Sodium Sulphate: Everyday Chemistry, Lasting Impact
Historical Development
Long before fancy labels and safety data sheets, folks across ancient Egypt and the Roman Empire spotted the convenience of certain mineral salts. Sodium sulphate earned a spot in traditional glassmaking as early as the sixteenth century, particularly after Johann Glauber described “Glauber’s salt” in the 1600s. Chemists and manufacturers alike grew attached to sodium sulphate since it combined availability, relatively easy extraction, and stable handling. The so-called LeBlanc and later Mannheim processes put this compound on the industrial railways—a shift away from simple mining, toward predictable and scalable output for a range of new goods.
Product Overview
Commercial sodium sulphate comes in anhydrous and hydrated forms. Industries count on its bulk for everything from detergents to textiles. The crystal’s reliable behavior during repeated heating and cooling—without decomposing—makes it an easy pick for process control in factories. Plus, easy solubility and non-toxicity have made it a mainstay in schools and labs long before other lab salts came along.
Physical & Chemical Properties
You won’t mistake sodium sulphate for table salt at the kitchen counter, but it looks like a pretty ordinary white powder or crystal. It dissolves in water and keeps its shape at standard pressure and temperature. The decahydrate melts around 32°C, making it fit for certain cooling packs and thermal storage, since it can soak up or give off heat without much fuss. Chemically, sodium sulphate sits pretty stable under neutral and basic conditions and shrugs off most mild acids and bases. What makes it useful—its stability—also keeps handling intuitive, even for large-scale operations.
Technical Specifications & Labeling
Packaged sodium sulphate runs by a whole host of technical grades: technical, purified, and food-safe. Each grade follows strict references—think ISO 9001 or specific ASTM guidelines. Every drum or bag gets a detailed label, not just for purity but also for water of crystallization, bulk density, and impurity limits (like iron and calcium content). These numbers matter for operations like papermaking or detergent compounding, where too much of the wrong trace element can throw machines or product quality for a loop. Most countries want this information upfront, not hidden, for both logistics and safety regulations.
Preparation Method
Producers often tap into two main sources: natural mineral extraction or synthetic production. Large deposits sit in evaporite lakes, so some sodium sulphate gets scooped or washed directly from the earth. Synthetic routes start with sodium chloride and sulfuric acid under heat, cranking out sodium sulphate and hydrochloric acid. In the world of chemical process plants, you’ll see this reaction happening in the Mannheim process—mix, heat, then collect the resulting salt. Yield, efficiency, and environmental load all depend on balancing these inputs and capturing byproducts before they float away or become waste.
Chemical Reactions & Modifications
In my time around chemical labs, the most striking thing about sodium sulphate is how predictably it reacts with strong reducing agents rather than most everyday substances. Exposure to charcoal and heat will convert it to sodium sulfide—a key reaction for dye and leather industries. It also stands in as an inert drying agent in organic synthesis, mopping up water without fuss or side reactions. Attempts to push it further, like with stronger acids or high temperatures, lead to breakdown products such as sulfur oxides, but the usual setting leaves it steady and unchanged.
Synonyms & Product Names
You’ll hear sodium sulphate called by several names, each floating through labs, factories, or the grocery store pharmacy. Names like “Glauber’s salt” point to the decahydrate. European and US regulations stick to either the sulphate or sulfate spellings, depending on tradition. Other times, you’ll find trade names that signal product grade or purity, which matters less to a high school science teacher and far more to a hydrometallurgy engineer planning to scale up a process.
Safety & Operational Standards
Safe handling of sodium sulphate draws more on common sense than emergency preparedness. Skin and eyes don’t love long contact, but you won’t see anything like the chemical burns from stronger acids or alkalis. Spills wash up with water, and the compound needs storage in a dry place to keep it from turning into mush. Factories flag the dust for respiratory irritation. Most technical guidelines—whether OSHA or local equivalents—highlight PPE like gloves and dust masks. Shipping rules label it as non-hazardous under most transit codes, so transport stays simple compared to many other chemical powders.
