Titanium Dioxide: Unpacking a Ubiquitous White
Historical Development
Titanium dioxide shows up in the story of industrial chemistry over a century ago. Factories began pulling titanium dioxide out of the earth’s crust on a large scale during the early 1900s, driven by a search for safer, brighter pigments than lead white. Experts in Norway and the United States dug into ilmenite and rutile ores, eventually isolating a compound that did not poison painters or flake off years after application. Over time, companies like DuPont and Kronos established huge production plants, and global use exploded. Governments and industrial watchdogs played a part too. The shift away from hazardous pigments pushed manufacturers toward titanium dioxide, cementing it as a paint, paper, and plastic staple. Some of the first serious research into its safety began in the 1960s, reflecting worries about consumer health long before microplastics or nanoparticles crept onto the scene.
Product Overview
Titanium dioxide, often known by names like titania, pigment white 6, or “TiO2” in labs and factories, takes on an almost mythical status in manufacturing circles. It appears as a pure, bright white powder, prized for covering power and non-toxicity compared with old-school pigments. This stuff isn’t rare—it ranks among the most widely used pigments worldwide, especially in paints, inks, food, and sunscreen. Chemists sort it mostly into two forms: rutile and anatase. Rutile sits at the top for UV resistance and weathering; anatase gets used where brightness or reactivity matter more. A simple ingredient list—titanium and oxygen—belies the complicated engineering behind every batch. Some products stay basic; others receive coatings of alumina or silica for extra stability or tailored surface properties. People know it in groceries as food additive E171 and see it in medicines and sweets just as often as in house paint or outdoor siding.
Physical & Chemical Properties
Titanium dioxide comes across as a heavy, flavorless white powder. Its density and brightness set it apart from most other pigments, giving paint layers opacity and hiding marks beneath. Chemically, TiO2 resists attack from acids and bases at everyday temperatures. This toughness lets it go into toothpaste, kitchenware, or architectural coatings without breaking down. Heat leaves its fingerprint too—TiO2 doesn’t melt easily, holding up to extreme processing temperatures in ceramics and plastics, only breaking down through intense reduction. Water barely affects it, and ultraviolet light can break bonds at the surface, sparking interest in self-cleaning coatings and photocatalytic air purifiers. With refractive index numbers above 2.7, few other mineral pigments reflect visible light with comparable efficiency.
Technical Specifications & Labeling
Manufacturers specify grade based on particle size, crystal structure, whiteness, tinting strength, and treatment. Paint-grade titanium dioxide usually shows a particle size between 200 and 350 nanometers; plastic and paper applications might lean larger or smaller, depending on the optical effect wanted. ASTM and ISO groups define standards for purity, brightness, and color, and a technical data sheet from any recognized producer spells out the trace elements by percentage. Labeling must include whether the batch is rutile or anatase, any surface treatment, typical humidity absorption rate, and identification numbers such as CAS 13463-67-7. Food and pharma grades face even tighter documentation, with heavy metals and contaminant levels tracking far below the levels allowed in construction or coating grades. Regulatory demands force raw material suppliers to publish thorough safety and technical dossiers for every formula, not only to satisfy compliance but to reassure downstream users about consistency.
Preparation Method
Two main routes pump out pure titanium dioxide on a commercial scale—the sulfate process and the chloride process. The sulfate method runs ilmenite through sulfuric acid, stripping out iron and yielding hydrated titanyl sulfate; this is then calcined to pure white pigment. The chloride process takes rutile or high-grade ilmenite, dissolves it in chlorine gas at high temperatures, and then removes impurities before the oxide is recovered by oxidation. Each process leaves a different crystal habit and range of trace impurities, so users choose based on what will work best for their product. Modern plants have cut emissions and byproducts significantly, thanks to advances in scrubbing technologies and closed-loop recovery for sulfuric acid or chlorine. Factory-scale labs tune heat profiles, milling speeds, and treatment techniques to pull just the right mixture of particle fineness and surface function, and investments in automation now let companies maintain purity at scales undreamed of in early 20th-century factories.
