Sodium Metabisulphite: Insights and Real-World Perspective
Historical Development
People started using sodium metabisulphite long before “chemical safety” became a household term. Back in the late 19th century, it popped up in food and beverage preservation, as wine makers found out it kept their product fresher, longer. With a sharp, acidic bite, it did more than keep grapes from turning. I’ve talked to family members who remember seeing this compound in the ingredient label of dried fruits as far back as the 1950s. Over time, the ability of sodium metabisulphite to control microbes, scavenge oxygen, and bleach made it a regular in industries like textiles, water treatment, and photography. The spread of large-scale food production especially brought it to the fore, since safety without refrigeration meant the difference between profit and public health scares.
Product Overview
What stands out with sodium metabisulphite is its powdery, solid form—a white or slightly yellowish crystal that gives off a sharp, sulfurous smell. This stuff gets loaded into drums or multi-layered bags to keep it dry, since it reacts quickly with moisture. It usually comes labeled for food, industrial, or technical grade, and the intended use dictates purity. While someone working in a candy factory looks for one kind, an engineer at a water treatment facility picks another grade entirely. The basics never really change; it’s still the same robust compound at its core.
Physical & Chemical Properties
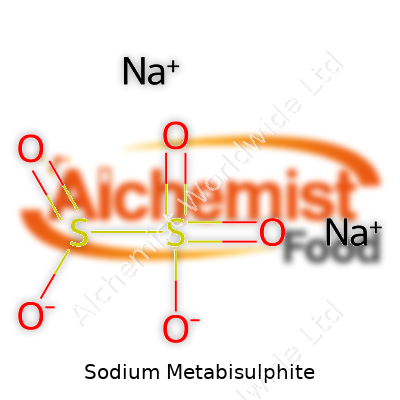
Sodium metabisulphite carries the formula Na2S2O5, with a molecular weight of about 190.10 g/mol. The crystals dissolve in water, creating an acidic solution, and the smell punches through most storage rooms. The melting point sits around 150°C, and it starts breaking down in damp air, releasing sulfur dioxide gas. It’s that gas that proves tricky: one whiff lingers in your nose for hours, and the residue gets everywhere. Chemically, it acts as a reducing agent, able to change the oxidation state of other substances. Water solubility ranks high, but alcohol leaves it mostly untouched.
Technical Specifications & Labeling
Labels on sodium metabisulphite containers call for close reading, especially if the product moves between countries. Purity shows as a percentage, often hitting 96% or higher for food and pharmaceutical markets. Heavy metals must stay below flagged limits, with iron, arsenic, and lead warranting regular testing. For food, labels feature the E223 tag in Europe. UN and CAS numbers show up in shipping paperwork, and hazard pictograms warn even the unwary to steer clear. In my experience working alongside warehouse teams, reading every line on a drum of this material saves countless headaches later. Out of spec shipments don’t just waste time; they raise questions with regulators no manufacturer wants.
Preparation Method
Factories prepare sodium metabisulphite by bubbling sulfur dioxide through a sodium carbonate or sodium hydroxide solution, cooling the resulting liquor, and letting crystals form out as the temperature drops. Workers shovel, scrape, and bag this by the ton. Each step depends on precise temperature controls. Once, touring a plant in southern China, I saw seasoned operators helping new recruits watch the color shift and the density of the mix for reliable results.
Chemical Reactions & Modifications
Sodium metabisulphite’s core feature remains its ability to release sulfur dioxide when wetted or heated. Adding acids boosts this reaction, pumping out enough SO2 to sanitize or bleach. In industrial processes, it offers a simple route for removing excess chlorine in water, and engineers rely on the speed of its reaction to ensure safety for downstream uses. Some research teams experiment with doping sodium metabisulphite with small amounts of other salts, aiming to fine-tune its behavior in custom food production or specialty chemicals. As a reducer, it pairs well with aldehyde removal or as a preservative ingredient where fast oxygen elimination matters.
Synonyms & Product Names
Switching between sodium pyrosulphite, disodium metabisulfite, and the simple “meta” among industry folks can leave newcomers confused. Some labels print it as E223, S223, or simply “preservative salt.” In the US, food-grade material often lands in retailers branded as “fruit preservative.” For shipping, you’ll see “sodium metabisulphite” on manifests, but regional preferences and translations result in varied spelling and terms. This naming mess complicates things: a friend importing wine additives once found himself stuck because customs flagged his container for unclear terminology.
