Sodium Selenite: Discovery, Use, and Moving Forward
Historical Development of Sodium Selenite
Chemists stumbled on elemental selenium early in the 19th century. It didn’t take long before they figured out that mixing selenium dioxide with sodium hydroxide gave a useful compound: sodium selenite. Factories found value in it through the years, first as a coloring agent for glass and ceramics. Scientific studies rolled in, revealing both its nutritional benefits in trace amounts and its dangers if handled poorly. Through the decades, work in labs and industry pushed for cleaner production, tighter control over quality, and more practical uses, especially in agriculture and medicine. Countries established regulations on import, sale, and use, due to public health concerns and contamination scandals. By the late 20th century, sodium selenite was recognized as both essential and hazardous, which led to stricter manufacturing standards and labeling requirements.
Product Overview
Sodium selenite comes as a white, crystalline powder appreciated for its solubility in water and clear, simple chemical composition—Na2SeO3. Labs keep it on hand for supplement formulation, research, and chemical syntheses. Animal feeds make use of selenite to supplement diets, especially in regions with selenium-poor soil. Pharmaceutical companies process it under strict regulation to prevent dangerous contamination. Glassworks rely on its reducing power to get rid of trace metals and correct greenish tints in products. Agricultural workers need to pay close attention to labeling, as misapplication can do more harm than good—too much can damage crops or poison livestock, so accurate dosage and clear instructions matter deeply.
Physical & Chemical Properties
On the lab bench, sodium selenite draws attention for its appearance: a pale powder or sometimes colorless crystals. It dissolves readily in water, which lets it slip easily into feed mixes or formulations. Boiling point sits high above room temperature, so simple heat won’t break it down. It resists combustion but melts with enough direct heat. In chemical storage, the crystalline form holds steady as long as containers remain dry, since moisture can degrade it over time. Left open, it can pick up water from the air. Chemically, it acts as a strong oxidizing agent under the right conditions, ready to react with a range of compounds. Its toxicity means that handlers use gloves, eye protection, and good ventilation, especially on large-scale operations.
Technical Specifications & Labeling
Manufacturers list sodium selenite by its purity, moisture level, and particle size. Most reputable suppliers certify material greater than 98% pure. Labels must list exact content, batch code, and date of production, since trace contamination—or a mix-up between selenite and other selenium compounds—brings serious risk. Appropriate storage instructions highlight dry, cool, well-ventilated areas away from strong acids, reducing agents, and materials susceptible to corrosion. For animal feed, each package warns users not to exceed recommended rates, and highlights regional maximums allowed by regulation. Pediatric use in supplements, if permitted at all, calls for strict dosage calculation and physician approval. I’ve seen feeds and supplements pulled from shelves simply because labeling failed to match local requirements.
Preparation Method
Commercial sodium selenite forms from the reaction of elemental selenium with sodium hydroxide, usually in water for better control. First, selenium reacts with oxygen—often by burning or heating in air—to create selenium dioxide. This dioxide then mixes with a hot sodium hydroxide solution, giving a clear reaction with strong fumes. The solution yields sodium selenite, which operators then crystallize through slow evaporation or cooling. Large producers use closed systems with scrubbers to reduce toxic fumes. Waste streams receive careful treatment to remove any traces of selenium that could threaten groundwater. Waste solid gets tested for safe disposal or recycling. For high-purity production, the entire process runs in stainless steel equipment with constant monitoring for contamination.
Chemical Reactions & Modifications
Sodium selenite acts as a flexible starting material for many chemicals. Reacting it with acids turns it back into selenium dioxide. Under reducing conditions, sodium selenite drops selenium out as an insoluble elemental form, letting labs or plants recover selenium for reuse. In industry, chemists look for ways to tune this compound into more complex selenium chemicals—like sodium selenate—by oxidation, or organoselenium compounds that feed pharmaceutical and imaging research. Glassmakers add it to melts to grab up green-causing iron impurities. Scientists sometimes push sodium selenite to react with organic building blocks, creating selenium-based molecules used in semiconductors. The broad reactivity means it’s always handled with care, since reactions can be fast and release dangerous gases.