Chemical Reactions & Modifications
Standard titanium dioxide doesn’t react with much outside of lab glassware unless subjected to extreme temperatures or chemical attack, but coating and doping techniques let chemists tweak its properties for tough applications. Coating particles with alumina or silica blocks photoactivity that can make plastics break down or paints fade. Surface treatment also guards against agglomeration—tiny granules less likely to clump give smoother dispersions in inks and films. Doping with metals like niobium or manganese can shift TiO2 toward semiconductor electronics or photocatalysts, making the pigment active in treating air or water pollution. Some research teams alter crystal size or shape at the nanoscale, chasing improvements in dye-sensitized solar cells or anti-bacterial coatings. These modifications create a more versatile pigment, but also raise deeper regulatory questions about what new forms might do in the environment or inside the human body.
Synonyms & Product Names
Beyond the technical “titanium dioxide,” the world knows this compound by code names like PW6, CI 77891, E171 for food and pharma, and several trade names from major manufacturers—Ti-Pure, R902, Tiona, or Kronos 2190, to name just a few. Labels tell buyers whether the pigment fits food, medicine, cosmetics, paints, or plastics. Product data sheets from the big names lay out detailed stats for color, purity, grind, and ease of dispersion. Different regions and regulatory bodies stick with their own naming conventions, but international trade has forced some convergence, so most pigment buyers recognize 77891 on a drum just as quickly as “titanium white.”
Safety & Operational Standards
Every safety watchdog, from the European Chemicals Agency (ECHA) to the FDA in the U.S., keeps close tabs on how titanium dioxide ends up in finished goods. Workplace rules require good ventilation and, for powder handling, respirators or dust extraction. Chronic inhalation of fine titanium dioxide dust can cause lung changes in animal models, leading regulators to call for strict airborne exposure limits. Manufacturers stay careful with labeling, ensuring that food and cosmetic grades carry markers for batch traceability in the event of recalls or health reviews. Handling instructions and spillage cleanup routines focus on preventing dust clouds, avoiding chemical burns from acidic intermediates, and keeping raw powders dry to prevent caking. Data sheets for industrial grades walk handlers through potential hazards, first aid, accidental release measures, and disposal requirements, based on up-to-date toxicological research and operational findings gathered directly from workplace injury data or field audits.
Application Area
The list of uses for titanium dioxide covers almost every aisle of a hardware or grocery store. Major paint brands rely on its unbeatable opacity and brightness for indoor and outdoor coatings. Plastics manufacturers use it to whiten ABS, PVC, and many polyolefins, while the paper industry loads it into high-end grades for sharp print and smooth feel. Beyond industrial users, personal care and food companies count on TiO2 as a whitening and thickening agent—think toothpaste, sunscreen, pills, confectionary, even salad dressings. Its ability to scatter ultraviolet radiation makes it a key ingredient in mineral-based sunscreens, offering broad-spectrum UV resistance. Recent years have seen titanium dioxide slide into roles in water purification, air filtration, and photocatalytic self-cleaning surfaces, where its semiconductor properties break down organic dirt under UV light. Its chemical stability and visual payoff simply make it hard to sideline in any market where lasting color, brightness, or safety matter.
Research & Development
Research never slows down, even for a mature pigment like titanium dioxide. Academic and industrial labs push for safer nanoparticle variants, aiming for the smallest grain possible while minimizing bioaccumulation or unforeseen health risks. Engineering teams also hunt for greener processing methods—seeking to cut down the tonnage of waste sulfuric acid or chlorine gas vented in traditional prep. Some companies now route effluent through advanced neutralization before reusing it in closed-loop production, reducing the ecological impact when compared to older approaches. Researchers experiment with hybrid materials, combining TiO2 with dopants or with graphene for chemical sensors, transparent coatings, or next-generation solar cells. The balance between performance and regulatory acceptance depends on real-world data, so universities and industry partners invest in long-term toxicity and environmental studies to find the sweet spot in nanoparticle properties.