Safety & Operational Standards
Sodium metabisulphite scores as both useful and hazardous, depending on how it’s handled. Inhalation of dust or sulfur dioxide gas burns the eyes and lungs. Occupational exposure limits, like OSHA and NIOSH guidelines, urge the use of gloves, goggles, and adequate ventilation. Emergency rooms regularly see workers with skin and respiratory irritation after spills or accidental mixing with acid. I’ve watched managers triple-check storage—dry places, sealed drums, and clear secondary containment to avoid both water contact and cross-contamination with oxidizers or acids. In packaging facilities, any standards slip-up becomes obvious quickly: acrid odors, signs of corrosion, or workers reporting headaches.
Application Area
In my time consulting with beverage producers and food manufacturers, sodium metabisulphite kept showing up as the go-to for preserving color and shelf life. Winemakers and brewers count on it for microbial control and stopping wild fermentation. Bakeries use it to bleach flour, while shrimp processors dip their catch to extend freshness. Out in the field, denim manufacturers add it to bleach and soften fabric. Pool and water plant operators use it to dechlorinate both incoming and outgoing water. Every few years, another sector finds a creative use, as in mining where it reduces cyanide in gold recovery.
Research & Development
Universities and companies test new uses or work to minimize downsides. In food tech seminars, researchers spotlight ways to curb its flavor impact and allergen potential while keeping the antimicrobial effect. New formulations and blends seek out better results, lower dosages, and improved safety. Over the past decade, many research programs focused on automated dosing, aiming to avoid the health risks tied to inhaling or handling the raw product. Papers published in toxicology journals dig into mechanisms of irritation and immune response, helping guide both regulations and label warnings.
Toxicity Research
No one takes the risks of sodium metabisulphite lightly, especially after high-profile cases of allergic reactions. Consumption above regulated limits triggers respiratory issues, especially for asthmatics. Acute oral and dermal toxicity studies, carried out in animals, flagged low but real risks. Chronic exposure pulls up more concerning results, such as sulfite sensitivity and exacerbation of existing conditions. In my contacts with allergists, they stress that undeclared metabisulphite in food poses a serious issue. Regulatory agencies continue to demand more transparency in food labels and clinical studies, and some suggest cap limits should drop again by 2030.
Future Prospects
Demand for sodium metabisulphite does not look to slow down. As food supply chains stretch across borders and extreme weather raises storage challenges, shelf life enhancers look increasingly valuable. The biggest pressure points fall around health and environmental impact. Biochemists join hands with product developers, working on lower-impact versions or replacements sourced from renewable inputs. In sustainable agriculture, sodium metabisulphite-free alternatives gain ground, but cost still tips the scales. Among policy makers, stricter labeling, worker protections, and exposure monitoring move from discussion to draft legislation. For those on the frontlines, practical steps—better PPE, automated dispensing, and smarter storage—promise to reduce risks without giving up the compound’s core benefits.
What is Sodium Metabisulphite used for?
Hidden in Plain Sight
If you ever checked the ingredients label of dried fruit or wine, you’ve seen sodium metabisulphite. Many winemakers reach for it to keep their reds and whites from turning funky. This stuff shows up in food so your apricots stay orange and your shrimp look fresh at the market. Most folks never worry about what keeps those golden raisins cheerful, but behind the scenes, sodium metabisulphite is quietly doing the heavy lifting.
Food Saver, Not Just Preserver
Commonly tossed into fruits and wines, sodium metabisulphite blocks bacteria, yeast, and molds, cutting down on waste. Work in food service long enough, you learn that spoilage eats into budgets fast. Keeping things out of the compost keeps restaurants and groceries open, especially for small operators. Sodium metabisulphite beats back oxidization, too, saving color and flavor when air would otherwise ruin a good batch. Nobody wants a lemon that looks like a turnip or wine that tastes like vinegar.
Beyond Eating and Drinking
My first job out of school landed me in a small textile storehouse. Some mornings we treated wool with sodium metabisulphite in the dye process. Without it, bright colors fade or go patchy. It wasn’t glamorous, but workers depended on reliable dye runs to put food on the table. In water treatment, I saw engineers drop buckets of sodium metabisulphite into big tanks, stripping chlorine out of drinking water. Hospitals and labs use it, too, sanitizing equipment or even processing blood samples. The public rarely gives a thought to chemicals that keep daily life ticking, but behind closed doors, industries lean on this powder for smooth running.