Synonyms & Product Names
Across texts and catalogs, sodium selenite picks up a few aliases. Sodium selenious acid salt, disodium selenite, and simply selenious acid, disodium salt, all refer to the same compound, though some names crop up more in research than in trade. In regulatory lists you might spot “sodium selenite pentahydrate” or “anhydrous sodium selenite,” labeling differences tied to water content. Product lines sometimes use coded numbers, or emphasize “feed grade” or “pharma grade” distinctions, but the chemical stays the same in the bottle.
Safety & Operational Standards
Safe handling for sodium selenite stands out as a top concern. Laboratories demand fume hoods and chemical gloves for weighing and mixing. Plants enforce PPE rules—masks, goggles, aprons—to guard against dust and skin contact. Sprays or powders in the air can cause lung and throat irritation. Immediate washing follows spills, removing the risk of skin absorption. Eating or drinking after exposure brings all sorts of risks, since the body needs little selenium and overdose kicks in fast. Disposal runs according to tightly controlled hazardous waste protocols. Any batch used in pharmaceuticals requires traceability down to the gram, with documentation signed off by trained supervisors. These measures come from bitter lessons—many workers and communities learned the hard way what can happen if these standards slip.
Application Area
Modern industries count on sodium selenite for specific needs. In agriculture, feed premixes include it to reduce selenium deficiency, which can cripple livestock productivity. Pharmaceutical companies keep tight watch on dosage for supplements, since selenium’s range from beneficial to toxic narrows quickly. Nutritionists sometimes use sodium selenite in vitamin formulations, guided by national health authorities, but also debate natural food sources versus synthetic supplements. Glass companies demand steady quality, since even trace mineral impurities change final glass color. Environmental labs monitor soils and water, tracking selenium compounds to ensure safe levels for crops and drinking water. Increasingly, researchers look for new uses in electronics, due to selenium’s role in semiconductors and imaging technology.
Research & Development
Researchers investigate sodium selenite for more targeted delivery in cancer therapies, using its ability to induce oxidative stress as a tool for attacking tumors. Several studies suggest that carefully dosed selenite may boost immune response in certain contexts, though results remain mixed and require more evidence. Technologists in chemical plants keep tweaking the preparation process to cut down on waste and make recovering spent selenium more efficient. Environmental scientists track break-down pathways in soils, looking for ways to reduce long-term buildup or unwanted mobility. In nutrition, the focus shifts to personalized medicine—supplement dosing adjusted not just for age and weight, but genetic differences in selenium metabolism.
Toxicity Research
Toxicologists have mapped out sodium selenite’s threat profile. Acute poisoning shows up fast: vomiting, abdominal pain, and in severe cases, death. Researchers tracked how even chronic low doses can cause selenium buildup and side effects like hair loss, nail changes, and nerve damage. Poison centers see animal cases linked to accidental overdosing in poorly mixed feeds. Antidotes for humans and animals aren’t straightforward, often relying on quick stomach pumping and supportive care. In environmental studies, high selenite in soil or water devastates aquatic life and disrupts food webs. Legislation now tightly limits how much can be used in agriculture and food, and new rules keep cropping up as science reveals lower safe limits than once believed.
Future Prospects
Sodium selenite is set to hold onto its spot in agriculture and glassmaking, but bigger changes hover on the horizon. Research groups hunt for safer selenium supplements with slower, more controlled release. Alternatives and biofortification—boosting selenium content of crops using natural uptake—could cut synthetic selenite demand but likely won’t replace it soon. Wearable sensors using selenium compounds, possibly born from selenite, point to new uses in healthcare and consumer technology. As countries keep toughening up environmental and food safety standards, producers will face pressure to get cleaner, more transparent, and more innovative in controlling contamination, recycling waste, and training workers on risk management. The gap between health benefit and danger remains tight, but ongoing research and better controls can keep people, animals, and the environment safer as the story of sodium selenite moves forward.
