Sodium Sesquicarbonate: More Than Just a Chemical Compound
Historical Development
Sodium sesquicarbonate didn’t show up in the marketplace by accident. Early on, folks found it naturally in deposits like trona and natron, often scattered around dry lake beds or mineral springs. Ancient Egyptians noticed its power as a cleanser, long before anyone worked out a chemical formula. By the 19th century, improvements in industrial chemistry made it much easier to produce the stuff in bulk. Chemical plants figured out practical synthesis methods, which fueled its spread into households and factories. Each step in its history builds on old knowledge and practical demand, not just theoretical curiosity.
Product Overview
You’ll usually spot sodium sesquicarbonate as a crystalline powder that feels a bit like baking soda in the hand. Some call it trisodium hydrogendicarbonate or trona, but in the supply trade, the old names stick. Unlike pure baking soda or soda ash, it combines sodium carbonate and sodium bicarbonate in a precise one-to-one ratio, resulting in a compound with distinct behavior in water and cleaning. Its chemistry sets it apart from its cousins, making it a go-to for cleaning, laundry, and pool treatment.
Physical & Chemical Properties
The technical crowd appreciates sodium sesquicarbonate for its slightly alkaline pH around 9.9, which comes into play for buffering and gentle cleaning. Solubility sits in the middle: it dissolves better than pure sodium carbonate, yet not as freely as sodium bicarbonate. The crystal structure, often monoclinic, means it holds up under rough handling and stores without caking if kept dry. In the lab, you can spot its identity by its tendency to release carbon dioxide when hit with acids, and to hold onto water molecules, forming stable hydrates when exposed to moisture.
Technical Specifications & Labeling
Regulators and suppliers know users need clear information to use sodium sesquicarbonate safely. Packaging shows grade, purity (often above 99% for cleaning products), and moisture content, with detailed batch information. Because the compound finds its way into laundry additives, detergents, and swimming pool treatments, the labeling must meet consumer protection standards. These days, the drive for transparency leads manufacturers to post safety data sheets, environmental profiles, and allergen status front and center. In my own experience reading bags for household use, nothing beats a clear label when managing a shed full of similar white powders.
Preparation Method
Most commercial sodium sesquicarbonate starts from trona ore, blended with controlled amounts of sodium carbonate and bicarbonate. The solution process usually involves dissolving a sodium carbonate source and bubbling in carbon dioxide at specific temperature and pH conditions, followed by slow crystallization and careful drying. Workers in chemical plants monitor the solution chemistry closely so the result falls within spec, neither too alkaline nor too weak. Refinement filters out impurities that could spoil finished detergents or food applications. I’ve heard from chemical engineers who say this “wet to dry” method runs smoothly when you keep water chemistry just right.
Chemical Reactions & Modifications
Sodium sesquicarbonate reacts energetically with acids, giving off carbon dioxide—a handy trait in cleaning and water treatment. It takes part in ion exchange, pulling heavy metals or hardness ions from solution. Sometimes, manufacturers modify the particle size or blend in additives to tailor performance for automatic dishwashers or eco-friendly cleaning sprays. For pool balancing, its mild alkalinity helps settle acidic shocks without swinging pH too far. Chemical tweaks, such as coating particles or blending in enzymes, shift its focus from raw cleaner to specialized product.
Synonyms & Product Names
Over the years, sodium sesquicarbonate has worn many labels—trona, trisodium hydrogendicarbonate, or soda crystals, depending on where and how it appears. Brand names fill supermarket shelves: Arm & Hammer markets it as a “washing soda alternative,” while pool supply shops sometimes tag it “alkalinity increaser.” Across languages and continents, users know it by whichever name solves their problem—be it cleaning grungy grout or keeping aquariums clear. Having juggled several brands over the years, I’ve learned to check both the ingredient list and the chemical formula—true peace of mind in a sea of overlapping names.
Safety & Operational Standards
Few things matter more than safety once you’ve got a chemical in hand. For sodium sesquicarbonate, contact with skin feels mildly abrasive but usually won’t sting unless you already have a cut. The dust can irritate eyes and lungs, so good ventilation and basic gloves go a long way, whether sprinkling it in the washing machine or mixing pool chemicals. Industrial settings adopt stricter rules—extraction hoods, goggles, even emergency showers. Disposal rarely poses headaches, since this compound breaks down to natural salts, but local guidelines deserve respect, especially in areas with fragile waterways or agricultural runoff.
