Sodium Metabisulfite: A Deep Dive Into Its Journey and Role
Historical Development
Sodium metabisulfite has roots stretching back to the early 19th century, arriving at a time when chemistry was making enormous leaps in both Europe and America. Chemists explored how sulfur could tweak the preservation of food and slow down unwanted chemical changes. Over the decades, folks in food, textiles, and paper saw clear benefits from this compound. In the 20th century, winemakers picked up sodium metabisulfite to control unwanted microbes and keep wine fresh in the bottle. The chemical industry soon adapted the compound for water treatment and as a reductant in dyes. I remember reading about old-school photographic processes, where sodium metabisulfite featured as a key fixer. Shifts in technology, taste, and global regulations since the 1970s widened research and led to well-documented industrial production. Today, the chemical no longer belongs only in the hands of scientists but shows up in everyday life — from your morning juice concentrate to municipal water treatment tanks.
Product Overview
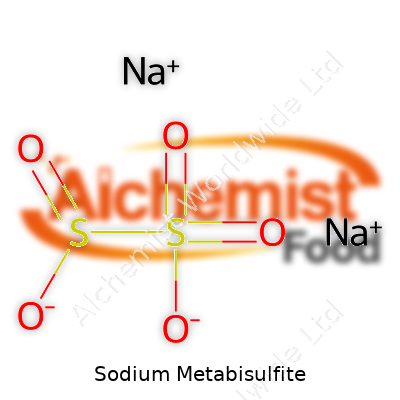
Sodium metabisulfite usually appears as a white or faintly yellow powder or crystalline solid, packing a sharp odor like sulfur dioxide. Chemists label it with the formula Na₂S₂O₅. If you've worked with food additives, you would know it's registered under E223. This compound gets shipped in bags, drums, or bulk tankers; users need to keep it sealed up tight and away from moisture, since it breaks down in damp air. The stuff gets a lot of use balancing out chemical reactions, acting as a preservative, or scavenging oxygen in closed systems. Familiar synonyms pop up in procurement documents: disodium disulfite, sodium pyrosulfite, and even the less fancy “metabite.” For industries that need big volumes of material that stays stable during long storage, sodium metabisulfite checks those boxes.
Physical & Chemical Properties
You may notice that this compound dissolves in water like sugar — quickly, and with a slight cooling effect. Its molar mass clocks in at 190.1 grams per mole. The melting point is tricky, since sodium metabisulfite decomposes to sodium sulfite and sulfur dioxide before reaching a true melt. That gas coming off offers a sour, choking smell, which says something about why working with sodium metabisulfite in a closed room feels uncomfortable. It reacts strongly with acids, kicking off sulfur dioxide gas, which can suffocate or cause pulmonary problems quickly. In dry form, it sits stable on a shelf, but moisture and heat trigger breakdown. For anyone handling it, gloves matter — the powder can leave the skin red and sore after long exposure.
Technical Specifications & Labeling
Industry standards stick close around sodium metabisulfite supplies. Pure grades must meet minimum sodium metabisulfite content, usually not less than 97% by weight, and impurities like heavy metals such as lead or arsenic can’t rise above a few ppm. Food-grade stocks have even tighter controls. Containers feature bright hazard pictograms warning about corrosive hazards and asthmatic reactions. Whether going into wine production, water treatment, or industrial bleaching, every shipment comes with a certificate stating origin, purity, batch number, and safety specifications. I’ve dealt with shipments where labeling mistakes brought regulatory headaches — fines and hold-ups because trace mercury content didn’t match the label. Compliance with standards from organizations like the US FDA, EU EFSA, or China’s GB means sellers and buyers keep a close eye on technical paperwork.
Preparation Method
Factories make sodium metabisulfite through a multi-step process. First, manufacturers dissolve sodium carbonate or sodium hydroxide in water, then bubble sulfur dioxide through the mixture. This step yields sodium bisulfite, which gets further reacted with more sulfur dioxide under controlled heat. The resulting solution is evaporated and crystallized, then centrifuged and dried. The big challenge comes with keeping impurities out: even low levels of iron, copper, or organic material can trigger off-odors or unwanted color reactions in later applications. Scale, water quality, and process temperature all matter. Automation helps, making sure CO₂ isn’t contaminating feed gas and that raw materials stay consistent batch to batch. Plants paying attention to these details land the best contracts with pharmaceutical and food partners.