What is Sodium Selenite used for?
Making Sense of Sodium Selenite
Sodium selenite doesn’t usually turn up in household conversations, but it keeps showing up in places that shape everyday health. As an inorganic compound built around selenium, this powdery substance plays a behind-the-scenes role from dietary supplements to animal feed. Anyone who’s mixed minerals into livestock feed, checked a nutrition label, or followed cancer research has brushed shoulders with sodium selenite.
Supporting Nutrition and Health
Most people learn about selenium as a trace element—something the human body only needs in tiny amounts. Still, those small doses pack a punch. Selenium helps the immune system, fights cell damage, and supports thyroid function. Foods like Brazil nuts, fish, and eggs carry some selenium, but gaps in soil quality or diet sometimes leave folks short. Here’s where sodium selenite steps up. Manufacturers add it to vitamins, fortified foods, and animal feeds to fill in the missing selenium, protecting against deficiency. For me, growing up on a farm meant watching as certain flocks and herds struggled unless secret formulas—mineral mixes containing sodium selenite—were tossed into the feed.
Sodium Selenite Outside the Kitchen
Hospitals take sodium selenite seriously in parenteral nutrition—the kind where nutrients go straight into the bloodstream. Cancer research teams keep an eye on it, too. Lab studies have tested sodium selenite for slowing down cancer cell growth, though they’ve hit snags with side effects at higher doses. Even glass factories use this compound to give color to glass, though you’re more likely to come across it in a pill or powder form than a stained-glass window.
Staying on the Safe Side
Selenium offers benefits, but too much brings trouble. Headaches, brittle hair, garlic-like breath, or even nerve issues can kick in if intake rises past safe limits. Health authorities like the National Institutes of Health set upper tolerable intake levels, warning that everyone—feed suppliers, supplement makers, and consumers—should respect the boundaries. I’ve seen livestock suffer from mineral overdoses when nutritional advice was ignored. The lesson repeats in human medicine, where a deficiency harms but an excess does damage too.
Challenges and Practical Solutions
Modern agriculture keeps pushing up production, which makes supplementation almost routine. Some soils have less selenium. Crops grown there pass along gaps straight into the food chain. Dairy farmers and crop scientists can boost selenium in animal diets or fertilize land with selenium-rich products. For the rest of us, good advice starts with reading labels, talking with doctors before starting supplements, and not chasing health trends blindly.
Supplement makers and hospitals both face pressure to stay transparent. In some parts of the world, regulation is light, so it falls to the end user to check product quality. Third-party testing, regulatory oversight, and honest labeling protect people. Whenever sodium selenite shows up in a formula, it deserves a closer look. Getting it right matters for both personal and public health.
Is Sodium Selenite safe for human consumption?
Looking at What’s on the Label
People come across sodium selenite in multivitamins, animal feed, and some food supplements. The scientific name makes it look intimidating, especially with words like "selenium" rooting memories of chemistry class. Selenium, though, plays a vital role in human health. It’s a trace mineral the body needs for thyroid function and to counteract oxidative stress. According to data from the NIH, adults require only about 55 micrograms per day. That’s less than a sprinkle.
The Good and the Bad
My own path with supplements taught me the line between "helpful" and "harmful" can shrink fast. Sodium selenite delivers selenium in a form the body can absorb, which helps prevent deficiency. Historically, areas with selenium-poor soils saw higher rates of certain heart conditions and immune troubles.
But the margin between benefit and risk grows thin. It reminds me of my impatient phase with sports supplements in college — thinking more would give that edge. With selenium, too much tips the scales toward toxicity. Signs like garlic breath, hair loss, stomach upset and even nerve damage can follow if intake runs too high. The European Food Safety Authority and FDA both set upper daily limits at 400 micrograms for adults. In practice, a “typical” multivitamin gives maybe 55 micrograms, nestled right in the safe zone.