Application Area
Walk through a detergent factory, and you’ll bump into sodium sulphate as a filler—it helps powder flow, prevents caking, and cuts costs without sabotaging cleaning power. Textile plants use it to push dyes evenly onto fabric, improving color sharpness and saving expensive pigment. Recycled paper lines make heavy use, harnessing its action to separate lignin from cellulose in the pulp bath. In medicine, older generations swallowed solutions of decahydrate as a laxative, though milder options have mostly replaced it now. The chemical finds occasional use in glassmaking, dye manufacture, and as a low-cost heat storage medium in modern energy grids looking for simple, reliable materials.
Research & Development
Teams across universities and industrial R&D labs keep finding unexpected ways to wring extra value from sodium sulphate. Materials scientists eye its phase change properties to craft better insulation and energy-efficient buildings. Environmental chemists study its role in water treatment, seeing if sodium sulphate can help neutralize acid mine drainage or mop up heavy metals from industrial runoff. Digital tech tracks purity and grain size in real time, with sensors guiding feedstock mixes for everything from paper mills to smart detergent plants. That ongoing cycle of improvement often lives in the background, but it transforms what might seem like “just another salt” into a platform for efficiency.
Toxicity Research
Sodium sulphate stands out for its low toxicological profile. Multiple animal studies, along with occupational health surveys, trace few negative outcomes unless someone confuses it for table salt and eats large amounts. Short-term ingestion in lower concentrations passes out of the body fast. Eyes and skin don’t appreciate high exposure, leading to minor irritation. These facts help push the compound into consumer goods and broad industrial use. Environmental scientists have dug into what happens when it flushes into waterways. High enough doses can harm aquatic life, but most municipal systems dilute it well below risky thresholds. Continuous monitoring in plants and wastewater facilities is the norm to keep that impact negligible.
Future Prospects
Where sodium sulphate fits in the next phase of manufacturing feels both familiar and full of new promise. As industries step up calls for sustainability and circular materials, sodium sulphate stands ready to shift from single-use roles to closed-loop systems. Thermal energy storage, eco-friendly building materials, or affordable water cleanup all call for compounds that perform under stress, stay stable, and avoid nasty byproducts. Researchers see chances to recover and reuse sodium sulphate from process wastes, lowering both cost and landfill burdens. Next-gen sensor and automation technologies let sodium sulphate products meet tighter specs, responding to real-time data rather than old-fashioned batch testing. With the pressure cutting across supply chains, energy grids, and clean manufacturing, this old standby never really fades from view.
What are the main uses of Sodium Sulphate?
Taking a Close Look at Laundry Detergent
Most people don’t notice sodium sulphate on a label, but this simple salt shows up heavily in laundry detergent. Washing powder manufacturers trust it as a filler. It isn’t there to clean. Its job is to help powder pour better and keep it dry. Without it, detergent would form clumps or become cake-like. Running out of dry, fluffable detergent in a household or hotel laundry room sparks frustration. With sodium sulphate, detergent holds up in humid conditions. If you have ever poured powder from a box, you owe that smooth flow partly to this substance.
Textiles and Dyeing
Sodium sulphate often makes dyeing fabric easier. During dyeing, proper absorption matters, and this salt helps dyes cling to cotton fibers instead of floating off in wastewater. Smooth, even colors sell more shirts and towels. In my own work with small clothing brands years ago, dyers shared how sodium sulphate controlled results and kept batches consistent. This matters to the big players too, from hospital bedding suppliers to high-end home brands.
Crafting Glass That Lasts
Glassmakers have counted on sodium sulphate since the 1800s. Its main draw is its ability to remove tiny air bubbles as molten glass forms. That means you get windows, bottles, and mirrors crystal clear. Without it, car windshields and storefronts would carry those tell-tale flaws. Many glass factories around the world purchase large quantities for this very job. From my experience watching production tours, the key has always been a clear, uniform product that buyers trust, and this compound quietly plays a part.
Papermaking and Industrial Uses
Pulp and paper plants rely on sodium sulphate, especially for making certain wood pulp grades. This helps produce paper that holds up to heavy ink or moisture. Inkjet copy paper, grocery bags, and specialty cardboard often contain pulp processed this way. Chemical supplies need to be both affordable and reliable at this scale, with millions of pages and packages shipped daily. Companies keep their operations running more smoothly thanks to predictable results from additives like sodium sulphate.