Toxicity Research
Titanium dioxide won regulatory trust for decades, but new data keeps shifting the conversation about nanomaterials in the human body and the wider environment. Animal studies link long-term respiration of the finest dust to lung overload and tissue change, especially in workers exposed during pigment production. Those results triggered the EU’s decision to classify TiO2 as a suspected carcinogen by inhalation in powder form, while oral exposure through food has not shown clear evidence of harm in humans at typical dietary levels. National authorities keep a close eye on new nano-forms that slip into sunscreens, toothbrushes, and cosmetics, calling for more data about how those particles cross biological membranes. Many food regulators, including the European Food Safety Authority, have pulled E171 from approved additive lists for use in foods, citing uncertainties about bio-distribution and DNA-level effects. That said, consensus in properly handled, non-respirable forms used on surfaces or trapped in plastics still supports titanium dioxide’s safety for workers and end-users, as long as dust generation and inhalation are kept under control.
Future Prospects
The path forward for titanium dioxide mixes ongoing innovation with tough questions about exposure, especially as more products lean on nano-technology. Research teams look at surface modification and smarter particle engineering, both to tweak performance and to shrink health risks. Bigger factories retrofit scrubbers and waste reduction systems not only to cut costs but to keep regulatory watchdogs satisfied. Some up-and-coming startups dig into alternatives or bio-derived pigments but still find it tough to match TiO2 for sheer durability and versatility. As regulations on nanomaterials get stricter and green chemistry incentives grow, future success in the pigment world could turn on mastering both technical know-how and transparency about safety at every link in the supply chain. That means buyers, sellers, and regulators have to keep talking, sharing data, and looking out for surprises as the science changes. The pigment’s next decades will surely look different than its last century, but its legacy and versatility keep it an industrial mainstay for now.
What is titanium dioxide used for?
Brightening the World Around Us
Walk into any supermarket and take a look at toothpaste, sunscreen, or even a box of powdered donuts. The bright white color almost leaps off the shelf, often thanks to titanium dioxide. This mineral, pulled from mined ores like ilmenite or rutile, turns dull products into something eye-catching. For years, food companies have added it to frostings and candy to score that clean, appealing look. Make-up brands blend it into foundations and powders to give them opacity. It hides flaws and gives things a clean finish.
Protection on the Skin
Plenty of us slap on sunscreen before heading outside, hoping to dodge a nasty sunburn. Titanium dioxide steps in here, too. It reflects and scatters UV rays, helping to safeguard skin from damage. Dermatologists often mention it for people with sensitive or reactive skin. It does not penetrate deep, which means less risk of irritation. For families with small children or people fighting off skin conditions, a sunblock with this mineral brings peace of mind and a practical way to enjoy a day at the park.
In the Medicine Cabinet and Kitchen
Pills look appealing and easy to swallow, often because of a snow-white coating made with titanium dioxide. The coating keeps tablets from sticking together and prolongs the shelf life. In the kitchen, it keeps some processed foods looking fresh, but health groups have started asking pointed questions. French regulators recently stopped letting food makers use it, linking it to possible risks. The US Food and Drug Administration says it's safe within limits, though. People who read ingredient lists sometimes swap in products that leave this mineral out, looking for a more natural option.
Modern Construction and Paints
Take a walk through any hardware store—titanium dioxide powers much of the paint aisle. It gives paints that dense coverage that hides old colors and flaws with fewer coats. The paint lasts longer and resists damage from the elements. In plastics and paper, it helps block out light, which slows fading and makes packaging stand out on store shelves. Architects and decorators rely on it for crisp, vivid colors in everything from office buildings to home interiors.
Weighing Risks and Choices
No question, this compound adds value in areas where durability and safety matter. As research unfolds, some scientists point out concerns with inhalation by people who work with fine powders in construction or manufacturing. Responsible companies give workers proper masks and ventilation. People outside of factories bump up against smaller risks, but the science keeps evolving.
Shoppers, cooks, parents, and workers all face choices. Making an informed decision sometimes means skimming a label and reading a bit more or choosing something designed for your comfort and safety. A future with smarter regulations, safe alternatives, and open communication between companies and families could let us all get the benefits, without worry about surprise side-effects.
Better Transparency, Fewer Surprises
Strong research and transparent ingredient lists help everyone understand what's in their products. Reliable oversight gives people the trust they need to pick products confidently. For now, titanium dioxide sticks around in our paint cans, sunscreens, and snack foods. Knowing how it works, and where scientists draw the line, keeps that sense of trust intact.