Natural Sources vs. Supplements
Diet delivers selenium without much fuss. Brazil nuts serve as the classic example, though one or two contain all you need for the week. Seafood, meats, eggs, and grains also chip in. Deficiency doesn't bother most healthy people eating a varied plate, and food sources rarely cause toxic buildup. Problems surface when supplements bypass the body’s normal regulation — like the fish tank I once over-dosed, believing a little extra nutrient would clear cloudy water faster. It didn't, and I learned that line matters.
Are There Real Risks?
Sodium selenite is a compound with research behind it. Multiple studies show its role in treating deficiencies and supporting immune health. Still, its place in the ingredient list keeps raising eyebrows. That worry often grows from stories of accidental overdosing or outdated research linking high levels to problems like diabetes or cancer, especially in long-term, high-dose use. Most studies agree: keeping intake near the recommended daily allowance supports benefits without inviting risk.
Some groups run a higher risk. People with kidney problems or those living in selenium-rich regions glide closer to excess just from food alone. In my own family, a relative with kidney disease received strict warnings about trace minerals. Their physician checked bloodwork regularly and cut down on all non-prescribed supplements, including those with sodium selenite.
Building Trust in Supplement Decisions
Labels give doses, but manufacturers vary. Third-party testing boosts accountability. Trusted names stick to public standards and publish testing results. For anyone shopping for supplements, I recommend researching the brand, and talking to healthcare providers, especially with underlying health issues.
Sodium selenite has a valuable role but real dangers pop up from too much or misguided use. The best safeguard is open conversation with your care team, attention to dose, and remembering there’s no substitute for a balanced, whole-food diet. Supplements can play backup, but they rarely replace the main game.
What is the recommended dosage of Sodium Selenite?
The Role of Sodium Selenite in Health
Sodium selenite draws attention for its place in human nutrition. It acts as an essential source of selenium, a mineral that the body uses to build important antioxidant enzymes. These enzymes help fight harmful oxidation and protect cells from damage. Over the years, I’ve seen how trace minerals like selenium often get overlooked, but science links deficiencies with higher risks of certain cancers, weakened immune response, and thyroid issues. Too much, though, spills over into toxicity—so balance matters.
Current Recommendations and Safe Intake Levels
For adults, the recommended dietary allowance (RDA) for selenium stands at 55 micrograms per day, according to the National Institutes of Health. Pregnant women need a bit more, around 60 micrograms, and those breastfeeding require 70 micrograms. One thing people often miss: not all selenium supplements are the same. Sodium selenite appears commonly in supplements and fortified foods, and it packs a punch. Only small amounts count toward your daily limit, as selenite absorbs efficiently.
According to health authorities, staying below 400 micrograms a day keeps things on the safe side. Over that, and the risk of selenosis creeps in. I remember reading about symptoms—hair loss, nail brittleness, digestive upset—that can hit if someone takes too much for too long. In my own experience tracking supplement use, it’s rare, but easy to stumble into unsafe territory with high-potency pills.
Sourcing Selenium from Diet and Supplements
Brazil nuts stand out as the all-stars for selenium, sometimes packing several hundred micrograms in a single nut. Seafood, meats, and some grains fill in the rest for most diets. Folks who eat varied diets usually don’t need to supplement, but those on restrictive diets may look to sodium selenite to fill the gap.
Supplemental sodium selenite often appears in multivitamins at 50-200 micrograms per serving. Dosing matters because the body does not store large amounts, so daily intake works best. Toxic effects build slowly with chronic overuse, not with a single high dose. Sometimes doctors recommend sodium selenite at higher doses for certain conditions, but that calls for careful monitoring and never self-dosing above recommended dietary intakes without medical supervision.