Chemical Reactions & Modifications
Chemists and industrial engineers get creative with sodium metabisulfite. Drop some in dilute acid, and you’ll see a real-time release of sulfur dioxide gas used for sanitizing wine barrels or controlling pH in swimming pools. Mix it into an alkaline solution, and the equilibrium shifts toward various sulfite anions, which end up reducing active chlorine or neutralizing oxidants. In organic synthesis, sodium metabisulfite often serves as a mild reducing agent; it helps snap double bonds, clean up aldehyde contaminants, or convert brominated alkanes to non-reactive, stable products. Textile labs use minor tweaks to the compound for specialized dye applications. There’s research on blending sodium metabisulfite salts with calcium or magnesium to make slow-release agents for water treatment. All that hands-on experimentation in local labs and factories ends up feeding new patents and industrial tweaks every few years.
Synonyms & Product Names
Suppliers and buyers worldwide deal with a string of product names and synonyms. You’ll hear sodium pyrosulfite in older European technical manuals. In other places, the term "disodium disulfite" shows up, and in Spanish-speaking markets, "metabisulfito de sodio" dominates. Bulk shipping paperwork and safety data sheets sometimes call it "E223," matching the European food regulatory system. Most large chemical distributors, such as BASF, Solvay, or Sinopec, maintain a careful product registry, avoiding confusion for cross-border orders. My own experience has shown that every synonym can reflect a slightly different regulatory or purity spec, and closely checking specifications avoids plenty of on-the-ground mishaps.
Safety & Operational Standards
Commercial and home environments both benefit from clear safety guidelines with sodium metabisulfite. Short-term or chronic inhalation of vapors can bring on acute asthma attacks, so personal protective equipment should be mandatory in handling zones. Most companies require gloves and eye protection since even tiny quantities can inflame skin or eyes. For food processing, maximum permitted levels are set and monitored by agencies like the FDA and WHO, with limits sitting around 100-200 ppm in finished foods, depending on the use. In case of spills, fast evacuation and ventilation matter more than fancy equipment — sulfur dioxide gas can build up rapidly, especially near drains. Fire and building codes treat mixing, transfer, and storage as “moderate hazard” activities, and I remember municipal inspectors shutting down a bottling line for keeping sodium metabisulfite in unsealed bulk totes. The rules aren’t there to slow down operations, but to keep workers and end-users out of the emergency room.
Application Area
Ask around any food plant, water treatment facility, or brewery, and you’ll hear plenty about sodium metabisulfite’s roles. Its job in food revolves around stopping bacterial growth and preserving the bright color of dried fruit or vegetables. In beverage processing, winemakers count on its ability to prevent wine from turning brown or taking on spoiled flavors. Water treatment plants count on its reducing power for scrubbing chlorine from pipelines before water enters natural streams. In pulp and paper mills, it helps bleach fabrics and neutralize leftover oxidizing agents after disinfection. Pharmaceuticals tap sodium metabisulfite to stabilize injectable drugs or remove trace oxidants. Textile and tanning operations use it to rinse dyes or prepare leathers for softening. Each sector balances cost, effectiveness, and safety, so sodium metabisulfite often wins out over pricier, less flexible chemicals.
Research & Development
Laboratories work hard refining sodium metabisulfite’s properties. Research teams focus on boosting safety for food and medical uses, finding ways to purify the chemical more cheaply, and investigating nano-scale applications for anti-microbial packaging. Process engineers ditch old, energy-heavy drying systems for more efficient technologies that yield finer, easier-to-dissolve powders. Some researchers look at blending options, pairing sodium metabisulfite with other antioxidants in complex food recipes, hoping to use lower overall doses. Academic papers talk about the environmental impact of waste metabisulfite in large-scale manufacturing, recommending capture and recycling instead of simple disposal. Others dive into the biology, studying how bacteria and fungi adapt to low levels of sodium metabisulfite in closed food systems, which matters for food security and shelf-life extension. The innovation isn’t just about a better product: it’s about doing more with less waste and risk.
Toxicity Research
Toxicologists have dug into sodium metabisulfite effects in humans, animals, and the environment. At low levels, most people tolerate the compound, but those with asthma or sulfite sensitivity may show symptoms after inhaling or ingesting small amounts, including shortness of breath and tightness in the chest. Food scientists monitor residue levels in commercial products, relying on strict international standards to keep daily intake well below risk levels for the vast majority. Studies show that the compound breaks down rapidly in the gut, with most adults safely metabolizing small exposures. Chronic workplace exposure tells a different story. Construction and pool maintenance crews sometimes develop skin conditions or aggravated asthma after years around sodium metabisulfite. Lethal doses for laboratory animals sit orders of magnitude above what one might see in daily life, but the science keeps up the focus on long-term low dose effects, and regulators update labeling requirements as new data comes in. The strong odor serves as a warning, but safety protocols and clear education handle most real dangers.
