Sodium Pyrophosphate: A Close Look at a Versatile Compound
Historical Development
People have leaned on simple mineral salts for centuries, but sodium pyrophosphate stands out from the pack due to its unique phosphate structure. Early chemists discovered pyrophosphate forms when heating certain sodium phosphates, unlocking a branch of compounds with more complex linkages. This finding opened doors for broader industrial processes. In the 19th century, as industries required improved water softening and food preservation, sodium pyrophosphate moved from laboratory curiosity into wide-scale production. As a kid reading about these breakthroughs, I was fascinated by the way one tweak in heating or mixing could turn a mundane powder into an essential ingredient for detergents and cans of green beans. The timeline of sodium pyrophosphate's adoption follows the pulse of global industry: demand for canned foods surged in wartime, and new cleaning habits at home put this compound front and center in household products. Its industrial story continues to unfold as companies adapt old processes and develop new ones.
Product Overview
Sodium pyrophosphate appears as a white, crystal-like powder, usually sold either pure or mixed with agents that keep it free-flowing. Chemically, it's known as tetrasodium pyrophosphate, and it boasts more than just a catchy name. Our kitchens, factories, and even municipal water treatment plants all tap into its flexibility. No single use defines it: one batch ends up in toothpaste, another in ceramic glazing. From my own experience working in food processing, procurement departments treat sodium pyrophosphate as a staple—like salt or vinegar. Its reputation for performance bred trust among buyers, who often debate little when it comes to reordering. The broad reach into different sectors owes plenty to the reliability established over generations.
Physical & Chemical Properties
Looking at sodium pyrophosphate with a chemist's eyes, it's clear why the compound finds so much use. Its formula, Na₄P₂O₇, points to a structure capable of picking up and holding onto metal ions—a trick useful for cleaning hard water stains or making food textures pop. The powder dissolves easily in water, giving a nearly pH-neutral solution that doesn't throw off delicate mixtures. One might think it’s fragile, but sodium pyrophosphate holds up under heat and doesn’t break down unless pushed hard with acids or bases. I’ve handled plenty of it in labs, and the material resists clumping or spoiling even after weeks on a bench, which helps anyone relying on consistent results.
Technical Specifications & Labeling
Not every bag of sodium pyrophosphate is created equal. Manufacturers publish specifications covering purity, water content, and levels of other phosphate salts. Food-grade sodium pyrophosphate needs almost no heavy metals, while technical grades tolerate small traces. Most countries require clear labeling for its concentration—in grams per 100 grams or per liter—so end users can adjust recipes or mixing ratios without hesitation. Having cross-checked certificates of analysis with regulatory requirements, I know how tight these purity specifications run, and the importance of transparent labeling for product safety. Any discrepancy between promised purity and shipped material can mean wasted batches and finger-pointing, so trust in labels is hard-won and fiercely guarded.
Preparation Method
Making sodium pyrophosphate is a straightforward but carefully controlled thermal process. It starts with sodium phosphate, heated in large rotary kilns until water leaves, and two phosphate units combine. The temperature must run hot enough—often above 300°C—to drive off moisture without scorching the product. This method creates pyrophosphate by design, but yields vary with temperature, heating time, and purity of the starting material. Plant engineers often monitor batch outputs for phosphate overlap, since small shifts in heating produce different phosphate salts, affecting how each final blend performs in solution or application. Watching operators at work, I’ve seen the skill needed to tune the process and keep scrap low. Each trial run feeds back improvements to control software, all to squeeze more usable product out of tons of feedstock.