Pitfalls of Self-Medication and the Importance of Medical Advice
It’s tempting to adjust doses based on internet advice or anecdotal stories, but blood tests show wide variation in people’s selenium status. Needs shift with age, health status, and even geography—soil levels shape how much ends up in local food. From my own experience in health forums and nutrition consults, vague symptoms sometimes lead people to over-supplement. Monitoring selenium through lab work, not just guesswork, provides clarity. If someone already eats foods high in selenium, adding a supplement could tip the balance in the wrong direction.
Solutions for Safe Selenium Intake
Learning to check supplement labels, sticking below 200 micrograms daily from all sources, and working with a healthcare provider go a long way to avoid problems. For those with specific health issues or absorption challenges, gathering medical advice before starting sodium selenite prevents missteps. Education matters—not every mineral works the same way, and selenium’s margin between helpful and harmful is narrow. I’ve found that people benefit most when they combine professional guidance with a real look at what their diet already provides.
Are there any side effects of taking Sodium Selenite?
What Happens When Selenium Levels Tip Over the Line
Sodium selenite, a common form of selenium, pops up in supplements and even some fortified foods. Selenium plays a part in metabolism, thyroid function, and immune support. I'm all for getting the minerals we need, but too much of anything creates new problems. Many of us eat foods like Brazil nuts or seafood that have a decent amount of selenium to start with.
Too much sodium selenite risks selenium toxicity. The most noticeable sign often comes from your hair. If strands start falling out more than usual, check that multivitamin label. It's no old wives’ tale—excess selenium can also create brittle nails or lead to that garlic-like breath some people start to notice. One friend of mine, after trying a high-dose supplement, started dealing with constant fatigue and odd tingling in her fingertips. These sorts of neurological symptoms usually sneak in when selenium levels build up over weeks, not days.
Adverse Health Impacts Beyond a Bit of Hair Loss
Digging into documented side effects, the body reacts to an overload with nausea, diarrhea, and sometimes muscle tenderness. Over months or years, high selenium can mess with blood sugar and might impact thyroid hormone production. Research from the National Institutes of Health points out that long-term high selenium links to increased risk of type 2 diabetes. At the same time, a few studies link high levels to a higher risk of prostate cancer in men taking supplements well above the recommended daily limit.
Some symptoms—metallic taste in the mouth, skin rashes, or irritability—appear without much warning. For those already dealing with chronic conditions, adding sodium selenite without a doctor's advice just piles more risk onto existing health challenges. Doctors keep an eye on selenium because it builds up slowly, and there isn’t much the body can do to get rid of extra once it’s there.
Why Oversupplementation Happens
Selenium gets marketed as a quick fix for everything from heart disease to low energy. Many supplement companies highlight benefits but gloss over the small window between “enough” and “too much.” Most adults need only 55 micrograms a day, as set by the National Academy of Medicine. Some supplements—especially those sold for immune boosting—go up to 200 micrograms or more. Multivitamins on top of a high-selenium diet inch intake even higher. The risk gets worse for people who already snack on selenium-rich foods.
Smart Strategies for Staying Safe
Most people won’t run into problems from natural food sources alone. Issues crop up when high-potency pills get added to the mix. Before adding any selenium supplement, a talk with your healthcare provider makes sense. Blood tests can confirm if you even need selenium. Sticking with modest doses avoids most health problems tied to sodium selenite.
Skepticism is healthy. There’s no benefit to pushing daily intake way past what’s recommended. The key is balance—getting enough, but not treating supplements as quick solutions. Knowledge, along with reading product labels, often keeps things safe and simple.
Can Sodium Selenite be used as a dietary supplement?
What We Know About Selenium
Selenium doesn’t get the spotlight like vitamin C or calcium, but it’s an essential micronutrient. The body uses selenium for many things—protecting cells from oxidative stress, maintaining thyroid function, and keeping immune defenses in working order. Foods like Brazil nuts, seafood, and grains carry selenium naturally. Where diets can fall short, some manufacturers offer sodium selenite, a form of selenium, blended into supplements and fortified foods.