Everyday Connections Beyond Industry
Some folks recognize sodium sulphate because it turns up as a laxative in the pharmacy, though it’s not often someone’s first choice these days. Still, its presence in over-the-counter remedies reflects its versatility and the trust people place in traditional compounds. Small quantities find use in photography as well, where it acts as a stabilizer during processes like developing film—though digital technology has reduced this need.
Looking at Sustainability and Solutions
In dozens of industries, keeping costs low while protecting the planet creates a challenge. Many companies now ask whether they could reuse sodium sulphate recovered from other chemical reactions. Closed-loop manufacturing, where waste gets recycled into new products, stands out as a practical answer. In the glass and detergent fields, efforts at recycling look promising. Others, like the textile world, rethink water use, since large-scale dye processes create salt-laden wastewater. Simple steps, like treating and reusing water on site, help both cut down total pollution and stretch one bag of sodium sulphate much further. As industries face scrutiny over waste, adopting responsible cycling of this compound helps balance practicality with stewardship.
Is Sodium Sulphate safe for human contact?
What Sodium Sulphate Is and Where You Find It
Sodium sulphate crops up in plenty of regular places. It gives a boost to soaps, detergents, and even laundry powder. Manufacturers use this salt to help their powders flow better and rinse away without a hitch. It also sits in some paper mills and in the textile world. So, if you’ve ever reached into a box of washing powder or added a scoop to a soapy sink, you’ve probably met sodium sulphate already.
Skin Exposure and Human Safety
People worry about chemicals in the home. After years of trying out cleaners on grimy kitchen counters and doing loads of hand-washing laundry, I’ve seen how most off-the-shelf detergents feel safe to touch. Sodium sulphate, as a rule, doesn’t cause much fuss. Research shows dry, non-irritated skin stays calm after casual or short encounters with household products containing it. Even the CDC lists it as a “low hazard” for skin and eyes.
There’s a catch with extra sensitive skin. Sodium sulphate may strip away some natural oils if used all day, every day, or if you have an existing rash. Anyone with eczema or cracked hands knows how quick chemicals can sting. If you scratch at your skin or suffer from allergies, even a tiny bit of dryness can make you worry, and sodium sulphate won’t help matters much under those conditions.
Swallowing or Inhaling: What Science Says
Swallowing this salt by accident happens sometimes—think of toddlers around the laundry tub or a carelessly washed cup. At small amounts, studies show little to worry about aside from mild stomach upset. High doses, used in medical settings as laxatives, don’t hang around in the body. Instead, the stuff passes through and leaves quickly.
Breathing in the powder can irritate lungs and throats, especially in a warehouse or during a spill. Dust masks and open windows help a lot in situations with heavy use. Nobody enjoys a coughing fit mid-cleaning, so take a little care when you come across big clouds of sodium sulphate dust in bulk.
Trust in Regulation and Testing
Regulatory bodies like the U.S. Food and Drug Administration call sodium sulphate “generally recognized as safe” for use in foods and pharmaceuticals. Industrial workers get guidelines on safe handling and proper ventilation. Consumer protection groups cross-check scientific research regularly, keeping the public and factory staff in the loop.
It’s true that no chemical earns a perfect safety record. Good habits make the difference between a careless accident and a healthy routine. Handwashing after exposure, reading product instructions, and storing bulk supplies away from children all help keep risks low.
Better Safe Practices and Real-Life Solutions
Sodium sulphate’s record with casual household exposure stands up to scrutiny. The biggest issue most folks face is dry skin after frequent use. Moisturize, keep gloves nearby for big jobs, and avoid accidentally rubbing your eyes after using powders. In workplaces, ventilation, personal protective equipment, and clear labels go a long way.
Learning about what’s inside your soap or cleaner gives you control. If you’re ever unsure, looking up product safety data sheets helps. Personal experience and scientific studies both show that sodium sulphate, used in typical daily routines, doesn’t wreck health. Respecting product labels and choosing the right routines for sensitive skin protect everyone under the same roof.