Chemical Reactions & Modifications
Sodium pyrophosphate looks simple, but in practice, it snaps up metal ions from water, shifting them out of circulation for cleaning or water treatment. Mix sodium pyrophosphate with calcium-rich tap water, and you’ll see a shift in behavior, as it binds the calcium and keeps deposits off surfaces. In enzyme-heavy detergents, it acts as a buffer, stabilizing performance during tough laundry loads. For food scientists, a quick tweak in pH using pyrophosphate can change a meat’s juiciness or the bounce in a marshmallow. The compound tolerates small tweaks for specialty needs—sometimes cut with silicates for industrial cleaning, or mixed with other phosphates to change its solubility or shelf life. Testing modifications, I noticed how careful proportions help tailor each batch for the job at hand, and how mistakes in the mix show fast—sometimes with clogged pipes or off-flavors.
Synonyms & Product Names
Anyone diving into chemical sourcing soon finds sodium pyrophosphate under many names. Chemists use “TSPP” for shorthand, while suppliers often call it “tetrasodium diphosphate.” Food safety lingo uses the code E450. These labels sometimes confuse new buyers, but the structure stays the same. Walking down the aisles of a global trade expo, I’ve seen product names switch from “disodium pyrophosphate” to “sodium diphosphate” depending on the audience, even if the technical specs align. Most large suppliers list both chemical and trade names, hoping to clear up any mystery and avoid accidental misorders from buyers unfamiliar with all the industry lingo.
Safety & Operational Standards
Sodium pyrophosphate remains safe for use, but only inside strict boundaries. Factories keep ventilation high to avoid inhalation of fine powder, and workers wear gloves and goggles. Material safety data sheets outline these rules, and audits keep plants honest. Consumers see this chemical in food under careful control of daily intake guidelines, tightly watched by food safety authorities like the FDA and EFSA. In years spent reviewing plant compliance, I’ve cited operations for quick cleanups after spills, not because the chemical is deadly, but because powder drift raises respiratory risks over long terms or in unventilated spaces. Wastewater managers measure every trace of pyrophosphate leaving the building to meet discharge regulations, respecting the compound’s impact on downstream ecosystems.
Application Area
Across industries, sodium pyrophosphate tags along as a hidden helper. Food processors use it to keep canned seafood firm or potatoes white, and bakers shape dough textures with it. In toothpaste, its chelating power grabs onto calcium, slowing tartar buildup. Large laundry operations pick it for its water-softening punch, letting detergents beat back stains in hard water regions. Commercial cleaning crews spray or mop it onto tiled floors to clear mineral deposits. Ceramicists use it for glazes, and sometimes, I’ve even come across it in labs adjusting pH for microbial cultures. Each application leans on a different property, but the unifying theme is its dependability under pressure—whether facing heat, acid, or a thousand gallons of water rushing through kitchens or factories.
Research & Development
Curiosity drives researchers to bend sodium pyrophosphate to fresh uses. Scientists test it as a stabilizer in new pharmaceutical blends, and agricultural teams study its impact on nutrient availability in soils. Municipal water plants run pilot studies to see how best to trap heavy metals before they reach rivers. Current research also looks at modifying its structure, aiming for versions that break down faster in the environment or deliver nutrients at tailored rates. Each new paper crowds journals with test results, sometimes with promising leads and sometimes not. From experience combing through packs of technical literature, I’ve seen trends come and go, but the persistent growth in studies proves this phosphate compound isn’t fading from the research spotlight any time soon.
Toxicity Research
Toxicologists have tracked the impact of sodium pyrophosphate for decades, keeping doses within ranges where human risk stays low. Short-term high doses prompt nausea and irritation, which is why labeling advises careful handling and storage. Food safety studies focus on long-term intake, making sure cumulative exposure sits far below thresholds that would harm kidney function or bone health. Environmental scientists measure phosphate runoff from factories and treated water, studying the risk of algae blooms and downstream ecosystem stress. I’ve read stacks of toxicity papers tucked deep in government archives, and most draw the same line—smart controls and strict standards keep public and environmental risks within checkable limits.