Application Area
Laundry boosters probably make up the biggest chunk of sodium sesquicarbonate’s day-to-day workload. It softens water, lifts stubborn stains, and keeps musty odors out of gym socks. Cleaning companies blend it into surface scrubs, while municipal pools dump it in bulk for pH and alkalinity control. Aquarium buffs rely on its buffering properties to steady water conditions for sensitive fish. You’ll also find it in personal care goods like bath salts, where it soothes skin and keeps fragrances stable. I’ve even used it myself to clean coffee pots and deodorize damp basements with impressive results—sometimes the classics work best.
Research & Development
Chemists continue to push the boundaries for sodium sesquicarbonate uses. They’re analyzing microplastic removal, pollution clean-ups, and new green formulations. In the push for safer consumer products, researchers blend sesquicarbonate with plant-based ingredients to lower allergens and toxicity. Laboratories map out interactions with surfactants, enzymes, and natural acids, chasing stronger cleaning power and lower environmental impact. The push for green chemistry gives sesquicarbonate a welcome boost, building from proven safety records and gentle chemistry.
Toxicity Research
Toxicologists argue that sodium sesquicarbonate counts among the milder compounds in regular use—with good handling, the risk stays low for workers and consumers. Ingesting large amounts causes upset stomachs, but normal exposure through cleaning or water treatment rarely triggers symptoms beyond mild irritation. Fish and plant studies in environmental science point to manageable risk, especially when used as directed and not dumped unchecked into rivers or streams. Regulatory checks, both at home and abroad, reaffirm its safety with periodic updates and transparent disclosures. I trust products with clear toxicity profiles, especially when children and pets share the house.
Future Prospects
Demand for responsible chemistry brings sodium sesquicarbonate back into the spotlight. More industries need gentle cleansers, phosphate-free water softeners, and low-carbon manufacturing. Chemical companies explore partnerships with eco-brand detergents, looking for ways to lower packaging waste and cut shipping energy. Advanced research may unlock new purification for pharmaceuticals or recovery of rare minerals from wastewater. With its long track record of safety and adaptability, sodium sesquicarbonate keeps finding new jobs to do, whether in cleaning, agriculture, or water care. Experience shows that old compounds don’t fade away; sometimes, they anchor the next wave of green technology.
What is Sodium Sesquicarbonate used for?
Everyday Cleaning and Laundry
Sodium sesquicarbonate ends up in a lot of cleaning supplies that live under most kitchen sinks. This compound looks like a cross between baking soda and washing soda. Some brands market it as “alkaline washing powder.” It helps break down greasy messes in laundry or household cleaning without causing the irritation that harsher chemicals often bring. I’ve tried mixing it into homemade cleaning sprays, and it lifted caked-on grime from stovetops and tile grout. It doesn’t scratch like scouring powders can. For people sensitive to strong cleaning agents, products using sodium sesquicarbonate often get recommended.
Laundry boosters and oxygen-based bleaches often list this ingredient on their packaging. The extra alkalinity it brings helps keep whites looking bright. Old-school laundry techniques relied on soda crystals; sodium sesquicarbonate gives that punch while being gentler on hands and fabrics. Its water softening power also matters wherever the tap water runs hard, because hard water minerals make detergents less effective. Adding it to the wash helps soap dissolve fully and rinses away chalky residue.
Pool and Water Treatment
Tap water and pool enthusiasts know it under a different light. Municipal water utilities use sodium sesquicarbonate to correct pH levels and keep drinking water safe. Pool owners add it by the pound to tweak the water’s balance. If the water gets too acidic, metal pipes can start corroding and chlorine breaks down far too quickly. With this compound, the pH stabilizes in a safe range for swimmers and plumbing alike.
Many people don’t think about the treatment of drinking water until there’s a problem. In my town, a sudden metallic taste led to a boil advisory last summer. The culprit traced back to imbalanced water chemistry, one of those invisible services that keep communities healthy every day. Sodium sesquicarbonate helps avoid those headaches, keeping water clear and palatable.
Food Additive and Baking
Some food companies list sodium sesquicarbonate as an acidity regulator or anticaking agent. It sometimes shows up in baking powder mixes or packaged snacks. As a food additive, it's considered safe by most regulatory agencies, provided it's used within certain limits. The compound helps control acidity in dough, improving texture and keeping things from getting too sticky. I’ve seen recipes for homemade baking powder that mention sodium sesquicarbonate as the magic touch for consistent muffins or cookies.