Safety Concerns and Realities
Sodium metabisulphite doesn’t come without risks. At high doses, it can cause breathing trouble, headaches, or allergic reactions—especially for people with asthma. Regulations cap the amount used in foods and drinks. Even with those limits, some people react to trace amounts. As someone who’s had asthma since childhood, I grew up checking labels, especially when eating out or trying new snacks. Consumers deserve honesty about ingredients, with transparent labeling and well-trained food workers who get how serious reactions can get. The chemical’s benefits should never outrank basic public health or trust.
Room for Improvement
Modern supply chains push for fewer additives, and some companies look for natural methods—good ventilation, smarter packaging, or improved refrigeration. Tech keeps advancing, so newer alternatives might side-step some health concerns. At home, more people now check packaging, preferring fewer preservatives. Education helps. By understanding why sodium metabisulphite shows up in so many products, shoppers can weigh the risks and benefits and press for safer options.
Why This Matters
Sodium metabisulphite sits at the crossroads between science and daily living. It’s in the wine we celebrate with, the snacks packed for a hike, even the water in our homes. People rely on it to reduce waste and keep costs down, yet health questions persist. Building awareness, using it responsibly, and hunting for alternatives won’t end the conversation anytime soon. The more we know about what preserves our food, colors our world, and keeps water safe, the better choices we can make for ourselves and our families.
Is Sodium Metabisulphite safe for consumption?
What Is Sodium Metabisulphite?
Sodium metabisulphite kicks around in all sorts of products, from dried fruit to wine. It’s a white, powdery preservative you often find tucked away in ingredients lists where it stops food from browning, keeps bacteria out, and helps those bright colors hang around. If you’ve ever eaten anything with “E223” on the label, you’ve tried it. It’s not just food, either—this compound pops up in winemaking, pharmaceuticals, and even wastewater treatment because of its knack for stopping spoilage and acting as an antioxidant.
How Does the Body Handle It?
Once you eat sodium metabisulphite, the body does its job. Sulphites break down mostly in the digestive tract and get flushed out. Most people don’t notice a thing, so from an everyday perspective, it’s no different than eating other common preservatives. The science backs this up. Regulatory bodies, like the Food and Drug Administration and European Food Safety Authority, studied its effects over decades. They both say sodium metabisulphite is generally safe up to certain levels—specifically, an acceptable daily intake of 0.7 mg per kg of body weight.
Where Risk Creeps In
Problems start for people who react to sulphites. Sulphite sensitivity mostly affects folks with asthma. Reactions might show up as wheezing, shortness of breath, hives, or even stomach upset. Severe cases don’t come around often, but they do happen. In my family, someone with asthma once had a mild episode after eating a fruit salad laced with preservative. For most people, though, a regular diet packed with pizza, packaged snacks, and wine might never bring these symptoms. The overall risk stays low, as long as you know your own body and pay attention to understanding food labels.
Numbers That Matter
It pays to check the numbers. Studies in the U.S. and Europe show average intake lands well below the strict limits. A typical meal with dried apricots, wine, and a slice of cake rarely puts anyone close to the daily maximum. Too much of anything can cause trouble, but real-world data shows most people never even get close. And, authorities require clear labelling if levels top 10 mg per kilogram in a food product. If you see “contains sulphites,” you know what’s included.
Industry and Consumer Choices
Plenty of businesses keep looking for alternatives, especially as more people get wary of processed ingredients. Some use natural antioxidants, like ascorbic acid (vitamin C), or experiment with shelf-stable packaging. Manufacturers already trim down how much preservative they use, and they run tests for quality every step of the way. For people looking to play it safe, reading labels and refreshing your food routine with more fresh or home-cooked meals reduces exposure without stress.
Building Trust and Transparency
It matters that people know what they’re eating and why. Brands and supermarkets step up by listing ingredients clearly and answering questions through customer service or online Q&As. No one needs to take unnecessary risks. Simple transparency and up-to-date information help consumers make smarter choices, especially those who need to watch sulphite levels for health reasons.
Looking Forward
Staying alert to new research and better alternatives helps everyone. Food science doesn’t stand still; it moves alongside research, government oversight, and changing public attitudes. Sharing solid facts and listening to honest questions keeps communities safe and informed.
What are the common applications of Sodium Metabisulphite?
