Sodium Sulfite: The Backbone Chemical Most Folks Overlook
Historical Development
Sodium sulfite has a backstory that stretches far beyond the everyday lab bench. In the late 19th century, paper mills started using it to break down wood pulp for paper manufacturing. The demand for smooth, bright paper pushed workers and scientists to refine sulfite-based pulping, laying down a foundation for industrial chemistry. Over time, this humble salt picked up new roles across water treatment, photography, and food preservation. The chemical industry’s growth after the world wars leaned hard on versatile compounds like sodium sulfite, with manufacturers seeking reliable reagents for newer processes and tighter product standards. Generations of chemists relied on simple, reproducible reactions, and sodium sulfite often served as a go-to for reducing or dechlorinating jobs. Nothing fancy, but plenty effective in meeting industrial and municipal needs.
Product Overview
Most sodium sulfite comes as a white, crystalline powder, though liquid concentrations have their niche. This compound has earned its spot in warehouses, science classrooms, and purification plants for its straightforward use and stable shelf life. For folks checking ingredient lists, it answers to several names—sodium sulphite, sulfurous acid sodium salt, or sometimes just E221 in the context of food regulations. Producers have developed grades with varying purity, but few stray from its core chemical profile. Sometimes the grade tells the story: tech grade for factories, food grade for canneries and bottlers, and photographic grade for the shrinking number of darkrooms.
Physical & Chemical Properties
Sodium sulfite has a no-nonsense makeup. This salt dissolves freely in water, forming clear, slightly alkaline solutions. It doesn't survive long in open air; exposure to oxygen gradually turns it into sodium sulfate. Its molecular formula, Na2SO3, sums up its core structure—two sodium ions balancing the charge of one sulfite group. In my time running simple water tests, I’ve watched its reducing power in action, scrubbing chlorine from city water or softening tough stains from laundry. Its limited reactivity with strong acids brings out the rotten-egg smell of sulfur dioxide, a reminder of its roots in old-school chemistry sets.
Technical Specifications & Labeling
Producers always slap a label on sodium sulfite drums listing purity (often above 96% for commercial specs), typical moisture levels, and content of trace contaminants like iron or heavy metals. Bulk bins line up with big-letter warnings about “oxidizer” properties, and safety data sheets call out proper handling, storage, and what to do in case of accidental inhalation or spills. Food-grade packaging adds details about approved uses and limits set by regulatory agencies such as the FDA or EU authorities—best if kept sealed, cool, and away from acids to keep undesired reactions at bay.
Preparation Method
Making sodium sulfite at scale doesn't require a labyrinth of fancy reactors. It starts with bubbling sulfur dioxide gas through cold sodium hydroxide or sodium carbonate solution. That direct approach keeps production cheap and scalable, with minimal leftover by-products. Operators need to monitor pH closely, because drifting too far acidic generates sodium bisulfite, while working at elevated temperatures sometimes causes unwanted decomposition. Factories monitor effluent for sulfur compounds and recycle as much chemical as possible, since discharge limits keep getting stricter. In small labs, I’ve mixed my own batches just by adding sodium carbonate to a vessel and trickling in SO2; the basics haven’t changed in over a hundred years.
Chemical Reactions & Modifications
Sodium sulfite stands out for its reducing punch. It pulls dissolved oxygen out of water, staving off corrosion in boilers and industrial pipes. Exposed to chlorine, it neutralizes disinfectants fast, a must-have in treating municipal water before it reaches taps. In biochemical labs, it serves as a gentle reducer for dyes and enzymes, tweaking delicate reactions without wrecking organic molecules. Mix it with acids and up comes sulfur dioxide—a reaction prized by winemakers for sterilizing barrels. Some manufacturers tweak the salt to produce sodium metabisulfite, dialing up the preservative effect or accommodating tighter storage requirements.