Eco-Conscious Products and Health Considerations
A lot of people searching for green cleaning or allergy-friendly laundry look for sodium sesquicarbonate as one of the main ingredients. Its origin traces back to natural mineral beds rather than synthetic petrochemicals. For households dealing with allergies or asthma, avoiding harsh scents or irritants matters. The cleaning power without the chemical burn draws interest.
Of course, no chemical comes risk-free. It shouldn’t be inhaled in powder form or left on skin. In food, strict limits keep intake low. Regulatory agencies such as the FDA and EPA weigh the evidence before approval, so checking labels stays important.
Alternatives and Future Choices
Sodium sesquicarbonate stands out for being cheaper and milder than heavy-duty cleaners or industrial water softeners. For folks in areas with very hard water, pairing it with vinegar rinses cuts back on detergent use and protects appliances. As cities look for sustainable answers to old problems, ingredients like this can help phase out more hazardous chemicals in everyday products. Knowledge about what’s inside the box or bottle matters, both for personal health and the planet.
Is Sodium Sesquicarbonate safe for cleaning purposes?
Everyday Encounters With Sodium Sesquicarbonate
Sodium sesquicarbonate pops up in laundry boosters, tile scrubs, and all sorts of DIY green cleaners. It lands somewhere between baking soda and washing soda in terms of cleaning muscle and chemical strength. Having tried it myself on stubborn kitchen stains, it works remarkably well and quickly, lifting grease without leaving a heavy chemical scent. Family members ask if it's any safer or different from regular table salt or other powders. The science says yes; sodium sesquicarbonate holds a balance between being gentle on surfaces and tough on dirt.
Real-World Toxicity and Skin Contact
The Environmental Protection Agency has weighed in, labeling sodium sesquicarbonate as a “low hazard” material under normal usage. This speaks volumes, since some heavy-duty cleaners come with strict warnings, but this compound doesn’t force you to ventilate your house or reach for gloves out of fear. The European Chemicals Agency examined it, too. They found that unless you’re handling industrial quantities or inhaling fine dust regularly, risks look minimal.
During at-home cleaning, I found that direct contact dried my hands after repeated scrubbing—much like using washing soda or strong soap. This isn’t surprising; anything that strips body oils, even hot water, can wear down skin over time. The solution’s simple: rinse your hands when finished, and use a dab of hand lotion. If worried about allergies or extremely sensitive skin, testing a little on a small patch is common sense for any cleaner, natural or not.
Environmental Perspective
Lots of people worry about what gets washed down the drain. Sodium sesquicarbonate breaks down in water to soda ash and bicarbonate, both common in nature. They don’t persist or build up in groundwater. The United States Food and Drug Administration considers it safe enough for incidental food contact, listing it as GRAS (Generally Recognized As Safe). You won’t find the same green light for heavy-duty disinfectants.
I’ve used leftover mop water to clean sidewalks and patio furniture, and there’s never been any stress over harming lawn or garden plants. Local water authorities deal with far tougher compounds daily, and sodium sesquicarbonate fits well within their tolerances.
Cleaner, Not a Cure-All
Despite its many positives, people sometimes ask if sodium sesquicarbonate can do it all—disinfect, deodorize, scour. It shines for washing, neutralizing acids, and removing grime, but it won’t take out viruses or high-level bacteria like bleach does. For normal house cleaning, it’s strong enough. If facing stubborn mold, highly infectious surfaces, or preparing for renovations, it makes sense to supplement it with heat or stronger disinfectants—just like you’d reach for oven cleaner to tackle baked-on grease.
No cleaner gets a perfect score, but for those who value safety and low environmental impact, sodium sesquicarbonate stands out. Following label instructions and observing simple hygiene like rinsing and avoiding eye contact keep things incident-free.
Simple Steps for Smarter Cleaning
If sodium sesquicarbonate is your pick, use it as part of a blend, just as you do in baking. Mixing it with vinegar for a fizzing bathroom scrub, or adding a scoop to the laundry, keeps surfaces fresh and the air in the home clear. The best defense against any risk comes from staying informed, reading up on what you’re using, and paying attention to your body’s feedback. Safer cleaning doesn’t ask for much more than that.
How is Sodium Sesquicarbonate different from baking soda or washing soda?