Future Prospects
Tech companies target sodium metabisulfite for environmental cleanup innovations, testing new sorbents that scrub heavy metal ions from industrial wastewater streams. Food industry experts bank on cleaner, lower-allergen versions and more robust labeling to satisfy global market trends. Energy-efficient production facilities and recycling programs can sharply reduce waste, giving sodium metabisulfite new green credentials. I’ve talked to researchers exploring ways to pair this compound with smart sensors, adjusting preservative dose in real time based on spoilage risk, which could save both product and money. Some see promise in blending sodium metabisulfite with bio-based synthetic agents, shrinking the overall chemical load needed for shelf stability. Given its long track record and adaptability, sodium metabisulfite looks set to keep a firm seat at the table, supporting innovations in everything from safer food to cleaner water and more sustainable manufacturing.
What is sodium metabisulfite used for?
How I Learned About Sodium Metabisulfite
My neighbor once asked why the apricots he dried at home didn’t look much like the ones in stores. They seemed dull, not golden-orange. That little conversation led both of us to the label on his bag of store-bought fruit, and right there: sodium metabisulfite. Seeing that name in so many places, I started paying attention. This chemical pops up in foods, wine, swimming pools, even in paper products.
Why Businesses and Households Use It
Food companies trust sodium metabisulfite to keep fruits and veggies looking good and tasting fresh longer. The key ingredient here helps prevent browning—think apples, apricots, or raisins—and stops bacteria and molds from turning those foods quickly. Most dried fruits on store shelves wouldn’t keep their color without it. Even a can of shrimp might contain a dash to keep the seafood firm and light pink.
Wine has its own story. Winemakers count on this chemical to keep their product from spoiling. After fermentation, a little sodium metabisulfite keeps unwanted bacteria away. Many brewers, especially small ones, rely on it to sterilize their equipment. Without it, the risk of a bad batch grows fast.
Beyond the dinner table, water treatment crews use sodium metabisulfite to reduce chlorine levels before sending water back to rivers. In large swimming pools, it lowers free chlorine, making water safer for people and less harsh on metal fixtures. Paper mills use it to bleach and process pulp.
Concerns Most People Feel
Despite the long list of uses, concerns about sodium metabisulfite keep showing up. People with asthma or sulfite sensitivity often react to foods with this preservative, sometimes with serious symptoms. In the U.S., the Food and Drug Administration (FDA) asks manufacturers to label foods containing sulfites larger than ten parts per million, so shoppers can make informed choices. In my own kitchen, I look for that label on dried fruit and wine because a friend reacts to sulfites.
The food industry faces a balancing act between safety, shelf life, and honest labeling. Sulfites don't cause trouble for most folks. For those who do react, even tiny amounts trigger real issues. Restaurants often have to train staff to answer questions about allergies and check ingredient lists for all processed foods they serve.
Choosing Safer and Cleaner Alternatives
Some companies go chemical-free. Freeze-drying and vacuum-sealing products create longer shelf life with fewer preservatives. Traditional drying methods, using sun and airflow without additives, work for certain fruits but don’t stop browning as well. In winemaking, a move to organic certification encourages growers and brewers to reduce or skip sulfites.
At home, storing fruits in airtight containers and the fridge cuts the spoilage risk, though it won’t keep food looking as bright as the ones coated in preservatives. Making jam or canning with heat offers another workaround. Water treatment systems now test alternatives like activated carbon or UV light to lower chlorine without chemicals.
The question comes down to what matters most: shelf life, safety, or keeping things simple. Sodium metabisulfite won’t disappear overnight because it solves tough problems in food and water. Still, listening to those with real health concerns shapes better labeling, safer kitchens, and sometimes, new methods that step back from chemicals altogether.
Is sodium metabisulfite safe for consumption?
Sodium Metabisulfite in Everyday Food
Most folks don’t notice sodium metabisulfite printed on the back of packages. Yet people have been eating foods with this preservative for generations. It gets tossed into dried fruit, wine, pickled veggies—even some bakery flour. It helps keep foods from turning brown and stops bugs from spoiling a batch. As someone who reads every label because my dad’s asthma flares up easily, those words on the bag grab my attention.