Is titanium dioxide safe for human consumption?
The Food Ingredient on Our Plates
Titanium dioxide shows up in places you might not expect: chewing gum, frosting, salad dressing, even toothpaste. It makes white foods whiter, brightens colors, and, for decades, few people asked questions about it. As an everyday eater and someone who has spent time reading ingredient lists in grocery store aisles, it’s hard to ignore how often titanium dioxide turns up, especially once you know to look for it.
The Science and Health Concerns
Titanium dioxide is a naturally occurring compound, often used as a pigment and additive under the name E171. Food manufacturers use it because it does not add taste or odor but delivers strong color. The World Health Organization and U.S. Food and Drug Administration have long considered it safe in small amounts used in food. Yet, research rarely sits still, and in recent years, toxicologists started to take a closer look.
Over the past decade, scientists tested how titanium dioxide particles behave in the body. Some find that the tiny particles, especially the smallest ‘nanoparticles,’ can pass from the gut into the bloodstream. Animal studies raise red flags: small doses cause gut inflammation and may disrupt DNA. These studies do not always translate to direct harm in humans, but they shift the conversation, and public trust shakes when animal studies generate more questions than answers.
Europe took the latest science to heart. The European Food Safety Authority reviewed the evidence in 2021, found gaps in the safety data, and recommended a ban on titanium dioxide as a food additive largely because the risk of genotoxicity (damage to DNA) could not be ruled out. France had already placed its own ban a few years earlier, after national agencies pointed to similar concerns. Shopping for groceries in France myself, I noticed food labels started to change, dropping E171 from confections and sauces. Europe’s actions led to waves in global markets, with companies moving toward reformulation across many regions, including the U.S.
Why This Matters for Consumers
As a parent, seeing "titanium dioxide" pop up in snacks aimed at kids gives me pause. Science does not give black-and-white answers most of the time. What’s clear is that people deserve a food system that puts long-term health first. The debate is not just about one chemical; it’s really about the confidence people have in what they eat every day.
Everyone trusts food less when headlines drop words like ‘potential carcinogen’ or ‘genotoxicity’ about familiar ingredients. Once that doubt creeps in, it sticks. The burden falls on those who govern food safety to give clear, independent answers, not just rely on old assumptions.
What Can Be Done?
Lawmakers and regulators can move faster to respond to public health questions by demanding up-to-date safety testing before approving such substances for continued use. Companies need to listen to customers—transparency shapes purchasing decisions, especially when safer alternatives exist. Consumers are getting savvier, learning to scan ingredient lists, push for clearer labeling, and ask for options in their favorite foods.
If changing regulations creates headaches for manufacturers, it also encourages real innovation. There’s plenty of food science talent out there that can find safer ways to make foods look and taste appealing, without relying on questionable additives. While scientists keep working, the simple move for families is to favor less-processed foods. Fewer ingredients often mean fewer unknowns.
The titanium dioxide story serves as a strong reminder that every food safety call comes down to trust, transparency, and acting on the best current evidence. That is what really keeps people protected at the table.
What are the potential health risks of titanium dioxide?
Titanium Dioxide Hides in Plain Sight
Look at a grocery aisle, and there’s a good chance you’ll spot titanium dioxide, lurking quietly in familiar foods and toothpaste. Over the years, companies found it handy for making things look whiter and cleaner — mints, chewing gum, pills, even sunscreen use it for that flawless finish. In modern kitchens and medicine cabinets, this little powder doesn’t exactly announce itself, but it’s more common than folks think.
Food and Cosmetics: Tiny Particle, Big Questions
Scientists started raising eyebrows when research found that titanium dioxide, especially in its nanoparticle form, could end up in the gut, lungs, and elsewhere after repeated contact or ingestion. Once it breaks past the digestive barrier or gets into lungs through powders and sprays, it doesn’t always leave quietly. Some animal studies showed damage to immune cells and the lining of the gut. France banned titanium dioxide as a food additive back in 2020, and Europe’s food safety agency called its safety into question, spurring more countries to take a closer look.