Understanding the Basics
People hear a lot about household powders like baking soda and washing soda. There's another player in the cleaning aisle: sodium sesquicarbonate. Its name isn't catchy, but it does a solid job. You’ll run across it in water softeners, some household cleaners, and even certain swimming pool products. Unlike baking soda and washing soda, it's a combination of both, sort of a chemical “mash-up” — a one-to-one mix of sodium carbonate and sodium bicarbonate. This makes it a bit of a workhorse, bridging the gap between gentle and tough cleaning jobs.
Baking Soda: The Classic All-Rounder
Baking soda, or sodium bicarbonate, goes into pantries, fridges, and many science fair volcanoes. It’s mild, with a pH close to 8. That’s gentle enough to use as toothpaste. People use it to freshen carpets, scrub kitchen sinks, and settle upset stomachs. It reacts with acids, which is why it’s essential for baking cakes that need to rise without yeast. If you sprinkle it on grimy pans, the fizzing gives a hand with baked-on messes. Because it isn’t caustic, people tend to reach for it first.
Washing Soda: Extra Muscle for Cleaning
Washing soda — sodium carbonate — cranks things up a notch. Its pH is around 11, making it much more alkaline. If baking soda takes on mild odors and stains, washing soda goes after grease and tough mineral deposits. It softens water by binding calcium and magnesium, turning laundry day from headache to breeze in hard-water areas. This extra alkalinity can irritate skin, so gloves matter. It acts quickly and can damage some delicate materials, so it suits heavy-duty cleaning: scouring stovetops, tackling outdoor grime, or prepping laundry for stain removal.
Sodium Sesquicarbonate: The Middle Ground
Sodium sesquicarbonate sits between the extremes — not as gentle as baking soda, not as strong as washing soda. This makes it handy for folks with sensitive skin who still want a deeper clean. Aquarium hobbyists often rely on it to adjust water conditions without shocking their fish. Historical laundry powders used it widely because it worked well in both soft and hard water. It acts as a mild abrasive, fights odors, and helps break up grease, all without being too harsh.
Cleaning the shower, many people reach for sodium sesquicarbonate when they want results better than baking soda can deliver but want to avoid the burn that can come from washing soda. It dissolves well, leaves behind less residue, and rinses clean. People with allergies use it as a replacement for strong detergents.
Why Bother Knowing the Difference?
Shrinking the number of chemicals in your home starts with knowing which ones pull double duty. Too many folks grab whatever powder is closest, only to find their project fizzled (sometimes literally). Choosing the right one doesn’t only save effort—it can save money, time, and even prevent skin irritation or ruined fabrics.
Reading the label and knowing these basic differences keeps cleaning simple, effective, and safe. Swapping out products can wreck a batch of cookies or dissolve a favorite shirt, so matching chemistry to the job matters in everyday life.
Ideas for Smart Use
A little research never hurts, and talking with others can help avoid mistakes. If you keep fish, look up safe water treatments. For laundry, start mild and only move up to stronger stuff if the stains stick around. Gloves protect hands, and rinsing thoroughly protects everything else. Most of us don’t need a laboratory full of powders. Just a little awareness, and the right tool for the job, makes home care much easier.
Can Sodium Sesquicarbonate be used in laundry detergents?
The Science Behind the White Powder
Sodium sesquicarbonate often comes up in cleaning conversations, but for good reason. It’s a compound that mixes sodium carbonate and sodium bicarbonate, taking traits from both sides. My own attempts to make greener household cleaners led me to this ingredient a few years back, mostly because it pops up in so many DIY formulas. With a low cost and solid track record for lifting grime, I started looking closer at how it could work in the bigger world of laundry detergents.
Why It Makes Sense in the Washing Machine
The job of any detergent is to get clothes clean, keep fabrics looking fresh, and avoid irritating skin. Sodium sesquicarbonate comes with the grit to tackle tough stuff like greasy collars and ground-in sports stains. Unlike plain baking soda, it steps up the alkalinity. This property helps shift proteins, oils, and odors, letting surfactants focus on carrying the dirt away. Research from household chemistry experts backs this up: studies show that alkaline boosters like sesquicarbonate help detergents work better in hard water by preventing scale and binding minerals.
Some of the most popular washing soda blends on the market use this ingredient, often as a builder. Builders help the soap part work harder rather than just fighting minerals in the water. In my washing machine, adding a spoon of this powder meant I could use less of the pricier detergent and still get good results—even in cold water. That makes a difference if you’re trying to stretch your cleaning budget.