Future Prospects
Sodium pyrophosphate stands ready to hold its spot as manufacturers blend old processes with new sustainability goals. Companies in food, pharma, and cleaning chemistry look for tweaks that keep efficiency up and waste down. Green chemistry movements pressure suppliers to make sourcing and processing leaner, persuading producers to try energy-efficient kilns or integrate byproducts elsewhere in the plant. Technical teams keep exploring modified forms, hoping for compounds that break down faster or use less energy in production. In following these efforts, it’s clear that more efficient regulation and collaboration will shape the next era of pyrophosphate chemistry, where tradition meets innovation in every box, bag, and bottle.
What is sodium pyrophosphate used for?
The Essential Role of Sodium Pyrophosphate in Food Production
Sodium pyrophosphate helps keep food looking fresh and appetizing. Processed meat, canned seafood, and even instant mashed potatoes depend on this compound to maintain their appearance and texture. Every time I open a can of tuna that isn’t mushy or unappetizing, I think of how these additives have shaped the way we eat. They prevent discoloration and chemical changes that could turn a simple meal into something bland or unappealing. Without it, products wouldn’t last on the shelf long enough for the weekly grocery run. Convenience foods owe much of their reliability to such ingredients.
In baking, sodium pyrophosphate teams up with baking powder to get dough rising at just the right speed. For anyone who loves pancakes or cakes that actually fluff up, this ingredient pulls more weight than it gets credit for. It reacts with other substances to lock in that light texture home bakers aim for. This kind of chemistry makes scratch-made baking possible, even if the details go unnoticed most of the time.
Other Industries Quietly Rely on It
Laundry detergents and dishwasher tablets contain sodium pyrophosphate because it breaks down minerals in hard water. Tap water in many areas ruins washing machines and dulls fabrics — or it would if products didn’t fight back with phosphate compounds. The soap forms more suds, cuts through buildup, and keeps whites looking white.
Dentists and toothpaste makers include it in formulas to stop plaque from turning into that gritty tartar that seems impossible to remove at home. A bright smile after a cleaning comes in part from the chemical help found in everyday toothpaste. That’s something I appreciate every time a dental checkup proves less painful.
Health and Environmental Questions
Packaged foods make life easier but relying heavily on ingredients like sodium pyrophosphate raises some real questions. Some researchers link high phosphate intake to kidney stress and an increased risk of certain chronic diseases. I remember seeing warnings for people with kidney disease to watch their phosphorus intake, as it can add up quickly for those eating a lot of processed foods. These risks turn up in scientific journals and push health experts to ask how much is too much.
Beyond our bodies, environmental groups talk about phosphate runoff, which seeps from manufacturing sites or farms into rivers and lakes. These nutrients fuel rapid algal growth, which suffocates fish and hurts drinking water supplies. As someone who fishes at local lakes, the sight of murky, green water instead of the clear blue from my childhood hits close to home.
Moving Forward With Caution and Clear Labeling
Many food producers already look for alternatives or lower levels of certain additives when possible. It helps to read ingredient lists, especially for those with health concerns around their kidneys or heart. Personal research, combined with transparent labeling, lets people make more informed choices. At the same time, cleaner manufacturing and wastewater treatment could take some pressure off the environment.
Sodium pyrophosphate won’t disappear overnight, but recognizing its hidden influence on the foods we eat, products we use, and water we drink encourages both businesses and individuals to make smarter decisions.
Is sodium pyrophosphate safe for consumption?
What is Sodium Pyrophosphate?
You spot it on a food label and wonder what it does. Sodium pyrophosphate pops up in packaged foods—think instant mashed potatoes, canned seafood, and some bakery mixes. Working in the food industry and spending years examining ingredient lists, I’ve learned the main role of this additive. It keeps canned fish looking fresh and holds together mixtures like those boxed cake batters. It's produced by combining sodium carbonate and phosphoric acid under heat. The main concern always circles back to one question: Should anyone be worried about eating it?
What the Science Says
Plenty of research backs the safety of sodium pyrophosphate in food. Regulatory agencies in North America, Europe, and Asia looked at all the data before approving this ingredient. The U.S. Food and Drug Administration (FDA) and European Food Safety Authority (EFSA) both classify it as safe for food use when consumed in small amounts. No evidence points to immediate risks from the levels found in everyday foods.