Synonyms & Product Names
Check any warehouse shelf and sodium sulfite goes by many aliases. Chemical catalogs list it as disodium sulfite, sulfurous acid disodium salt, or sometimes soda sulfite. Bulk shipments may arrive under trade names specific to each region, depending on formulation or additive content. These alternative names reflect both tradition and marketing spin, but beneath the label you’ll find the same core compound doing honest work for a variety of trades.
Safety & Operational Standards
Anyone handling sodium sulfite should trust the advice on those thick safety binders. Skin and eyes need protection—this salt can irritate when mishandled. Breathing in the fine powder sometimes triggers respiratory issues, especially for folks with underlying conditions or allergies. Operators keep it far from strong acids, since that's the fast track to stifling sulfur dioxide fumes. Strict storage guidelines call for air-tight containers, dry rooms, and clear hazard signage. Regulatory bodies, including OSHA and the European Chemicals Agency, call for comprehensive hazard assessments, emphasizing personnel training and emergency plans. In my own experience, common-sense steps—gloves, goggles, and fume hoods—keep most risks at bay.
Application Area
Few chemicals enjoy jobs as varied as sodium sulfite. Paper mills rely on it during pulping and bleaching, loosening lignin bonds to produce clean white sheets. City waterworks use it as a dechlorinating agent, stripping residual chlorine before water meets people or aquatic environments. Food processing plants trust its preservative punch to slow spoilage in dried fruits and bottled juices, with usage checked by global food safety regulators. Back in the day, film developers and photographers relied on sodium sulfite to extend developing bath life, preserving photographic masterpieces in the process. Dry cleaners and textile workers appreciate its stain-removing skills, and industrial boilers need it to mop up oxygen and cut rust. No matter what role it takes on, the chemical’s reliability explains its steady presence.
Research & Development
Modern laboratories continue to poke and prod sodium sulfite, smartening up both its usefulness and safety profile. Investigators study degradation rates, interactions with new water contaminants, and hybridization with surfactants or catalysts. Recent research turns an eye toward green chemistry, hunting for ways to reclaim or upcycle spent sulfite solutions rather than dumping them. Some teams use advanced sensors to monitor sodium sulfite’s performance in real-time, creating feedback loops that trim waste and improve efficiency. Newer processing lines look for methods to cut sulfur dioxide release, blending tighter emission controls with old practices. Published research from journals like Industrial & Engineering Chemistry demonstrates the ongoing push to tune every step, reduce worker exposure, and stretch each dollar spent on reagents.
Toxicity Research
Decades of toxicology work show most folks tolerate sodium sulfite at low exposure—but questions linger for those with asthma, sulfite sensitivity, or underlying respiratory conditions. Animal studies nail down target organs, warning against repeated inhalation or high-dose ingestion. In the food world, regulators have kept limits tight, thanks to rare but severe allergic reactions tied to sulfite preservatives. Inhaled dust triggers mild to acute symptoms based on dosage and duration; personal protective equipment and local exhaust fans remain the first line of defense. Environmental research digs into how spent sulfite influences aquatic life, as concentrations build up in some waste streams. Ongoing work tracks biodegradation rates and safe disposal methods, aiming to cut down on long-term ecosystem impact.
Future Prospects
Industries that rely on water treatment, paper pulping, and food preservation won’t drop sodium sulfite anytime soon. The challenge comes from balancing traditional use with stricter safety and sustainability demands. Environmental regulators ask chemical companies to lower emissions and find greener disposal routes. Researchers explore less toxic alternatives for specific preservation jobs, testing organic acids or enzymes. Some speculate about adapting sulfite cycles for circular economy projects, where every by-product finds another purpose. Digital monitoring systems make tracking and dosing smarter, and recycling initiatives keep spent solutions in the production loop. With regulations evolving and supply chains tightening, those using sodium sulfite must keep pace with new standards, smart management, and cleaner processes. Real change takes a mix of established know-how and willingness to adapt, moving this steadfast compound from industrial stalwart into the future's leaner and safer workflows.