Food Preservation and Safety
Walk through any commercial kitchen or restaurant, and you’ll likely run into sodium metabisulphite without even realizing it. For decades, food processors have used this compound to keep dried fruit, potato products, and wines fresh. It prevents browning, fighting off bacteria and mold before they can settle in. Having grown up around family friends who ran a small dried fruit business, I remember the process well—the big steel vats, the glimmering slices of apricot, the careful measuring of white powder before sealing everything away. This method keeps color and texture intact, making the food visually appealing and safe long after packaging. The FDA keeps close tabs here, too, requiring food labels for any use of sulphites due to allergy risks.
Cleaning and Disinfection
Sodium metabisulphite goes beyond the kitchen; janitors and industrial cleaners rely on it as a bleaching and sanitizing tool. In breweries or juice factories, it breaks down stubborn residues in pipes and tanks. It kills germs and keeps unwanted wild yeast strains from causing trouble. Growing up in a small town with several local breweries, I saw first-hand how stubborn stains can be on brewing equipment—and how efficiently sodium metabisulphite could clean it all out with a quick soak and rinse. For home brewers, it offers a simple solution for clean equipment: dissolve, soak, rinse, get on with the fun part.
Water Treatment
Municipal engineers and maintenance crews regularly turn to sodium metabisulphite to treat water and wastewater. After chlorine has done its job killing off bacteria in water meant for drinking, this compound helps neutralize leftover chlorine so that water doesn't carry an unpleasant taste or odor. Towns with aging water systems have to manage this balancing act every day, ensuring water is disinfected but also safe and palatable by the time it reaches homes and schools. For me, hearing city workers talk about “dechlorination” brought these chemical processes down to earth—it’s about safe water with no strange aftertaste.
Mining and Industrial Uses
In mining operations, sodium metabisulphite acts as a key ingredient during ore processing. Miners use it to separate precious metals like gold and copper from raw rock, reducing unwanted substances and increasing recovery rates. These operations can have big environmental footprints, so the push for safer handling and responsible waste management never lets up. Industrial chemical suppliers often recommend handling and disposal procedures not only for the workers’ sake, but also out of concern for soil and groundwater nearby.
Potential Risks and Better Practices
Despite its many strengths, sodium metabisulphite prompts some concern about health and environmental safety. People with asthma or sulphite sensitivity can experience severe reactions even to small amounts, so food safety rules keep this risk front and center. In workplaces, personal protective equipment and good ventilation cut down on accidental exposure to fumes and dust. Tackling industrial runoff calls for treatment systems that trap or neutralize waste before it seeps into rivers or farmland. These steps turn a useful chemical into a safe tool—for businesses, families, and communities alike.
Looking Ahead
Innovation continues around this unassuming powder. Researchers explore safer alternatives for food preservation and water treatment. Producers keep looking for more efficient ways to recycle or treat waste output. For anyone who buys dried fruit, sips on wine, or enjoys clean drinking water, understanding what goes into everyday products and systems turns technical jargon into something real—a reminder that science, safety, and daily life constantly intersect.
How should Sodium Metabisulphite be stored?
Understanding the Substance
Sodium metabisulphite pops up in all sorts of industries – food, photography, and even water treatment. It works as a preservative and an antioxidant. From all my years working around chemicals, I know it packs a punch for such a small compound. But it also demands respect. Breathe in too much dust or let it mix with the wrong stuff, and you end up with problems – from breathing trouble to caustic fumes. Handling it with care makes all the difference.
Choosing the Best Storage Area
A cool, dry spot keeps sodium metabisulphite stable. Humidity turns it soggy and gets reactions going. At my old workplace, we learned the hard way: a leaky roof brought on clumping and a harsh sulfur smell that clung to the building for days. Dry, well-ventilated rooms with low humidity levels don’t just slow down spoilage. They help protect workers’ lungs too.
Keep it away from sources of heat and sunlight. Heat breaks it down and triggers chemical changes that nobody wants inside the warehouse. Early in my career, a supplier stored it near a skylight to save floor space. Sunlight crept in, the temperature rose, and half the product spoiled before anyone caught on.
Sealed and Secure Containers
Use tough containers with air-tight lids. I prefer high-density polyethylene drums, but steel with a protective lining also works well. Cardboard and thin plastic don’t hold up – one mishap with a slightly damp floor and you’ll have a sticky, stinging mess to clean up. Every reputable supplier knows to ship sodium metabisulphite sealed tight in bags made to handle chemicals. Once you open those bags, transfer everything to a secondary container right away, and label it clearly.