What is the chemical formula of Sodium Sulphate?
Understanding Sodium Sulphate’s Structure
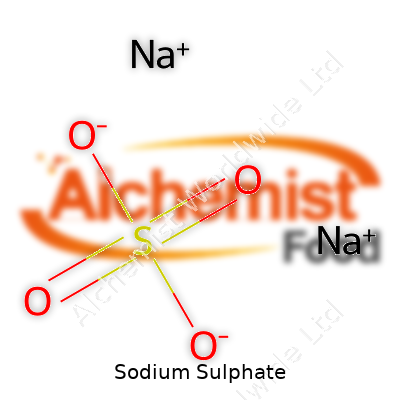
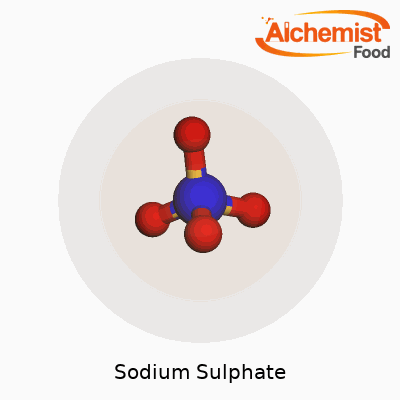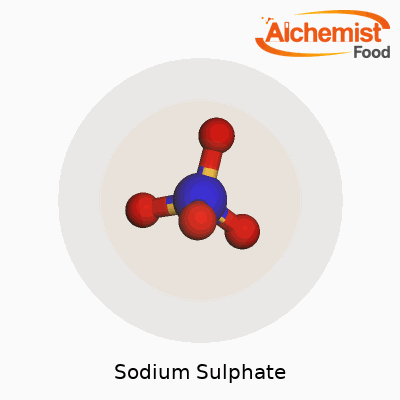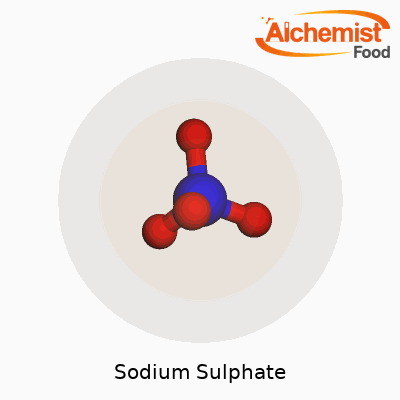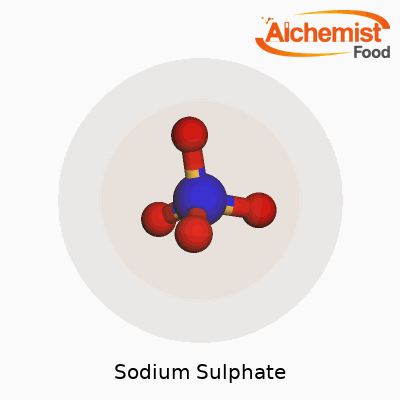
Sodium sulphate shows up across several industries and even at home, but most folks don’t think too much about how it’s built. Chemically, it’s written as Na2SO4. On the surface, it’s just a simple combination of sodium ions and the sulphate ion, but the way these elements link up does more than give it a name. Whenever I see Na2SO4, I’m reminded of just how specific chemistry can be — if you mix two parts sodium with one part sulphate, you land on this reliable, colorless solid that pops up everywhere from powdered laundry detergent to glass manufacturing. That little subscripted formula captures a lot of the world’s everyday activity, even if most people never realize it.
Role in Industry and Life
Few chemicals play as many behind-the-scenes roles in daily life as sodium sulphate. Glass producers use it to get rid of small air bubbles in molten glass. The detergent aisle at the supermarket brims with boxes made bulkier and lighter by its presence. Even in textiles, sodium sulphate gets cloth ready to soak up dyes evenly. The reason all these uses matter comes down to the chemical’s actual formula: two sodium atoms provide the right charge balance to work with one sulphate ion, forming a salt that dissolves and reacts just enough to clean surfaces or clear up glass. I’ve often read how glass factories can’t even operate at scale without this trusty compound managing impurities. The fact that such demands rest on a single formula underlines just how much thought goes into structuring chemical recipes — a point I think folks don’t appreciate until they’ve seen a process break down when an ingredient is missing or off-balance.