Why It’s Used—And Where it Gets Risky
Sulfites fight spoilage hard, which means less waste and fresh-tasting snacks longer. Old cookbooks sometimes talk about “campden tablets”—those are pure sulfites. They work so well that even big commercial winemakers use them in vast tanks, not just home brewers. Here’s the trouble: for a small but real group—mainly folks with asthma—they can spark allergic reactions. My dad found out the hard way in the ’90s, after he nearly wound up in the ER eating restaurant salad sprinkled with sulfites.
Most healthy adults don’t notice a thing. For those with breathing issues or true allergies, eating food with sodium metabisulfite can mean hives, itchy throat, or even wheezing. Children with asthma face the biggest risk. Hospitals have seen this pattern for decades; medical organizations confirm the link. Europe and the U.S. both say food labels must list sulfites over a tiny threshold. In my view, most of us appreciate clear warnings, just like peanuts or gluten shout-outs.
What the Science Tells Us
Plenty of research points toward “generally safe” at the usual levels found in modern foods. The U.S. Food and Drug Administration tests sodium metabisulfite and sets firm upper limits. The acceptable daily intake floats at about 0.7 milligrams for every kilogram you weigh. Health Canada and the European Food Safety Authority both land on a similar number. Scientists have poked and prodded for years and studies don’t link regular, moderate intake to cancer or birth defects.
Problems multiply if someone chugs lots at once, or ignores allergies. In huge doses, sulfites can upset stomachs, lower blood pressure, or worse. That applies not just to sodium metabisulfite but to all sulfite preservatives. Food safety people have seen outbreaks with unlabeled bulk foods—like bins of trail mix where no one lists ingredients.
Improving Safety and Awareness
From my side, the answer involves a little common sense and a sharper eye. Allergy-prone families treat labels as gospel. Restaurants need better staff training so salad makers don’t slip additives in without warning. Companies can bold their ingredient lists or set aside additive-free options for sensitive eaters. As a parent buying dried fruit for school, I appreciate brands that wave the “no sulfite” flag.
Doctors warn their at-risk patients to ask questions and carry inhalers. Consumer groups push for new research on allergy rates and better education. Not every food needs a chemical preservative—sometimes you just pay a little more for fresher snacks and eat them quick. Sulfites play their role, but they aren’t magic. Real food safety isn’t about trusting any chemical; it’s about knowing what’s in your food and looking after the folks at your table.
How should sodium metabisulfite be stored?
Understanding Sodium Metabisulfite Risks and Realities
Anyone who’s worked with cleaning agents, brewing supplies, or industrial chemicals probably knows sodium metabisulfite. Its crisp, sharp odor, quick dissolving power, and strong preservation properties make it a reliable staple. Behind the scenes, though, this powder packs real risks if left in the open or stashed without care. Growing up in a family that used it for wine-making, I learned firsthand what happens after one careless spill: corroded tools and a musty, eye-burning cloud in the pantry. Ruined gear and stinging noses show that treating this chemical casually can turn a simple routine into a mess or even a medical emergency.
Simple Storage Rules That Actually Work
Fresh, dry, and cool describe where sodium metabisulfite always belonged in our house. Whether in a factory storeroom or a home cabinet, keep it in airtight, moisture-proof containers. Even small amounts of humidity start to break it down, change its texture, or trigger the production of sulfur dioxide gas. Poor storage makes it not just less effective, but also hazardous. Moldy smells or visible clumps in the container are red flags you should never ignore.
Direct sunlight spells trouble for chemicals like this. I learned after finding a yellowed, hard mass in a see-through plastic jar one summer. Anyone stacking supplies near a window or other hot spot will shorten its shelf life and possibly spark a dangerous reaction. The solution comes down to locating a shaded, temperature-stable spot. Store it in original, clearly labeled packaging so nobody confuses it with flour, sugar, or other powders—a real risk in small kitchens or hobby rooms.
Health Hazards: No Room for Sloppiness
The fumes from this powder irritate eyes, throat, and lungs. OSHA counts sodium metabisulfite among substances that need thoughtful handling. Some people, including those with asthma, can land in the hospital after breathing it in. In workplaces, suitable gear—gloves, goggles, and masks—offers solid protection. At home, airing out the area and handling the powder gently matter most. Never mix with acids or water in a rush—violent bubbling, fizzing, and sharp fumes follow.
Practical Storage Setup
Arrange chemicals away from any acids, food, and drink. Make it a habit to check containers for cracks, leaks, or swelling lids every few months. If you spot signs that the powder changed color or clumped, replace it right away. Safe disposal includes mixing with lots of water in a well-ventilated area before diluting it into waste drainage guided by local rules—not just tossing it in the trash.