Cancer Risk: Evidence and Uncertainty
The cancer picture remains cloudy. The World Health Organization flagged titanium dioxide as “possibly carcinogenic” if inhaled in dust form, especially for workers in factories making paints or powders. Most regular folks don’t scoop up clouds of the stuff, but any chemical that lingers in the body deserves attention. Chronic inhalation in large quantities has raised red flags, leading to tighter factory controls, but swallowing small amounts from food or toothpaste hasn’t shown such clear effects. That said, questions still hang in the air about long-term low-level exposure.
Children, Chronic Illness, and Cumulative Effects
Kids face more risk because their bodies are growing and they tend to eat or chew on things without thinking twice. Some studies now suggest that people with gut issues like inflammatory bowel disease could react differently to titanium dioxide in food, possibly facing higher harm. The chemical’s effect on gut bacteria and immune function hasn’t been fully nailed down, but some animal tests showed changes in gut health after regular exposure.
Solutions Worth Considering
Many shoppers these days scan ingredient lists, skipping anything unfamiliar. Caring about what ends up in our bodies turns into a practical act. Food companies, faced with consumer pressure, started looking for alternatives — rice flour or calcium carbonate do a similar whitening job, without sparking the same debates. Regulators would do well to set clearer limits and demand longer-term safety data, especially on nanoparticles and long-term cumulative exposure. Doctors and nutritionists tell patients to stick to whole foods rather than heavily processed items, keeping unneeded chemicals out of dinner and snacks.
Trust takes time to build, especially once safety questions catch the news cycle. Honest labeling, strict regulation, and open access to new science are steps that build that trust. As new studies come in, folks deserve straight answers from companies and regulators about what they’re eating, swallowing, or putting on their skin. For now, checking ingredients and asking questions might be dull, but everyone wants to feel secure about what goes in and on their bodies.
Is titanium dioxide a natural or synthetic ingredient?
The Roots of Titanium Dioxide
Titanium dioxide exists in the world’s rocks and sands. It comes from minerals like ilmenite and rutile. Miners scoop these out of the earth, so, yes, this stuff has a base in nature. The process doesn’t stop there, though. To end up with that bright white powder found in food, sunscreen, and paint, manufacturers crush, treat, and purify it. Through heat, chemicals, and filters, they turn raw ore into something almost unrecognizable from its rock origins. That shift isn’t just a small upgrade—it makes a world of difference for both safety and performance.
Synthetic or Natural: What’s in Your Products?
Some shoppers look at ingredients and see titanium dioxide, assuming it’s something cooked up entirely in a lab. In reality, nearly every source starts as a mineral but transforms through a pretty aggressive refining process. Synthetic, in our daily sense, usually means something like aspartame or artificial fragrance—molecules built from scratch. Titanium dioxide straddles a line: it’s pulled from natural sources, then heavily processed. No one walks through a forest and finds it in this sparkling form. That’s the twist—it’s born from nature but shaped by industry.
Real Uses, Real Questions
Most people brush against this ingredient without even realizing it. Chewing gum, toothpaste, vanilla frosting, and that tube of sunscreen in the beach bag—titanium dioxide lends “whiteness” or UV protection. Painters and manufacturers rely on it because it works well and doesn’t cost a fortune. Parents give it a side-eye today because recent studies have raised concerns about eating it in large quantities. In France, regulators banned it in food a few years back, citing uncertainty about safety. Food safety agencies in the US and Canada still call it ok for now.
I have spent time reading ingredient labels, mostly after seeing something alarming in the news or online. Sitting with cookbook authors, health bloggers, or food scientists, I’ve listened to frustration over the confusion. Titanium dioxide isn’t plucked straight from the earth and sprinkled over cupcakes. It gets refined, heated, sorted based on crystal shape, and sometimes ground down to nano size. Those steps lead to cleaner color, but also to debates about possible health impacts.
What Science Tells Us
We don’t have slam-dunk proof about all risks. Animal studies show digestive inflammation at high doses but not everyone agrees on the big picture. One thing is clear: the size and purity of the particles might matter. The European Union wants more research on very tiny, nano-sized titanium dioxide before ruling it safe in food. That’s not fearmongering. Shoppers should know where their food and sunscreen ingredients come from, and what those processes look like.