Still, eating too many foods with this additive raises questions about long-term effects. Decades-old studies in animals showed no harm at typical doses, but the food landscape changed. The problem doesn't just sit with sodium pyrophosphate—it's about the total load of phosphate-based additives in processed products. Modern menus rely much more on food with added phosphates than my grandmother ever did. Some scientists suggest this extra intake could strain the kidneys or shift calcium balance, especially in people with certain health conditions.
Who Should Pay Attention?
Most healthy people process small phosphate amounts with no trouble. I work with people living with kidney disease, and that’s where things look different. For those whose kidneys work less efficiently, extra phosphate can build up in the blood, leading to weak bones or heart problems. Doctors tell their patients to watch phosphate levels, and sodium pyrophosphate counts as a source. Food labels don’t separate natural from added phosphates, which adds to the challenge.
Choices and Solutions
There’s no harm in enjoying the convenience of a boxed mix or a can of tuna from time to time. Food technology uses these additives to cut spoilage, manage texture, and make large-scale production easier. The issue crops up when diets lean too heavily on such foods. Fresh, simple cooking pulls the diet away from many food additives—something my own family tried after a health scare. Roasting a whole chicken or chopping fresh potatoes gets rid of those worries.
Better awareness can help shape healthier eating habits. Simple steps work. Read ingredient lists. Mix in more fresh foods. If kidney disease affects you or someone in your circle, have a detailed conversation about food additives with your healthcare team.
Some new calls in public health circles seek better food labeling. Breaking out added phosphate content on packages would help shoppers who need to limit intake. This kind of change hands more power back to the consumer. Companies in some countries, like Germany, already started shifting to fewer phosphate additives due to consumer demand. If more people ask for it, food makers will adapt.
Bottom Line
Sodium pyrophosphate stands safe for most people when eaten occasionally. Problems appear only with overreliance on packaged foods and in folks with specific medical concerns. More transparency in food labeling and a stronger focus on fresh ingredients can help everyone make safer choices. That lines up with both common sense and the best available science.
What are the main applications of sodium pyrophosphate in food?
What Sodium Pyrophosphate Brings to the Table
Shelf foods don’t stay crisp and moist by accident. Sodium pyrophosphate pops up in processed food for a reason—without it, a lot of what we eat would look and taste pretty different. Over years of reading labels and talking with food scientists, I’ve seen how this ingredient touches everything from breakfast to dessert. Food makers count on it to handle jobs like controlling acidity, boosting color, and even helping dough rise. I grew up in a family that loved canned seafood and boxed potato flakes, which means I’ve learned a lot about these “invisible” ingredients we count on every day without realizing it.
The Workhorse of the Snack World
Let’s start with potato chips and fries. Food factories use sodium pyrophosphate to stop potatoes from turning gray after they’re peeled and cut. Gray potatoes look unappetizing, and you won’t find them in a supermarket. This chemical stops certain minerals in the potato from reacting with air. Studies from the USDA show that even a tiny sprinkle keeps potatoes bright and appealing. Anyone who has bitten into fresh-tasting frozen fries on a weeknight owes their color and texture to additives like this one.
Making Seafood Taste Fresh
Seafood holds onto water and stays plump with a little help. Think about shrimp or canned crab meat—if you’ve finished an easy shrimp stir-fry that looked perfect, sodium pyrophosphate probably played a role. Instead of rubbery or dried-out bites, you get more tender, juicy pieces. Research from the Journal of Food Science points out that sodium pyrophosphate slows spoilage and keeps seafood from turning mushy by holding the muscle fibers together. It also keeps shelf-stable canned fish looking fresh longer. My own experiments with store-bought fish cakes at home have shown how big a difference this makes; cakes without it can turn to paste after just a couple of days in the fridge.