Health, Safety, and Environmental Bits
Sodium sulphate is no stranger to the environment. It shows up when lakes dry out, and tons of it get produced each year for industrial supply chains. Unlike some harsher chemicals, sodium sulphate ranks pretty safe; major health agencies haven’t linked it to major toxicity concerns when using it as intended. That’s a comfort, and it means that the chemical’s structure isn’t prone to releasing anything nasty under normal circumstances. I’ve come across plenty of safety datasheets in my career, and the lack of big red warning labels on sodium sulphate stands out. Still, putting anything in excess into water or soil disrupts the natural balance — and with wide industrial use, it pays to keep disposal and runoff in check. Choosing the right disposal practices helps keep excess sodium and sulphates from pooling in waterways, harming aquatic life. Factories investing in closed-loop water systems give a good example of how industry and environment can live side by side without big fallout.
Science in the Details
The chemical formula of sodium sulphate isn’t just trivia for science class. It’s a shorthand for the way chemistry shapes industry, consumer goods, and even the environmental footprint of modern manufacturing. Thinking about Na2SO4 in tangible terms brings out the everyday impact that science has, connecting global supply chains with the chemistry in a box of detergent or a pane of clean, clear glass. That formula stands as proof that the details, right down to the last atom, end up steering how materials work — and how industries keep supply moving safely and efficiently across the world.
How should Sodium Sulphate be stored?
Why Proper Storage Matters
Walk into any chemical storage area and it’s clear that keeping things organized protects both the product and the people handling it. Sodium sulphate often looks harmless – that white, grainy, almost salt-like material – yet even the mildest chemicals can pose problems without smart care. Over the years, I’ve seen plenty of frustration caused by simple mistakes. A little care in storage stops accidents, reduces waste, and means less cleanup in the long run. Nobody enjoys sweeping up a caked-on, waterlogged mess in the warehouse after a moist sack tears open.
Moisture is the Biggest Enemy
Every time sodium sulphate mixes with water, it tends to clump. Humid air alone can slowly turn a stack of perfectly powdery product into damp lumps that clog up machines or refuse to dissolve in solutions. Dry storage – that’s the cornerstone here. Too many folks ignore the humidity until it starts ruining their supplies. In my experience, a room with no leaks, good ventilation, and a dedicated dehumidifier works far better than hoping for the best. Even if the factory sits in a dry climate, a sudden summer storm or a leaky roof can undo everything in a week.
Choose Containers Wisely
Loose sacks – especially thin paper ones – tear easily and sweep up every drop of moisture they can find. Polyethylene-lined bags or well-sealed drums offer more protection. I’ve seen companies rely on old packaging just to save a little, only to watch their investment turn to rock-hard blocks. The chemicals stay safe when you avoid direct contact with concrete floors, stash the product away from outside doors, and stack bags on pallets, never bare on the ground.
Keep It Clean, Keep It Separate
In shared warehouses, accidents happen. Someone thinks it’s fine to jam everything in together, but cross-contamination ruins more batches than spills. Storing sodium sulphate near strong acids or oxidizers opens the door to dangerous reactions if leaks occur. Separation isn’t just a safety habit; it keeps inventory easy to track and reduces headaches at audit time. In my own work, dedicating clear zones and using color-coded signage makes mistakes harder to slip past busy staff.
Fire Risk: Not a Major Threat
Sodium sulphate doesn’t burn or fuel a fire. That tempts some to relax standards, but that attitude misses the point. Fires in warehouses cause chaos – sprinkler systems kick in, dousing everything. Products like sodium sulphate, harmless in dry form, create a sludgy hazard after such events. Plan to keep the chemical away from pipes that might burst, overhead storage, and any area likely to take water in an emergency.
Documentation and Access Control
Good records prove their worth every year. Marking every batch with date codes and source suppliers helps with recalls or tracking quality. Limiting who handles the storage area stops careless stacking and rough handling. It never pays to let just anyone move things around. Training staff – showing them what a sealed bag should look like, and reminding them why moisture control matters – often pays for itself quickly.
