineral needs. Neighborhood clinics and dietitians can help clear up confusion. Calcium and phosphate balance shows just how complicated supplement advice can get—one size doesn’t fit all, and context from labs and diet history matters.
What Works for Whom?
Choosing a supplement resembles building a grocery list: personal preferences, existing health, diet patterns, and budget all weigh in. Tricalcium phosphate finds a niche in liquid supplements, vegan-friendly formulations, and shelf-stable foods. Parents, athletes, older adults, and those with chewing challenges may notice a difference. Doctors and registered dietitians rely on blood work and interviews, not marketing buzz, to make recommendations with real effect.
| Names | |
| Preferred IUPAC name | Tricalcium phosphate |
| Other names |
Tribasic calcium phosphate Bone phosphate of lime TCP Calcium orthophosphate Tricalcic phosphate Phosphoric acid calcium salt (3:2) |
| Pronunciation | /traɪˌkælsiəm ˈfəʊsfeɪt/ |
| Preferred IUPAC name | tricalcium phosphate |
| Other names |
Tricalcium orthophosphate Calcium phosphate tribasic TCP Bone phosphate of lime Phosphoric acid calcium salt Tricalcic phosphate |
| Pronunciation | /traɪˌkæl.si.əm ˈfoʊs.feɪt/ |
| Identifiers | |
| CAS Number | 7758-87-4 |
| Beilstein Reference | 1861547 |
| ChEBI | CHEBI:9766 |
| ChEMBL | CHEMBL1201760 |
| ChemSpider | 14122 |
| DrugBank | DB11348 |
| ECHA InfoCard | 100.029.343 |
| EC Number | 40-216-9 |
| Gmelin Reference | 13768 |
| KEGG | C00878 |
| MeSH | D013612 |
| PubChem CID | 5460356 |
| RTECS number | TC9677000 |
| UNII | V3R023EJ38 |
| UN number | UN3078 |
| CompTox Dashboard (EPA) | DTXSID8036022 |
| CAS Number | 7758-87-4 |
| Beilstein Reference | 816918 |
| ChEBI | CHEBI:43474 |
| ChEMBL | CHEMBL1201758 |
| ChemSpider | 14121 |
| DrugBank | DB11239 |
| ECHA InfoCard | 18e13ae1-7f4a-47ef-a338-28a6fdf07d76 |
| EC Number | 231-840-8 |
| Gmelin Reference | 13354 |
| KEGG | C00738 |
| MeSH | D015809 |
| PubChem CID | 5460341 |
| RTECS number | TC8485000 |
| UNII | 3KX7XUP3BG |
| UN number | UN 3264 |
| CompTox Dashboard (EPA) | DTXSID5020095 |
| Properties | |
| Chemical formula | Ca3(PO4)2 |
| Molar mass | 310.18 g/mol |
| Appearance | White, odorless powder |
| Odor | Odorless |
| Density | 3.14 g/cm³ |
| Solubility in water | Almost insoluble |
| log P | -4.6 |
| Vapor pressure | Negligible |
| Basicity (pKb) | 12.7 |
| Magnetic susceptibility (χ) | −85.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.63 |
| Dipole moment | 0 D |
| Chemical formula | Ca3(PO4)2 |
| Molar mass | 310.18 g/mol |
| Appearance | White, odorless powder |
| Odor | Odorless |
| Density | 3.14 g/cm³ |
| Solubility in water | 0.002 g/100 mL (25 °C) |
| log P | -4.4 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 11.6 |
| Basicity (pKb) | 12.76 |
| Magnetic susceptibility (χ) | −54.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.637 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 116.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −4007 kJ/mol |
| Std molar entropy (S⦵298) | S⦵298 = 206.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -4007 kJ/mol |
| Pharmacology | |
| ATC code | A12AA04 |
| ATC code | A12AA04 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | No signal word |
| Hazard statements | Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Lethal dose or concentration | LD50 oral rat 2,500 mg/kg |
| LD50 (median dose) | > 2,500 mg/kg (Rat, oral) |
| NIOSH | YT2450000 |
| PEL (Permissible) | 10 mg/m3 |
| REL (Recommended) | 70 mg/kg bw |
| Main hazards | May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P304+P340, P312, P305+P351+P338, P337+P313, P403+P233, P501 |
| Lethal dose or concentration | LD50 (oral, rat): > 10,000 mg/kg |
| LD50 (median dose) | > 2,500 mg/kg (oral, rat) |
| NIOSH | GTQ8345000 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 70 mg/kg bw |
| Related compounds | |
| Related compounds |
Calcium phosphate Dicalcium phosphate Monocalcium phosphate Hydroxyapatite Calcium carbonate |
| Related compounds |
Monocalcium phosphate Dicalcium phosphate Hydroxyapatite Calcium oxide Calcium hydroxide Calcium carbonate Calcium sulfate |