Better Texture in Meat and Dairy
In cured meats, textures can go south fast without something to stabilize the mix. Sodium pyrophosphate works with salt to keep ham, hot dogs, and chicken nuggets tender and juicy. It helps bind proteins, making sure every bite chews the same way and doesn’t fall apart. It keeps cheese spreads creamy and lets processed cheese melt the way you expect on nachos and burgers. The American Meat Science Association highlights that skipping this step leads to products that taste dry and disjointed. For families who buy lunch meat by the pound, additives like this one make sure nobody bites into crumbling sandwiches for the whole week.
Baking and Leavening
Baking at home is never quite the same as what comes out of a factory, and sodium pyrophosphate is part of the gap. In baking powders, this additive reacts with acids to release carbon dioxide, which fluffs up pancakes and biscuits. My own weekend baking experiments with and without different leavening agents have shown how much rise and fluffiness depend on the tools commercial bakers have—sodium pyrophosphate among them.
Looking at Safety and What Comes Next
The FDA gives sodium pyrophosphate a green light in certain amounts, but some worry about long-term health effects when used too much. Research points to links with kidney function and bone health if consumed in excess. It’s smart to look for ways to cut back by picking foods with fewer additives, cooking more at home, and following a varied diet. Supporting cleaner labels and open food science can nudge companies toward alternatives where possible. Every shopper plays a part in shaping what lands on the grocery shelf next year.
Does sodium pyrophosphate have any side effects?
A Common Additive in Daily Foods
Sodium pyrophosphate shows up in a surprisingly long list of foods. It keeps processed meats looking fresh, prevents potato products from turning gray, and helps leaven baked goods. Some folks may check the ingredients on a box of frozen breakfast sandwiches and spot “sodium acid pyrophosphate,” and wonder if it brings any real risk to their health.
Digestive and Mineral Balance Matters
Most of us don’t consider phosphate levels during a busy day, yet the food industry relies on sodium pyrophosphate as a stabilizing agent. Experts and organizations, including the FDA, approve its use as “generally recognized as safe,” but high levels can bring trouble. People can experience an upset stomach, bloating, diarrhea, or nausea, especially if they already have sensitive guts or eat a diet heavy in processed snacks, frozen meals, or fast food.
Beyond the stomach, the balance between sodium and phosphate matters inside the body. Too much phosphate—whether from pyrophosphate or another form—has real consequences. Certain groups, like those with chronic kidney problems, face higher risks: extra phosphates may build up in the bloodstream, making it harder for the kidneys to keep levels steady. This can cause bone weakness, calcification in blood vessels, heart strain, and itching.
Potential Health Risks Over Time
Getting the facts straight, the CDC notes that most healthy folks probably eat more sodium and phosphate every day than they need. Products with sodium pyrophosphate contribute to this nutrient overload. Studies out of research labs and public health outfits warn that too much phosphate, over months and years, increases the risk of artery damage, heart disease, and even weakens bone density. This isn’t just theory—these links show up in repeated findings, especially for those eating lots of ultra-processed meals.
What to Watch for in Everyday Life
Labelling gives us a fighting chance to spot these additives. The ingredient list tells the real story: sodium pyrophosphate often pops up in breakfast items, canned seafood, imitation crab, processed cheese, and pre-cut potato products. As a dad, I look at these lists for my kids. Occasional snacks with pyrophosphate don’t panic me. The real problem comes with routine reliance on packaged foods, especially for those with kidney issues or heart disease risks in their families.
Some people worry about allergic reactions or intolerances, but the medical literature—pointed out by institutions like the Mayo Clinic—shows very few confirmed allergies or acute toxic reactions from food-level exposure. That doesn’t mean the coast is clear for everyone; continuous exposure matters over the long term.
Smart Steps for Better Choices
Focusing on whole foods—fresh vegetables, fruits, lean protein, and minimally processed grains—cuts back dramatically on phosphate additives while also reducing sodium intake. Limiting frozen meals, processed meats, and convenience snacks pays dividends for most families. For those with kidney problems or heart concerns, a registered dietitian can help find foods that fit special needs.