What is sodium sulfite used for?
Working Behind the Scenes in Food and Photography
Growing up in a family with a strong food industry background, I remember watching relatives prepare cured meats and dried fruits for market. One powder kept recurring: sodium sulfite. It seemed to go unnoticed, but it allowed foods to look bright and appetizing long before arriving on store shelves. This simple compound helps prevent discoloration and spoilage in dried fruit, shellfish, and even certain wines. By holding back unwanted browning, sodium sulfite makes it possible for consumers to enjoy food that looks good and stays fresher a bit longer.
The food world isn’t its only home. As a camera enthusiast, I spent evenings in darkrooms where sodium sulfite keeps developer solutions stable. Photographic images wouldn’t hold up nearly as well without it. Before digital photos, everyone who cherished print images benefited from this quiet chemical helper.
Sodium Sulfite in Industrial Solutions
Every so often, I visit a water treatment plant as part of my freelance work. The staff often mention sodium sulfite’s knack for removing leftover chlorine from municipal drinking water. Chlorine does its job fighting contaminants, but nobody wants a chemical aftertaste. Sodium sulfite neutralizes the chlorine, allowing tap water to taste clean and safe.
If you’ve ever worked with textiles or paper, you’ve encountered sodium sulfite’s influence as well. In pulp and paper mills, it steps in to break down lignin, easing the process of turning wood into soft, clean pulp. The result: smoother, brighter paper for writing or wrapping. Textile workers use it as a bleaching agent, but also to maintain fibers’ strength and appearance. These daily conveniences—safe tap water, crisp paper, soft clothes—often trace back to this simple additive.
Safety and Health: Keeping Use in Check
For all its benefits, sodium sulfite is not risk-free. Sulfite-sensitive folks, especially asthmatics, can react badly to even trace amounts. Regulators in many countries require food products to disclose this additive if amounts surpass a defined limit. The Food and Drug Administration keeps a close eye on sulfite use, particularly in foods frequent on kids’ menus.
The real challenge is balance. Removing it from food preservation altogether might shorten shelf life and make mold and bacteria tougher to control naturally. The push for fewer additives, though, encourages both the food industry and chemical makers to research safer or more natural alternatives. Some companies now explore ascorbic acid (vitamin C) or naturally derived antioxidants. Promoting these new methods can limit sulfite exposure and widen food choices for those with sensitivities.
Transparency and Consumer Choice
The best move for now: keep labeling transparent, enforce safety limits, and push for cleaner technologies wherever practical. Knowledge empowers families to choose suitable foods, and it motivates manufacturers to improve. Sodium sulfite carries a big load in several industries, but informed consumers and responsible producers together have the power to protect public health while still using chemistry to keep life running a bit more smoothly.
Is sodium sulfite safe for consumption?
Spotting Sodium Sulfite on Labels
Sodium sulfite appears on a surprising number of ingredient lists—canned mushrooms, dried fruit, some seafood, even certain wines and beers. Anyone who scans labels may notice it’s one of those additives with a reputation for being both useful and controversial. This compound helps preserve color and keeps food from spoiling too quickly. For food manufacturers, this means less waste and fresher-looking products on store shelves.
Why Food Makers Use It
I’ve come to appreciate why food processors lean on sodium sulfite. Nobody likes opening a can of fruit and seeing it turn brown before they’ve even tasted it. Food waste also drives up grocery costs. In my own kitchen, a little browning on an apple might not matter, but at the factory scale, oxidation ruins the batch. Sulfites not only stop this but also inhibit bacteria, mold, and yeast. The U.S. Food and Drug Administration (FDA) gives sodium sulfite a thumbs-up as a food additive, under certain restrictions. This isn’t a free-for-all; there are strict limits to how much can end up in what we eat.