Simple Solutions Go a Long Way
The answers tend not to cost much. Use plastic-lined bags, store above ground, limit humidity, and keep the area clean. Add training, strong shelves, clear separation, and honest recordkeeping. Every experience with chemical storage reinforces the same truth: cautious habits beat wishful thinking every time, and nobody gets stuck cleaning up avoidable messes.
What industries commonly use Sodium Sulphate?
Everyday Products Powered by Sodium Sulphate
Talk about household products, and sodium sulphate has a quiet but strong presence. Think laundry detergents: manufacturers toss in a hefty dose as a processing aid and filler, helping powders flow smoothly. If you’ve noticed some laundry powder sweeping up easily and staying fluffy instead of clumping, sodium sulphate does the heavy lifting. Researchers found this compound in more than half the laundry detergents sold worldwide, especially in older or value brands where making bulk batches stretch is a priority.
Beyond detergents, look inside your favorite glassware. Glass producers support melting and refining raw materials with sodium sulphate. This ingredient breaks up imperfections, reducing bubbles and flaws in bottles, windows, and even touchscreen panels. Since large-volume glass factories use every edge they can get, sodium sulphate helps save on energy and raw material costs. Industry statistics point to millions of tons of sodium sulphate landing in glass plants every year.
Pulp, Paper, and Everyday Convenience
Paper manufacturing leans hard on this salt, especially during a stage called the “Kraft process.” In this step, mills use sodium sulphate to produce the chemicals that break wood chips down into pulp. Without it, stronger grades of paper—think grocery bags and shipping boxes—would look much weaker and tear more easily. As someone who grew up in the Midwest, I remember seeing enormous paper mills surrounded by rail cars stacked with white crystals destined for these chemical vats.
Paper and board producers also count on sodium sulphate to balance pH and clean out leftover residue from recycled fibers. Outages due to gunky build-up or off-color paper mean big losses, so they trust these tried-and-true chemical aids to keep lines running smooth.
Textiles and Dyeing: Behind Brilliant Colors
Textile plants love sodium sulphate for its role in dyeing cotton and blends. Every T-shirt, bedsheet, or towel with rich, even color owes a bit to this ingredient. The compound helps carry dyes into fabric, giving textile mills better dye take-up. I've talked with textile technicians who say that, without sodium sulphate, they’d run into blotchy batches, which means more waste and higher costs.
Chemicals, Pharmaceuticals, and Beyond
Chemical manufacturers use sodium sulphate to create other compounds, including sodium silicate, which later appears in adhesives and fireproofing materials. Pharmacies and hospitals touch it in small doses. Laxatives and saline solutions sometimes list sodium sulphate, though large-scale industrial use far outpaces medical needs.
Environmental and Supply Chain Concerns
While sodium sulphate rarely harms the environment directly, huge factories sometimes dump leftover solutions into waterways. Down the line, researchers worry about the buildup of dissolved minerals, which can upset fragile wetland balances. Some governments monitor these byproducts and call for better collection and treatment.
Prices swing depending on mining, recycling, and demand from various sectors. Natural deposits account for most of the supply, but recycled sodium sulphate from chemical processes helps lower emissions and cut costs. Forward-thinking companies focus on reclaiming more from industrial byproducts, pushing to create a tighter, cleaner loop.
Innovation on the Horizon
Cleaner technology brings new possibilities. Detergent makers push toward low-phosphate or phosphate-free formulas, raising questions about the future demand for sodium sulphate as a supporting ingredient. On the glass and paper front, more efficient recycling methods could shift volumes downward. Smart factories adopt closed-loop chemical recovery, reducing raw sodium sulphate needs and making production leaner and less polluting.