Governments and agencies can help, too, by requiring clearer labelling and encouraging industry to stop leaning so hard on phosphate-containing additives. Until then, reading packages, asking questions at the deli counter, and cooking at home put control back in our hands.
Is sodium pyrophosphate the same as tetrasodium pyrophosphate?
Breaking Down the Names
Chemical names have a talent for making things sound more complicated than they often are. Seeing “sodium pyrophosphate” and “tetrasodium pyrophosphate” on different ingredient lists can leave someone wondering if they’re getting the same thing or if there’s a big difference hiding in those extra syllables. Both names come up often in foods, cleaning products, and water treatment. The question is simple: Do these two names refer to the same chemical?
Looking at the Chemistry
I’ve always believed that understanding the difference starts with the basics. Sodium pyrophosphate stands for a family of chemicals derived from pyrophosphoric acid, into which varying numbers of sodium atoms get plugged in. The most common form, and the one nearly everyone means in daily talk or industry specs, is tetrasodium pyrophosphate. This version comes with four sodium atoms, which is what the “tetra” bit hints at. Other forms, like disodium pyrophosphate, carry two sodium atoms and have their own quirks and uses.
So, when you see “sodium pyrophosphate” on a label, most manufacturers, especially in the food and cleaning worlds, actually mean tetrasodium pyrophosphate. The word “tetra” sometimes gets dropped for the sake of space or simplicity. In rare cases, chemists will specify another form, but that’s not common in regular consumer products.
Safety and Regulation Matter
Having cooked quite a bit and read the back of countless boxes in my kitchen, these differences aren’t just word games. Chemical form changes how a compound interacts with the rest of a recipe, or what happens inside a dishwasher, or even what ends up in the water after a rinse. The U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) both keep close watch over the use of pyrophosphates in food, including their acceptable limits and the exact forms allowed. The forms with fewer sodium atoms, like disodium pyrophosphate, act a little differently—sometimes as leavening agents, sometimes to keep food from turning brown. Tetrasodium pyrophosphate, the star of most “sodium pyrophosphate” labels, often helps with texture, stability, or water retention in foods like canned tuna or marshmallows.
In water treatment, tetrasodium pyrophosphate softens water or prevents mineral buildup. Each application counts on the chemical behaving just the way it’s designed to, and switching to the wrong form risks throwing off the product or the safety of what we eat and use every day.
Consumer Clarity and Solutions
Misunderstandings tend to fuel anxiety. Reading a long, unfamiliar name on a food label or a detergent box can spark confusion. Some shoppers worry about additives, and the mix-up between “sodium pyrophosphate” and “tetrasodium pyrophosphate” only muddies the water. Companies who care about gaining trust ought to put clearer information on their ingredient lists. If a product contains tetrasodium pyrophosphate, it helps to say so directly. Even a parenthetical “(as tetrasodium pyrophosphate)” after “sodium pyrophosphate” could cut down on customer confusion or internet searches.
There’s room for better education, too. School science texts, food safety campaigns, and customer support lines can do a better job explaining what’s really on that label and why it’s there. Building trust starts with clarity, and those details matter, especially to people managing health conditions that require keeping an eye on sodium or food additives.
Focusing on Trust and Transparency
Easy access to clear information about what goes into food and household goods is the foundation of an informed society. As someone who checks labels for personal reasons, I find relief in seeing full names and detailed explanations—less mystery in what I’m actually bringing to the table or using around the house. Scientists, regulators, and brands have to keep consumers in mind each step of the way, from naming conventions to educational outreach.