Keep Away from Water and Acids
Anyone who lets sodium metabisulphite come near water or acids without controls isn’t just risking product loss – they’re risking their health. Contact with water releases sulfur dioxide gas, and mixing with acids cranks up that reaction. You do not want a cloud of choking gas in a storeroom. Clean spills right away, and never leave it where a leak or flood can reach it.
Always Mark and Manage Properly
Clear labeling should include the name, handling instructions, and hazard warnings. In facilities I’ve worked with, the safest storage rooms don’t just have signs. Staff get regular reminders and access to protective equipment right at the entrance. Gloves, goggles, and dust masks help with both handling and emergencies.
Regular checks guard against leaks, damaged packaging, and improper stacking. Starting and ending each shift with a quick sweep kept our team safe for years. Storing incompatible chemicals apart, such as acids, took only a bit of extra planning but saved a lot of stress.
Supporting Safety with Good Habits
Training matters most. Workers who know how to store and handle sodium metabisulphite spot risks sooner and react quicker. In my experience, keeping up with local regulations – like OSHA’s chemical storage requirements – leads to fewer accidents and a more confident team.
Safe storage builds on routine, not luck. Dry, cool, labeled, and away from water – these basics work day after day, no matter the industry. Regular checks and the right containers help keep sodium metabisulphite useful and people healthy.
What are the potential health hazards of Sodium Metabisulphite?
Why This Chemical Pops Up So Often
Sodium metabisulphite often shows up in places that fly under the radar for most people. It keeps dried fruits brightly colored on grocery shelves, prevents bacterial growth in wines and beers, and hides in water treatment plants. You probably walk by a drum of it on a factory tour without even knowing it. I still remember finding it on a food label and having no clue what it meant, and that's where some problems can start.
What Actually Happens to the Body?
Start with the basics: sodium metabisulphite is a powerful preservative, but once it mixes with water (or your lungs, eyes, or digestive system), it turns into sulfur dioxide gas. If you've ever been hit by a plume of the stuff, you know it's more than just a bad smell. Inhaling even small amounts can tickle your throat, squeeze your chest, and stir up coughing. I've met workers in food processing and warehouses who had to step away after handling a spill. People with asthma or lung problems often bear the brunt — their bodies react fast and hard, sometimes with scary asthma attacks that send them to urgent care.
Everyday Reactions Aren’t Always Obvious
Not everyone winds up with dramatic symptoms, and that sometimes leads folks to brush off the hazards. Headaches, skin rashes, and hives can sneak up after eating food preserved with the chemical. According to the FDA, hypersensitivity reactions are well-documented, and the numbers go up in places with heavy reliance on processed foods. In a busy restaurant kitchen, stray powder drifting from an open bag can leave people rubbing their eyes for hours or breaking out in red blotches on their skin. This isn't just theory; I've seen it in kitchens during college jobs and later, talking with allergy sufferers who avoid certain snack aisles.
High Doses Come at a Cost
Accidental overexposure has even more severe consequences. Swallowing too much sodium metabisulphite can bring nausea and vomiting. Workers splashed in the eyes need quick attention to avoid vision damage. The Centers for Disease Control and Prevention detail cases of corneal burns and permanent eye injuries. Repeated workplace contact cracks skin and brings painful dermatitis. The facts don’t leave much room for sugarcoating: this isn't a gentle or forgiving chemical.
Small Actions Lower the Risks
Read food labels with suspicion if you notice unexplained headaches or skin reactions. Allergic folks can ask restaurants which preservatives make it into sauces or baked goods. Employers can fit out staff with gloves, goggles, and working hoods. Awareness isn't costly — most reactions get worse because people don’t link their symptoms with the preservative in question. Even simple washing and ventilating work areas goes a long way toward keeping people safe. Schools and companies can remind workers that just because a powder comes in a food-grade container, it can still put your health in the crosshairs if you aren’t careful.
Keep the Risks From Getting Lost in the Fine Print
Sodium metabisulphite isn’t a mystery, but the way it shows up in daily life can catch people off guard. Taking the time to learn and share the facts matters — in the grocery aisles, at work, and in restaurants. I’ve seen people get sick from ignoring warnings and watched others steer clear simply by paying attention. As more processed food lines the shelves, and industries look for quick solutions, remembering the real health trade-offs keeps everyone a bit safer and a little wiser.