In the big picture, sodium sulphate stays essential for now. Factories can’t just flip a switch and walk away from what works. Yet, as technology and regulation evolve, businesses may shrink their appetite for this once-quiet workhorse. The story of sodium sulphate reflects both the complexity of industry and the opportunities for creative solutions.
| Names | |
| Preferred IUPAC name | Sodium sulfate |
| Other names |
Disodium sulfate Soda sulfate Sulfuric acid disodium salt Glauber’s salt Thenardite Mirabilite |
| Pronunciation | /ˈsəʊ.di.əm ˈsʌl.feɪt/ |
| Preferred IUPAC name | sodium sulfate |
| Other names |
Disodium sulfate Salt cake Glauber’s salt Sulfuric acid disodium salt |
| Pronunciation | /ˌsəʊdiəm ˈsʌlfeɪt/ |
| Identifiers | |
| CAS Number | 7757-82-6 |
| Beilstein Reference | 3929239 |
| ChEBI | CHEBI:32141 |
| ChEMBL | CHEMBL1355 |
| ChemSpider | 21514 |
| DrugBank | DB09462 |
| ECHA InfoCard | 100.029.176 |
| EC Number | 231-820-9 |
| Gmelin Reference | 11400 |
| KEGG | C01738 |
| MeSH | D012981 |
| PubChem CID | 25100 |
| RTECS number | WA1900000 |
| UNII | 0YPR857T8G |
| UN number | UN3077 |
| CAS Number | 7757-82-6 |
| Beilstein Reference | 3589909 |
| ChEBI | CHEBI:32139 |
| ChEMBL | CHEMBL1355 |
| ChemSpider | 27383 |
| DrugBank | DB09462 |
| ECHA InfoCard | 100.028.864 |
| EC Number | 231-820-9 |
| Gmelin Reference | Gmelin Reference: **1104** |
| KEGG | C01438 |
| MeSH | D013206 |
| PubChem CID | 24846 |
| RTECS number | WE1650000 |
| UNII | XF417D3PSL |
| UN number | UN3077 |
| Properties | |
| Chemical formula | Na2SO4 |
| Molar mass | 142.04 g/mol |
| Appearance | White, crystalline powder or granules |
| Odor | Odorless |
| Density | 2.66 g/cm³ |
| Solubility in water | Easily soluble in water |
| log P | -3.7 |
| Vapor pressure | Negligible |
| Magnetic susceptibility (χ) | −20.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.468 |
| Dipole moment | 0 D |
| Chemical formula | Na2SO4 |
| Molar mass | 142.04 g/mol |
| Appearance | White, crystalline powder |
| Odor | Odorless |
| Density | 2.67 g/cm³ |
| Solubility in water | Freely soluble in water |
| log P | -3.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | ~14 |
| Basicity (pKb) | NaN |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.468 |
| Dipole moment | 0 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 149.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1387.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1387.1 kJ/mol |
| Std molar entropy (S⦵298) | 149.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1387 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1387.1 kJ/mol |
| Pharmacology | |
| ATC code | A06AD18 |
| ATC code | A06AD18 |
| Hazards | |
| Main hazards | May cause respiratory irritation |
| GHS labelling | GHS07, Warning |
| Pictograms | GHS07,GHS09 |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 Oral Rat 5891 mg/kg |
| LD50 (median dose) | 'LD50 (median dose) Oral (rat): 5989 mg/kg' |
| NIOSH | WF8575000 |
| PEL (Permissible) | PEL: 10 mg/m³ |
| REL (Recommended) | 2,000 mg/kg |
| IDLH (Immediate danger) | Non-listed |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | Not a hazardous substance or mixture according to the Globally Harmonized System (GHS) |
| Pictograms | GHS07, GHS09 |
| Hazard statements | No hazard statements. |
| Precautionary statements | Wash hands thoroughly after handling. Avoid release to the environment. |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Explosive limits | Not explosive. |
| Lethal dose or concentration | LD50 (oral, rat): 5989 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 5989 mg/kg |
| PEL (Permissible) | Not Established |
| REL (Recommended) | 400 mg/kg |
| IDLH (Immediate danger) | Not Listed |
| Related compounds | |
| Related compounds |
Sodium sulfite Sodium bisulfate Sodium thiosulfate Potassium sulfate Magnesium sulfate |
| Related compounds |
Sodium sulfite Sodium thiosulfate Potassium sulfate Magnesium sulfate Calcium sulfate |