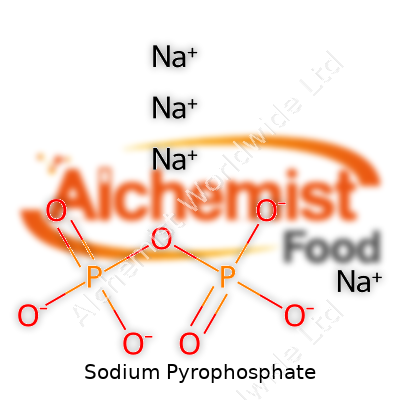

| Names | |
| Preferred IUPAC name | Disodium dihydrogen diphosphate |
| Other names |
tetrasodium pyrophosphate TSPP disodium dihydrogen diphosphate sodium diphosphate sodium phosphate, tetrabasic |
| Pronunciation | /ˌsoʊdiəm paɪˈrɒfəsfeɪt/ |
| Preferred IUPAC name | disodium diphosphate |
| Other names |
Disodium diphosphate Disodium pyrophosphate Tetrasodium pyrophosphate Sodium diphosphate Sodium phosphate, pyrophosphate |
| Pronunciation | /ˌsoʊdiəm paɪˈrɒfəsfeɪt/ |
| Identifiers | |
| CAS Number | 7722-88-5 |
| Beilstein Reference | 0109365 |
| ChEBI | CHEBI:18300 |
| ChEMBL | CHEMBL1377 |
| ChemSpider | 53552 |
| DrugBank | DB09114 |
| ECHA InfoCard | ECHA InfoCard: 035b363e-af7f-4a69-9dfe-fcd29fd8be6f |
| EC Number | 231-767-1 |
| Gmelin Reference | 11130 |
| KEGG | C02573 |
| MeSH | D011007 |
| PubChem CID | 24815 |
| RTECS number | UX8970000 |
| UNII | K8QBT1A664 |
| UN number | UN 3077 |
| CAS Number | 7722-88-5 |
| Beilstein Reference | 1811237 |
| ChEBI | CHEBI:18300 |
| ChEMBL | CHEMBL1201571 |
| ChemSpider | 11755 |
| DrugBank | DB11152 |
| ECHA InfoCard | ECHA InfoCard: 034-050-00-6 |
| EC Number | 231-767-1 |
| Gmelin Reference | 13796 |
| KEGG | C00690 |
| MeSH | D011165 |
| PubChem CID | 24857 |
| RTECS number | UI7450000 |
| UNII | O352J1J9HX |
| UN number | UN3278 |
| Properties | |
| Chemical formula | Na4P2O7 |
| Molar mass | 221.94 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.86 g/cm³ |
| Solubility in water | soluble |
| log P | -4.71 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.3 |
| Basicity (pKb) | 11.63 |
| Magnetic susceptibility (χ) | -59.0e-6 cm³/mol |
| Refractive index (nD) | 1.451 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.57 D |
| Chemical formula | Na4P2O7 |
| Molar mass | 221.94 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.534 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -8.64 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.3 |
| Basicity (pKb) | 11.95 |
| Magnetic susceptibility (χ) | −59.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.45 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.64 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 266.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2881 kJ/mol |
| Std molar entropy (S⦵298) | 266.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2910 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2889.7 kJ/mol |
| Pharmacology | |
| ATC code | A09AA06 |
| ATC code | A01AD11 |
| Hazards | |
| Main hazards | May irritate eyes, skin, and respiratory tract. Harmful if swallowed. |
| GHS labelling | **GHS07, GHS09** |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 Oral Rat 3,100 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 7100 mg/kg |
| NIOSH | WH2600000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 860 mg/kg |
| Main hazards | Harmful if swallowed; causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 0, Instability: 0, Special: - |
| Lethal dose or concentration | LD50 (oral, rat): 4,100 mg/kg |
| LD50 (median dose) | LD50 (median dose): 4,100 mg/kg (rat, oral) |
| NIOSH | WT0825000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 6 mg/kg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Disodium phosphate Tetrasodium pyrophosphate Trisodium phosphate Sodium triphosphate |
| Related compounds |
Disodium pyrophosphate Tetrasodium pyrophosphate Potassium pyrophosphate Calcium pyrophosphate Pyrophosphoric acid Sodium phosphate |