Safety for Most People—But Some Need Caution
Most healthy adults eat foods with sulfites and never notice. Reports suggest fewer than one in a hundred experience problems. For folks with asthma, or anyone who’s sulfite-sensitive, reactions can range from mild rashes to breathing trouble. On a personal note, a close friend with asthma had to skip out on dried apricots at a family get-together. She realized only later that the culprit was those invisible preservatives. The FDA requires packaged foods with more than 10 parts per million of sulfites to list them clearly—helping people who are sensitive steer clear.
Health Concerns and Science
People sometimes worry sodium sulfite might sneak up and harm them over the long run. The data doesn’t back up these fears for most of the population. Extensive studies, considered by groups like the World Health Organization and the FDA, find no cancer risk or organ damage from approved levels. Most negative reactions happen in a small, susceptible group.
Still, we don’t shrug away concerns. These additives have no place in foods for babies. The FDA banned sulfites in most fresh produce after people reported serious allergic reactions. Out of caution, schools and hospitals often use additive-free food for their most vulnerable people.
What Can Shoppers Do?
Awareness goes a long way. Reading labels helps anyone avoid unwanted ingredients, whether due to allergies or just personal preference. Fresh, unprocessed foods won’t have these additives. Cooking from scratch puts control in your own hands. For those who can’t avoid packaged snacks or ready-to-eat meals, looking for products labeled “sulfite-free” or “no added preservatives” works as another safeguard. The food world is vast, and healthy options come in every aisle.
The Takeaway
Sodium sulfite keeps food fresh and safe for most. Those living with asthma or known sensitivity have extra cause to check labels and talk with healthcare providers if unsure. Manufacturers and regulators have a shared duty to inform consumers, and so do writers and shoppers at the checkout.
What are the storage requirements for sodium sulfite?
Sodium Sulfite: Common, Yet Demanding Safe Storage
Sodium sulfite helps preserve food, treat water, and acts as a bleaching agent in paper production. Many labs and processing facilities use it often, trusting its straightforward chemistry. Because of how often it pops up in industry, anyone handling it should think seriously about how to store it safely. Skipped steps have a habit of catching up—especially with tricky chemicals.
Moisture and Air: The Two Main Threats
One of the most important lessons I learned working in an industrial warehouse is that sodium sulfite loves to soak up water from air. Humidity turns it into mush or even a liquid mess. If dampness reaches the bags or barrels, clumping and slow decomposition follow. So, somebody always checks that storage areas have low humidity. Sometimes, a decent dehumidifier does the trick. It makes a difference, too, if you keep this chemical away from big temperature swings. High heat speeds up its breakdown, sometimes with a rotten egg smell—nobody wants that drifting into other rooms.
Oxygen also poses a problem. Exposed sodium sulfite oxidizes over time, forming sodium sulfate. The result? The chemical loses effectiveness. Quality slips and batches can go to waste. Here’s where tightly sealed containers make a real difference—steel drums with screw lids, lined fiber drums, or even heavy-duty high-density plastic. Out in the field, plenty of folks just keep a careful eye out for leaks or ripped packaging. It saves a lot of hassle later.
Staying Safe and Compliant
Not all sodium sulfite comes pure—sometimes you deal with blends or contaminated residues. Labels—clear, legible, without smears—mean everyone knows what’s inside the drum. In my early days, I saw what happens when someone stores similar-looking powders in unmarked containers—bad news, all around. Regulatory inspectors often want to see careful stacking on well-marked shelves, never at risk of collapsing or falling onto workers. OSHA puts out broad chemical storage standards, and reputations rest on following those rules. That’s not empty bureaucracy, either. Fines appear for storage mistakes, but worse, real people can get hurt if standards slip.
Fire Hazards: Rare but Real
Sodium sulfite doesn’t catch fire easily, but contact with strong acids gives off toxic sulfur dioxide gas. In crowded storage rooms, leaks from elsewhere can trigger chemical reactions right when folks aren’t watching. So, acids sit far away, often in their own locked cabinets. I’ve seen managers act fast on this—no exceptions, no last-minute shortcuts. Plus, secondary containment trays help catch anything that spills, and cleanup supplies stay close at hand.