Safety and Comfort: How It Affects Skin and the Environment
Concerns about safety and environmental effect deserve honest attention. Pure sodium sesquicarbonate is a natural mineral, so it’s less likely to trigger reactions compared to some harsh chemicals. I have sensitive skin, so I checked out safety data on it. Most sources report low toxicity and low environmental impact. Water treatment experts also note that it breaks down easily after use. However, large doses or direct contact can still dry out or irritate skin, especially for anyone with eczema. Gloves keep things comfortable if you use the pure powder directly.
What About the Drawbacks?
Every cleaning ingredient has its limits. Sodium sesquicarbonate by itself won’t kill bacteria or lift every stain in sight. It shines as a supportive ingredient, not a miracle cleaner. For pure whites or delicate fabrics, it pays to check how your shirts or sheets react before tossing it into every single wash. If your local water is extremely hard, this powder helps reduce soap scum but won’t totally solve mineral build-up in your machine over time. It leaves a powdery residue if rinsing stays minimal, so always let the rinse cycle run long enough.
Finding the Right Balance
Choosing ingredients like sodium sesquicarbonate comes down to understanding what your laundry routine really needs. It’s not about jumping onto a trend but about supporting clean clothes and healthy households. Manufacturers keep looking for ways to cut down on harsh chemicals while keeping cleaning standards high. As someone who’s mixed up loads of homemade soap blends, I say this—sodium sesquicarbonate belongs on the short list of simple tools that work for most families. For anyone looking to reduce costs and chemicals at home, it’s worth a closer look and a small-scale test in your next laundry day.
Is Sodium Sesquicarbonate safe for use around pets and children?
What Is Sodium Sesquicarbonate?
Sodium sesquicarbonate shows up in laundry boosters, cleaning powders, and even some pool products. Sometimes called “double salt,” it’s a mix of baking soda and washing soda, which often makes people think it’s pretty harmless. I’ve found it in off-brand bath bombs and even as a suggestion for pet odor control. With so many uses, it’s fair to ask: what happens when curious little hands or paws get into it?
Health Effects—What the Facts Say
Hands-on experience with household cleaners gives a sense of trust, but looking into actual research always helps. The Environmental Working Group rates sodium sesquicarbonate with low overall hazard. Most risks come from irritating eyes or skin, especially if the powder stays on there a while. Ingesting a small amount by accident probably causes a mild upset stomach. But this isn’t a chemical that builds up in the body or causes cancer.
A report from the Journal of Veterinary Emergency and Critical Care highlighted accidental ingestion cases involving baking soda and similar compounds in pets. While a big dose can throw off blood chemistry, most animals recover with routine care and supportive treatment. For kids, the American Association of Poison Control Centers says minor stomach upset is the most common problem when exposed to household cleaners containing this compound.
Why Safety Measures Matter
Anyone who's lived with a curious toddler or a dog that sniffs everything knows trouble can start fast. Keeping containers with strong cleaning ingredients up high or locked away matters more than any label warning. I’ve moved all mine after seeing my kid drag a chair over to the laundry shelf. Sometimes the simplest reminder is the most effective: if you wouldn’t let a child touch it unsupervised, keep it out of reach.
During cleaning, powdery ingredients can get everywhere. Wiping down counters and mopping after using sodium sesquicarbonate makes a big difference. It doesn’t cling quite as stubbornly as bleach, but a few stray grains on the floor could end up in a crawling baby’s mouth or a cat’s paws. For pet bedding and litter boxes, rinsing or vacuuming any excess keeps pets from rolling in or licking up the powder.
Common Sense Solutions
Home safety relies on habits, not just product labels. Choosing unscented, dye-free products cuts down on extra chemicals. Reading packaging closely helps, since a mix of ingredients might pack in something harsher than expected. Label containers clearly if you transfer powders out of original boxes—it avoids confusion and protects visitors, too.
Ventilation helps when cleaning—open windows, turn on fans, and keep animals and kids away until the job is done. Pets, especially birds and small mammals, have more delicate respiratory systems. Quick wipe-downs after using powdered cleaners keep everyone safer. The American Veterinary Medical Association advises reaching out to a vet immediately if any pet appears weak, wobbly, or drooling after possible chemical exposure.
Based on what scientists say and my own experiences, sodium sesquicarbonate isn’t something to panic about. Used with care and stored safely, it barely raises more risk than table salt or baking soda. But all strong cleaners deserve respect—curiosity in kids and pets can’t be underestimated.