The Science and the Risks
Sodium selenite enters the scene as a source of inorganic selenium. Studies recognize that it absorbs well, delivering the trace mineral the body needs. Both the World Health Organization and the US National Institutes of Health point out selenium’s narrow safety margin. Adults only need about 55 micrograms a day, and the gap between meeting requirements and ending up with a toxic dose isn’t wide.
Taking too much sodium selenite brings its own dangers. Selenium toxicity can spark symptoms ranging from hair loss, brittle nails, and stomach upsets to nerve problems. Chronic overexposure is linked to a risk of diabetes and may interfere with some medications. Always use caution before grabbing high-dose supplements or adding selenium on top of an already balanced diet.
Supplements and Real Lived Experience
A quick trip to the drugstore reveals a crowd of supplements boasting trace minerals, including sodium selenite. It’s easy to think more is better, but experience shows that the body runs best with nutrients in the right range—not pushing the upper limits. Talking to older relatives, you often hear stories about how a varied diet was enough. I’ve seen cases where friends took multiple “support” pills and ended up bloated, jittery, or flat-out sick. Piling on extra selenium can be tempting if you hear it might give you more energy, but too much does more harm than good.
Who Benefits from Extra Selenium?
Most people eating a balanced diet in regions where the soil contains enough selenium won’t see much benefit from adding sodium selenite. Certain conditions can cause real deficiencies, including some chronic illnesses, strict vegetarian diets with limited variety, or living in areas with naturally low-selenium soil. Doctors sometimes recommend supplementation for patients in these cases, but they guide the amount closely.
Regular lab tests offer the clearest guidance for anyone worried about their selenium status. Self-diagnosis often leads to overuse, and blood checks clear up whether more is really needed.
Possible Solutions and Smarter Choices
Food wins out over pills for most nutrients, and selenium is no exception. For people not getting enough selenium from their meals, smart fortification of staples—flour, salt, animal feed—helps close the gap without raising danger levels. Doctors and registered dietitians can steer patients toward safer strategies, such as dietary changes or targeted short-term supplements rather than casual, ongoing use.
Before adding sodium selenite supplements to a daily routine, check actual needs and risks. Look for trusted brands, check dosing, and remember that skipping out on a perfectly good varied meal for the sake of a pill rarely pays off in the long run. Eating well and getting nutrients from food stays the best bet for lasting health.
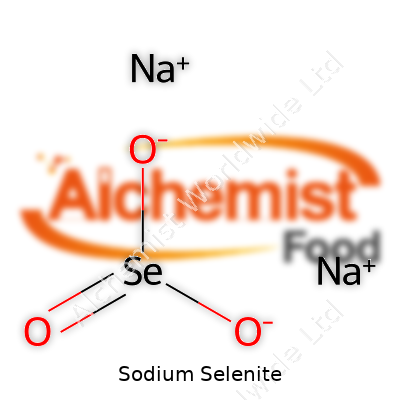
| Names | |
| Preferred IUPAC name | Sodium oxoselenate(IV) |
| Other names |
Disodium selenite Selenious acid disodium salt Sodium selenite pentahydrate |
| Pronunciation | /ˌsəʊdiəm səˈliːnaɪt/ |
| Preferred IUPAC name | Sodium selenite |
| Other names |
Disodium selenite Selenious acid disodium salt Disodium selenite pentahydrate Sodium selenite anhydrous Sodium selenite pentahydrate |
| Pronunciation | /ˌsəʊdiəm səˈliːnaɪt/ |
| Identifiers | |
| CAS Number | 10102-18-8 |
| Beilstein Reference | 3563789 |
| ChEBI | CHEBI:24968 |
| ChEMBL | CHEMBL1201562 |
| ChemSpider | 23241 |
| DrugBank | DB11131 |
| ECHA InfoCard | ECHA InfoCard: 100.030.216 |
| EC Number | 3.1.1.6 |