| Names | |
| Preferred IUPAC name | sodium disulfite |
| Other names |
Disodium disulfite Sodium pyrosulfite |
| Pronunciation | /ˌsəʊdiəm ˌmɛtəˈbaɪsʌlfaɪt/ |
| Preferred IUPAC name | sodium disulfite |
| Other names |
Disodium Metabisulfite Sodium Pyrosulfite SMBS E223 |
| Pronunciation | /ˌsəʊdiəm ˌmɛtəˈbaɪsʌlfaɪt/ |
| Identifiers | |
| CAS Number | 7681-57-4 |
| Beilstein Reference | 3592228 |
| ChEBI | CHEBI:38101 |
| ChEMBL | CHEMBL1357 |
| ChemSpider | 9470 |
| DrugBank | DB14527 |
| ECHA InfoCard | 100.028.258 |
| EC Number | 231-673-0 |
| Gmelin Reference | 774937 |
| KEGG | C00719 |
| MeSH | D017646 |
| PubChem CID | 24846 |
| RTECS number | UX8225000 |
| UNII | 0O7P44A5SP |
| UN number | UN 3077 |
| CAS Number | 7681-57-4 |
| Beilstein Reference | 0860462 |
| ChEBI | CHEBI:38101 |
| ChEMBL | CHEMBL1353 |
| ChemSpider | 6926 |
| DrugBank | DB14526 |
| ECHA InfoCard | 100.012.178 |
| EC Number | 231-673-0 |
| Gmelin Reference | Gmelin Reference: 9284 |
| KEGG | C07343 |
| MeSH | D017190 |
| PubChem CID | 24406 |
| RTECS number | UX8225000 |
| UNII | AGG2FN16EV |
| UN number | UN 3077 |
| CompTox Dashboard (EPA) | DTXSID4020089 |
| Properties | |
| Chemical formula | Na₂S₂O₅ |
| Molar mass | 190.107 g/mol |
| Appearance | White or pale yellow crystalline powder |
| Odor | Slight sulfur dioxide odor |
| Density | 1.48 g/cm³ |
| Solubility in water | soluble |
| log P | -3.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | Acidity (pKa): 1.2 |
| Basicity (pKb) | 6.6 |
| Magnetic susceptibility (χ) | 'Slightly diamagnetic' |
| Dipole moment | 1.45 D |
| Chemical formula | Na2S2O5 |
| Molar mass | 190.107 g/mol |
| Appearance | White or pale yellow crystalline powder |
| Odor | Faint smell of sulfur |
| Density | 1.48 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | Acidity (pKa): 7.0 |
| Basicity (pKb) | 6.6 |
| Magnetic susceptibility (χ) | **−62.0·10⁻⁶ cm³/mol** |
| Refractive index (nD) | 1.448 |
| Viscosity | Viscous when dissolved in water |
| Dipole moment | 1.29 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 143 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -941 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -944.7 kJ/mol |
| Std molar entropy (S⦵298) | 149 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -941 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -944 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | V03AB02 |
| ATC code | A16AX14 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes severe skin burns and eye damage. May cause allergy or asthma symptoms or breathing difficulties if inhaled. May cause an allergic skin reaction. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07,GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H318, H335 |
| Precautionary statements | P264, P270, P271, P301+P330+P331, P304+P340, P305+P351+P338, P306+P360, P312, P330, P340, P391, P403+P233 |
| NFPA 704 (fire diamond) | 2-0-1-W |
| Autoignition temperature | > 440°C |
| Lethal dose or concentration | LD₅₀ oral rat: 1,130 mg/kg |
| LD50 (median dose) | 1310 mg/kg (rat, oral) |
| NIOSH | NIOSH: WS5600000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium Metabisulphite: 5 mg/m³ |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | 570 mg/m3 |
| Main hazards | Harmful if swallowed. Causes serious eye damage. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H318, H334 |
| Precautionary statements | P264, P270, P271, P280, P301+P330+P331, P304+P340, P305+P351+P338, P312, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 2-0-1-W |
| Lethal dose or concentration | LD₅₀ (oral, rat): 1,540 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 1,130 mg/kg |
| NIOSH | RW6400000 |
| PEL (Permissible) | PEL (Permissible): 5 mg/m³ |
| REL (Recommended) | 400 mg/kg |
| IDLH (Immediate danger) | > 0.5 mg/m³ |
| Related compounds | |
| Related compounds |
Sodium bisulfite Sodium sulfite Sodium thiosulfate Potassium metabisulfite Sulfur dioxide |
| Related compounds |
Sodium bisulfite Sodium sulfite Potassium metabisulfite Sulfur dioxide Sodium thiosulfate |