The Road Ahead: Better Information, Smarter Choices
It helps when scientists, regulators, and companies share details in plain language. Labels often don’t say how refined or what size particles end up in the product. Parents and consumers deserve more transparency. If researchers see a reason for caution in food, there could be steps like clearer warnings or phasing it out of kid-friendly treats. Paint and sunscreen rely on different forms—those products need their own reviews and rules. Bringing people with chemistry and public health know-how into the conversation works better than fear or rumors online.
Talking honestly about titanium dioxide and how it gets to store shelves serves everyone. People can decide what to trust and what to skip, based on facts instead of marketing or viral panic. That’s the real ingredient missing from the debate: real, plain evidence and practical talk that lets people navigate the world, not just blank reassurance or alarm.
In which products can titanium dioxide be found?
Where Titanium Dioxide Pops Up
Walk into almost any store, and you'll probably buy something with titanium dioxide. This white pigment shows up across shelves in ways that don’t stand out until you start paying attention. Look at sunscreen bottles, tubes of toothpaste, boxes of powdered doughnuts, tubes of paint, and even makeup. Its bright, white color has become part of daily life, touching food, personal care, medicine, art supplies, and construction.
Food and Sweets
Those crispy mints in a glass bowl at the bank? Many of them contain titanium dioxide as a whitening agent. This additive, known as E171 in Europe, gives a clean, consistent look to candies, chewing gum, and even some cupcake frostings. Even salad dressings and low-fat dairy can have a dash of it. According to the U.S. Food and Drug Administration, its use in food can’t exceed 1% by weight due to safety limits. European officials have recently backed away from this approval, citing possible health risks after new studies raised concerns about long-term exposure and gut absorption.
Personal Care
Brushing teeth would feel different without titanium dioxide. Toothpaste gets its bright white look from this ingredient. It also enhances opacity in whitening strips. Skincare products—especially sunscreen—depend heavily on it. Titanium dioxide acts as a physical sunblock, reflecting UV rays off the skin. This is a big deal in countries with strong sunlight. Its non-irritating qualities helped sunscreen formulas move away from harsher chemicals. I’ve seen more parents reach for “mineral” sunscreens lately, which usually list titanium dioxide on the label, since these tend to be kinder for sensitive skin.
Medicine and Supplements
Pharmaceutical tablets and capsules often use this pigment in their coatings. Decorative coatings serve more than cosmetic purposes; they protect the pills from light and moisture. For example, the bold colors on allergy medicine or vitamins rely on titanium dioxide for that bright finish. Some manufacturers are looking for alternatives now, especially in Europe, but most over-the-counter and prescription medicines in the US still contain it.
Paints, Plastics, and Building Products
Anyone who has painted a room knows that classic “ceiling white” owes its punch to titanium dioxide. It’s tough, resists fading from sunlight, and spreads color evenly. This makes it valuable in high-traffic public spaces and homes alike. Plastic packaging, from yogurt tubs to appliance casings, often relies on this pigment for brightness and protection. Even paper—think greeting cards and receipts—gets some of its opacity and vivid look from it.
The Current Debate and Safer Futures
Growing evidence suggests we should pay attention to where titanium dioxide lands in our food. Regulators argue about whether eating a little bit every day over decades leads to health issues. France led a ban on food uses in 2020, and the European Union agreed with that move. In contrast, North American regulators still consider it safe, though consumer demand for clear labeling is pressing companies to rethink recipes.
For people with concerns, reading ingredient lists helps. Brands respond to public pressure by offering alternatives: chalky calcium carbonate, rice starch, or even natural colorants fill some of the same spots. The push for cleaner labels shows that consumers can shape what goes into products through steady feedback—and choosing brands that listen.