Steps for Responsible Storage
People working with sodium sulfite learn quickly the value of keeping records. Tracking lot numbers, arrival dates, and inspections lets staff notice if a drum looks off. Old stock rotates out before new stock moves in. Every shipment gets checked—hard clumps signal that water seeped in somewhere. Opened packages get resealed right away. In smaller labs, folks just double-bag leftovers and label dates by hand.
Good storage for sodium sulfite isn’t just about keeping up appearances. It’s protection for workers and a safeguard for the company’s bottom line. Safe, dry, labeled, and separated from acids—these habits save property and, more importantly, lives. They keep operations smooth, help the product do its job, and let everyone go home at the end of the day in the same shape they arrived.
What are the hazards or side effects of sodium sulfite?
Understanding Sodium Sulfite’s Place in Daily Life
Sodium sulfite pops up in a lot more places than people think. Take a look at the ingredient lists on some dried fruit, wine bottles, or photography supplies, and you’ll see it there. In the food industry, it works as a preservative, keeping colors bright and shelf lives long. The laundry world uses it for bleaching. While this compound does a lot of jobs, safety questions keep coming up, especially as people learn more about chemical sensitivities and allergies.
Breathing Trouble and Asthma
Certain people discover sensitivities fast—especially folks with asthma. Inhaling sodium sulfite dust or fumes, even in smaller amounts, can irritate the nose and throat, making it tough to catch a full breath. Asthma attacks sometimes show up in workplaces where sodium sulfite gets used, and public health agencies like OSHA recognize the risk. Young children and older adults tend to feel these effects more. Sneezing, runny noses, or even a burning sensation in the throat can point toward exposure.
Allergic Reactions and Food Sensitivities
Not everyone knows about sulfite allergies until after a reaction shows up. Eating foods with sodium sulfite can lead to hives, itching, or even swelling of the throat for sensitive individuals. The U.S. Food and Drug Administration (FDA) estimates that as many as one in a hundred people react to sulfites, and the risk climbs for asthmatics. Back in the 1980s, after several bad incidents, rules started requiring clear labels so shoppers could spot sulfites in their food.
Eye and Skin Irritations
Anyone who’s ever mixed the wrong chemical cleaner and gotten a whiff or splash knows the discomfort. Sodium sulfite in the eyes causes redness, pain, and sometimes temporary blurred vision. Skin contact can lead to rashes or red blotches, especially after longer handling without gloves. Routine use around the home doesn’t usually create big problems, but those who work in factories or photography labs see much higher levels—and more frequent issues.
Potential Changes in Blood Chemistry
The body deals with sulfites by using specific enzymes. Some folks don’t produce enough of these enzymes, often due to a rare deficiency, so sulfites hang around in the blood longer and can cause headaches, nausea, or—in severe cases—shock. Research from the National Institutes of Health shows these cases remain rare, but awareness matters, especially for doctors treating unexplained allergic reactions.
Safe Handling and Reducing the Risks
Wearing gloves, eye protection, and a proper mask in work environments helps cut down on exposure. At home, reading labels closely helps people avoid accidental ingestion. Many countries set daily intake limits, generally around 0.7 milligrams per kilogram of body weight, to protect public health. Food companies follow these rules to keep risk low, but homemade or imported goods might slip through, so staying informed pays off.
Moving Toward Safer Use
Big organizations like the FDA and OSHA keep updating guidelines as new research surfaces. For those who already live with asthma or allergies, carrying emergency medication and choosing whole foods can sidestep most problems. Companies are also experimenting with alternative preservatives and warning labels have improved over the past decade. Looking for “sulfite-free” on packages or asking before buying bulk foods brings an extra layer of safety.
What is the chemical formula of sodium sulfite?