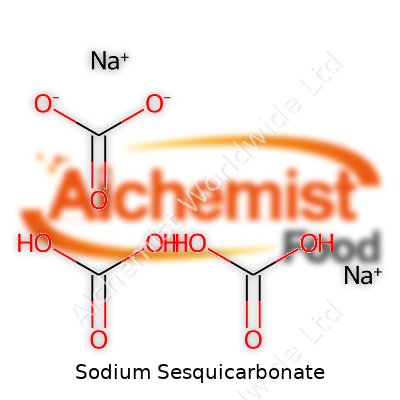
| Names | |
| Preferred IUPAC name | Trisodium hydrogen carbonate carbonate |
| Other names |
Trisodium hydrogendicarbonate Sesquicarbonate of soda Sodium acid carbonate Disodium carbonate bicarbonate Sodium carbonate monohydrate |
| Pronunciation | /ˌsəʊdiəm ˌsɛskwiˈkɑːbənət/ |
| Preferred IUPAC name | Trisodium hydrogencarbonate carbonate |
| Other names |
Trisodium hydrogendicarbonate Trisodium sesquicarbonate Disodium carbonate Sesquicarbonate of soda Sodium acid carbonate |
| Pronunciation | /ˌsəʊdiəm ˌsɛskwiˈkɑːbəneɪt/ |
| Identifiers | |
| CAS Number | 533-96-0 |
| Beilstein Reference | 1723499 |
| ChEBI | CHEBI:9120 |
| ChEMBL | CHEMBL1201561 |
| ChemSpider | 83622 |
| DrugBank | DB09467 |
| ECHA InfoCard | 100.027.225 |
| EC Number | 208-041-5 |
| Gmelin Reference | 6038 |
| KEGG | C14317 |
| MeSH | Sodium Sesquicarbonate |
| PubChem CID | 86288873 |
| RTECS number | VZ0950000 |
| UNII | BYC3K2J00J |
| UN number | UN3378 |
| CompTox Dashboard (EPA) | DTXSID3035783 |
| CAS Number | 533-96-0 |
| Beilstein Reference | 1713887 |
| ChEBI | CHEBI:9120 |
| ChEMBL | CHEMBL1201195 |
| ChemSpider | 53411 |
| DrugBank | DB09462 |
| ECHA InfoCard | 100.011.030 |
| EC Number | 208-580-9 |
| Gmelin Reference | 13776 |
| KEGG | C14547 |
| MeSH | D013476 |
| PubChem CID | 86117 |
| RTECS number | WT3786000 |
| UNII | 5ZV43GS7PO |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID7020644 |
| Properties | |
| Chemical formula | Na₂CO₃·NaHCO₃·2H₂O |
| Molar mass | 186.11 g/mol |
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 2.11 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.38 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.3 |
| Basicity (pKb) | pKb: 3.67 |
| Magnetic susceptibility (χ) | -49.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.470 |
| Viscosity | Viscous powder |
| Dipole moment | 0 D |
| Chemical formula | Na2CO3·NaHCO3·2H2O |
| Molar mass | Na₂CO₃·NaHCO₃·2H₂O: 226.99 g/mol |
| Appearance | White crystalline solid or powder |
| Odor | Odorless |
| Density | 2.10 g/cm³ |
| Solubility in water | Moderately soluble |
| log P | “-2.6” |
| Acidity (pKa) | 10.06 |
| Basicity (pKb) | pKb ≈ 3.2 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.484 |
| Viscosity | Viscous powder or crystals |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 207.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2107 kJ/mol |
| Std molar entropy (S⦵298) | 199.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2076.0 kJ/mol |
| Pharmacology | |
| ATC code | V03AS02 |
| ATC code | A02AA02 |
| Hazards | |
| Main hazards | Irritating to eyes, skin, and respiratory system. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P280, P301+P312, P330, P305+P351+P338, P337+P313 |
| Lethal dose or concentration | LD50 Oral Rat 4090 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 4090 mg/kg |
| NIOSH | SAF91500 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 mg/kg |
| Main hazards | Irritating to eyes, skin, and respiratory system. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 Oral Rat 4090 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 4090 mg/kg |
| NIOSH | WN3675000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium Sesquicarbonate: Not established. |
| REL (Recommended) | 26% |
| Related compounds | |
| Related compounds |
Sodium carbonate Sodium bicarbonate |
| Related compounds |
Sodium carbonate Sodium bicarbonate Potassium sesquicarbonate |