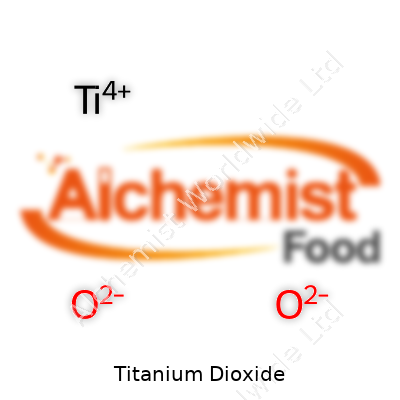
| Names | |
| Preferred IUPAC name | Titanium dioxide |
| Other names |
Titanium(IV) oxide Titania Rutile Anatase Brookite CI 77891 |
| Pronunciation | /taɪˌteɪniəm daɪˈɒksaɪd/ |
| Preferred IUPAC name | Dioxotitanium |
| Other names |
Titania Titanium(IV) oxide Pigment White 6 CI 77891 Titanic anhydride |
| Pronunciation | /taɪˌteɪniəm daɪˈɒksaɪd/ |
| Identifiers | |
| CAS Number | 13463-67-7 |
| Beilstein Reference | 1361814 |
| ChEBI | CHEBI:32234 |
| ChEMBL | CHEMBL1207297 |
| ChemSpider | 10042589 |
| DrugBank | DB11050 |
| ECHA InfoCard | 03-2119604319-46-0000 |
| EC Number | 236-675-5 |
| Gmelin Reference | 83322 |
| KEGG | C06752 |
| MeSH | D013978 |
| PubChem CID | 26042 |
| RTECS number | XR2275000 |
| UNII | 9XV404QY2V |
| UN number | UN3077 |
| CAS Number | 13463-67-7 |
| Beilstein Reference | 3857250 |
| ChEBI | CHEBI:32234 |
| ChEMBL | CHEMBL1201759 |
| ChemSpider | 10042589 |
| DrugBank | DB11050 |
| ECHA InfoCard | 100.031.198 |
| EC Number | 231-791-2 |
| Gmelin Reference | 668 |
| KEGG | C00350 |
| MeSH | D013980 |
| PubChem CID | 26042 |
| RTECS number | XR2275000 |
| UNII | LEY7VHYEAT |
| UN number | UN3077 |
| Properties | |
| Chemical formula | TiO2 |
| Molar mass | 79.866 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | Density: 4.23 g/cm³ |
| Solubility in water | Insoluble |
| log P | -0.42 |
| Vapor pressure | Negligible |
| Magnetic susceptibility (χ) | −0.8×10⁻⁶ |
| Refractive index (nD) | 2.61 |
| Dipole moment | 0 D |
| Chemical formula | TiO2 |
| Molar mass | 79.866 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 4.23 g/cm³ |
| Solubility in water | Insoluble |
| log P | -0.98 |
| Vapor pressure | Negligible |
| Magnetic susceptibility (χ) | +120.0e-6 |
| Refractive index (nD) | 2.61 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 50.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −944 kJ/mol |
| Std molar entropy (S⦵298) | 50.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −944 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D03AX03 |
| ATC code | D03AX03 |
| Hazards | |
| Main hazards | Suspected of causing cancer |
| GHS labelling | Warning, H statement: H319, P statement: P264, P305+P351+P338, P337+P313 |
| Pictograms | GHS02, GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | H372 Causes damage to organs through prolonged or repeated exposure |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P304+P340, P312, P362+P364, P501 |
| NFPA 704 (fire diamond) | 0-0-0 |
| Lethal dose or concentration | LD50 Oral Rat > 5,000 mg/kg |
| LD50 (median dose) | > 10,000 mg/kg (rat, oral) |
| NIOSH | NIOSH: XR2975000 |
| PEL (Permissible) | 15 mg/m3 (total dust); 5 mg/m3 (respirable fraction) |
| REL (Recommended) | 0.01 mg/m³ |
| Main hazards | May cause cancer by inhalation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08,GHS09 |
| Signal word | Warning |
| Hazard statements | Harmful if inhaled. Suspected of causing cancer. |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P362+P364, P403+P233, P405, P501 |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 Oral Rat: > 5,000 mg/kg |
| LD50 (median dose) | > 10,000 mg/kg (rat, oral) |
| NIOSH | NIOSH: XR2975000 |
| PEL (Permissible) | 15 mg/m3 (total dust) |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Zinc oxide Tin(IV) oxide Titanium(III) oxide Titanium oxysulfate Titanium(II) oxide |
| Related compounds |
Titanium tetrachloride Titanium(III) oxide Titanium(II) oxide Titanium oxysulfate Zirconium dioxide |