Sodium Sulfite: Formula and Naming
To many, sodium sulfite might sound like another obscure chemical in a long list. In fact, it’s one of those compounds that shows up in more places than we realize. The chemical formula is Na2SO3. Each unit of sodium sulfite contains two atoms of sodium, one atom of sulfur, and three atoms of oxygen. The “Na” stands for sodium, a reactive silver-white metal, while “SO3” refers to the sulfite ion, made up of sulfur surrounded by three oxygen atoms. This combination gives sodium sulfite its recognizable chemical identity.
Real World Roles and Impact
Sodium sulfite finds its way into everyday life through food, water, and even the paper we use. Over the years, I’ve talked with folks working in food production and water treatment. They often mention sodium sulfite’s power as an antioxidant. That means it helps stop foods from turning brown and water from carrying an odd taste or developing unwanted microbes. In the paper industry, it acts as a pulping agent, separating cellulose fibers for smoother, whiter sheets. In my own kitchen, I noticed dried fruit often lists “sulfites” on the package—these compounds help keep fruit from spoiling and improve shelf life.
Scientific literature backs up its effectiveness. Studies from the Food and Drug Administration note that sodium sulfite can preserve color and freshness in processed foods, reducing waste and making products more appealing. On a municipal scale, treatment facilities add sodium sulfite to help clean water, clearing up leftover bleach after disinfection steps. As reported by the Environmental Protection Agency, correct usage of sodium sulfite reduces risks involved with chlorine in drinking water and cuts down on environmental impact.
Health Considerations and Safe Use
While sodium sulfite does plenty of good, awareness about sensitivity matters. I’ve heard from friends with asthma or food allergies who need to check labels closely. For many people, sulfites don’t cause trouble, but the FDA estimates that about 1 in 100 people have some level of sensitivity, especially those with asthma. Even so, the benefits for food preservation and industrial processing serve millions each day—careful labeling and regulation make a real difference. My advice: if you have a concern or you’re shopping for someone who does, give that ingredient label a quick check.
Responsible Manufacturing and Use
Modern producers focus on balancing usefulness and safety. Some companies invest in refining manufacturing steps to keep purity high and impurities low. Regulations across Europe, Asia, and the US require documentation on how much sodium sulfite is added to food and water. This transparency helps keep consumers informed and safe. I’ve seen manufacturers adjusting their processes, installing better scrubbers and waste treatment systems, to protect workers and the environment. Regular checks and clear public information build trust in a substance that sees such wide use.
Ideas for Smarter Solutions
As science and technology advance, researchers investigate substitutes where sensitivity causes concern. Food scientists look into new preservatives with a lower risk of triggering allergies, and engineers design alternatives for industrial cleaning that break down even faster than sodium sulfite. Yet for now, its low cost and strong performance make it tough to replace entirely. Education, clear labeling, and strong quality standards go a long way toward addressing concerns. From what I’ve seen, continued collaboration among scientists, regulators, and manufacturers helps everyone reap the benefits while reducing risk.