| Gmelin Reference | 778 |
| KEGG | C01405 |
| MeSH | D017674 |
| PubChem CID | 24936 |
| RTECS number | VS7700000 |
| UNII | 2DI9HA706A |
| UN number | UN2630 |
| CompTox Dashboard (EPA) | DTXSID2020183 |
| CAS Number | 10102-18-8 |
| Beilstein Reference | 385873 |
| ChEBI | CHEBI:50006 |
| ChEMBL | CHEMBL1351 |
| ChemSpider | 74913 |
| DrugBank | DB11131 |
| ECHA InfoCard | 05e0c6bb-6e41-4e6c-a1ca-4b72d1e7a6ab |
| EC Number | 231-907-1 |
| Gmelin Reference | Gmelin Reference: 14108 |
| KEGG | C00268 |
| MeSH | D014460 |
| PubChem CID | 24814 |
| RTECS number | VS7650000 |
| UNII | 1KGS709BXS |
| UN number | UN2669 |
| CompTox Dashboard (EPA) | DB11162 |
| Properties | |
| Chemical formula | Na2SeO3 |
| Molar mass | 172.94 g/mol |
| Appearance | White to slightly yellow crystalline powder |
| Odor | Odorless |
| Density | 2.62 g/cm³ |
| Solubility in water | Soluble |
| log P | -4.0 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.0 |
| Basicity (pKb) | 11.5 |
| Magnetic susceptibility (χ) | '+245×10⁻⁶ cm³/mol' |
| Refractive index (nD) | 1.451 |
| Viscosity | Viscosity: 0.913 cP (25 °C, water solution) |
| Dipole moment | 0 D |
| Chemical formula | Na2SeO3 |
| Molar mass | 172.94 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.62 g/cm³ |
| Solubility in water | soluble |
| log P | -4.41 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.2 |
| Basicity (pKb) | 3.40 |
| Magnetic susceptibility (χ) | -39.0e-6 cm³/mol |
| Refractive index (nD) | 1.509 |
| Viscosity | Viscous liquid |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 124.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -688.4 kJ/mol |
| Std molar entropy (S⦵298) | 128.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -850.7 kJ/mol |
| Pharmacology | |
| ATC code | A12CE02 |
| ATC code | A12CE02 |
| Hazards | |
| Main hazards | Toxic if swallowed. Causes severe skin burns and eye damage. May cause cancer. Suspected of damaging fertility or the unborn child. Very toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS05, GHS06, GHS08 |
| Pictograms | GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H301 + H331: Toxic if swallowed or if inhaled. |
| Precautionary statements | P260-P264-P270-P273-P301+P310-P304+P340-P305+P351+P338-P308+P311-P330-P501 |
| Lethal dose or concentration | LD50 (oral, rat): 7 mg/kg |
| LD50 (median dose) | LD50 (median dose): 7 mg/kg (oral, rat) |
| NIOSH | WQ4950000 |
| PEL (Permissible) | 0.2 mg/m³ |
| REL (Recommended) | 0.05 - 0.10 mg/day |
| IDLH (Immediate danger) | 1 mg/m³ |
| Main hazards | Toxic if swallowed. Causes severe skin burns and eye damage. May cause cancer. Suspected of damaging fertility or the unborn child. Very toxic to aquatic life with long lasting effects. |
| GHS labelling | GHS02, GHS06, GHS08 |
| Pictograms | GHS05,GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H301 + H331: Toxic if swallowed or if inhaled. |
| Precautionary statements | P260, P264, P270, P271, P273, P301+P310, P304+P340, P308+P311, P330, P403+P233, P405, P501 |
| Lethal dose or concentration | LD50 (oral, rat): 7 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (rat) 7 mg/kg |
| NIOSH | TT4270000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium Selenite: 0.2 mg/m³ (as Selenium) |
| REL (Recommended) | 0.034 mg |
| IDLH (Immediate danger) | 1 mg/m3 |
| Related compounds | |
| Related compounds |
Sodium selenate Selenium dioxide Selenous acid Sodium sulfate Sodium tellurite |
| Related compounds |
Sodium selenate Selenous acid Sodium tellurite Sodium sulfate |