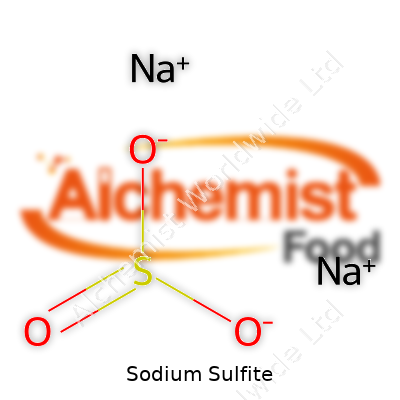
| Names | |
| Preferred IUPAC name | Sodium sulfite |
| Other names |
Disodium sulfite Sodium sulphite Sulfurous acid, disodium salt Sodium sulfurous Sulfite of soda |
| Pronunciation | /ˈsəʊdiəm ˈsʌlfaɪt/ |
| Preferred IUPAC name | sodium sulfite |
| Other names |
Disodium sulfite Sulfurous acid disodium salt Sodium sulphite |
| Pronunciation | /ˈsəʊdiəm ˈsʌlfaɪt/ |
| Identifiers | |
| CAS Number | 7757-83-7 |
| Beilstein Reference | 1713887 |
| ChEBI | CHEBI:28673 |
| ChEMBL | CHEMBL1357 |
| ChemSpider | 21514 |
| DrugBank | DB09466 |
| ECHA InfoCard | 100.028.870 |
| EC Number | 231-821-4 |
| Gmelin Reference | 811 |
| KEGG | C00245 |
| MeSH | D012985 |
| PubChem CID | 24437 |
| RTECS number | WE2150000 |
| UNII | AWK298130E |
| UN number | UN1384 |
| CAS Number | 7757-83-7 |
| Beilstein Reference | 1908226 |
| ChEBI | CHEBI:28673 |
| ChEMBL | CHEMBL1353 |
| ChemSpider | 23010 |
| DrugBank | DB09399 |
| ECHA InfoCard | 173839 |
| EC Number | 231-821-4 |
| Gmelin Reference | 11393 |
| KEGG | C00919 |
| MeSH | D013007 |
| PubChem CID | 24437 |
| RTECS number | WR8250000 |
| UNII | 790I21D1ZE |
| UN number | UN1384 |
| Properties | |
| Chemical formula | Na2SO3 |
| Molar mass | 126.04 g/mol |
| Appearance | White crystalline powder or granules |
| Odor | Odorless |
| Density | 2.63 g/cm³ |
| Solubility in water | 27.0 g/100 mL (20 °C) |
| log P | -4.11 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7–9 |
| Basicity (pKb) | 12.9 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.450 |
| Viscosity | 50 – 70 cP |
| Dipole moment | 0.00 D |
| Chemical formula | Na2SO3 |
| Molar mass | 126.04 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.63 g/cm³ |
| Solubility in water | 27.0 g/100 mL (20 °C) |
| log P | -4.0 |
| Vapor pressure | <0.1 mm Hg (20°C) |
| Acidity (pKa) | pKa ≈ 7-9 |
| Basicity (pKb) | 12.63 |
| Magnetic susceptibility (χ) | −23.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.520 |
| Viscosity | 30 cP (20°C) |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 126.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1387 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −1387 kJ·mol⁻¹ |
| Std molar entropy (S⦵298) | 126.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1387 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1387 kJ/mol |
| Pharmacology | |
| ATC code | A12CE02 |
| ATC code | A12CE02 |
| Hazards | |
| Main hazards | May cause respiratory irritation, causes eye irritation, may cause allergic skin reaction. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "May cause respiratory irritation. Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | > 535°C (995°F) |
| Lethal dose or concentration | LD50 (oral, rat): 3,560 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 248 mg/kg |
| NIOSH | The NIOSH of Sodium Sulfite is `"WE1900000"`. |
| PEL (Permissible) | PEL = 15 mg/m3 (total dust) |
| REL (Recommended) | 340 mg |
| Main hazards | Harmful if swallowed; may cause irritation to skin, eyes, and respiratory tract. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: "Avoid breathing dust. Wash thoroughly after handling. Use only outdoors or in a well-ventilated area. IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER or doctor if you feel unwell. |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 3560 mg/kg |
| LD50 (median dose) | Sodium Sulfite LD50 (oral, rat): 3,560 mg/kg |
| NIOSH | 1317 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Sodium Sulfite: 15 mg/m³ (total dust) |
| REL (Recommended) | 16~18 |
| Related compounds | |
| Related compounds |
Sodium bisulfite Sodium thiosulfate Sodium sulfate Potassium sulfite Calcium sulfite |
| Related compounds |
Sodium bisulfite Sodium sulfate Potassium sulfite Calcium sulfite Sodium thiosulfate |