Better Knowledge, Safer Handling
Even experienced workers and hobbyists sometimes shortcut best practices. Reviewing chemical labels, safety sheets, and recent guidance pays off. Communicate to family or coworkers what you’re storing and what to do if a spill happens. Proper habits, updated information, and honest respect for sodium metabisulfite prevent harm, give longer product life, and protect everyone who might come into contact with the chemical. The habits you form today in storing and handling this substance could save money, health, and the environment tomorrow.
What are the potential side effects or hazards of sodium metabisulfite?
Sodium Metabisulfite in Everyday Life
Sodium metabisulfite pops up more often than most realize. It preserves dried fruits, works as a bleaching agent in the paper industry, and helps sterilize beer and wine equipment. Despite its usefulness, this chemical carries real risks for health and safety, especially if handled carelessly. Years spent in kitchens and workshops have shown me that one careless moment can lead to itching eyes or a harsh cough, and a little knowledge can save a lot of trouble.
Short-Term Effects: Immediate Issues from Exposure
Accidental exposure—through skin, eyes, or inhalation—can hit hard. People working in food processing or cleaning breweries talk about watery, stinging eyes and scratchy throats soon after handling the powder. High concentrations in the air cause coughing, headaches, and even difficulty breathing. Asthma sufferers run an even greater risk. Studies in allergy clinics point out that sulfites prompt wheezing and shortness of breath, sometimes ending in a full-on asthma attack. It can sneak up on customers too. For some eating out, food preserved with sodium metabisulfite causes hives or swelling—a classic allergic reaction.
Long-Term Risks from Continuous Use
Many assume the worst effects pass once the powder settles or the food leaves the shelf. Research shows that isn’t always the case. Occupational health reports detail skin rashes that linger for weeks, even eczema that needs prescription creams to clear. Factory workers—especially those packing dried fruits or working in fermentation—talk about recurring respiratory problems after years of exposure. A British medical journal outlined an unusual case where long-term breathing of sodium metabisulfite dust led to ongoing lung inflammation.
Hazards Beyond the Body
Besides personal health, this chemical’s strong reactivity can spark trouble in the wrong environment. It reacts with water to throw off sulfur dioxide gas—a substance with a choking, rotten-egg odor. Leaks or spills in tight storage rooms quickly make the air unbreathable. Emergency room doctors report treating cases of severe lung irritation after such accidents. Products labeled “sulfite-free” don’t just cater to picky eaters—the regulations came after medical groups tracked plenty of genuine emergencies, including anaphylactic shock.
Simple Steps Toward Safer Use
Solutions don’t have to be high-tech or expensive. Wearing snug-fitting gloves and eye protection makes a real difference. Fans that move air and direct exhaust vents cut down on invisible dust. In restaurants or bakeries, staff training sessions help everyone spot symptoms and know which products use preservatives. Labels need clear warnings about sulfites, not vague “preservatives added.” In the food aisle, shoppers with asthma or allergies benefit from better labeling and fewer “hidden” ingredients. For regulators and manufacturers, better packaging cuts down on emergency calls and hospital bills over the long run.
Why It’s Worth the Effort
Plenty of chemicals play a part in modern life, but familiarity shouldn’t breed carelessness. Watching how a co-worker’s asthma nearly sent her to the ER taught me that some hazards belong out in the open. Health depends just as much on reading a label as on trusting the experts. The cost of caution, measured in gloves and ventilation hoods, comes up cheap compared to hospital visits and lost workdays. Sodium metabisulfite accomplishes real work—it just asks for respect in return.
Can sodium metabisulfite be used for water treatment or disinfection?
Looking at an Old Chemical in a New Light
Sodium metabisulfite pops up in plenty of places, from winemaking to food preservation, and sometimes even in the treatment of water. People who deal with chemistry every day tend to see it as a catch-all reducer, especially when it comes to removing leftover chlorine. Anyone running a municipal water system, or even running a boiler, knows the trouble chlorine causes if left unchecked. Sodium metabisulfite gets the job done. It soaks up free chlorine and protects pipes and equipment. In small, planned doses, it really has no substitute for that part of the process.
Not Every Fix Is Foolproof
It’s tempting to see an effective compound like this and imagine it can handle any disinfection job out there. That’s where real-world experience and technical know-how come into play. Sodium metabisulfite neutralizes chlorine, but that doesn’t mean it wipes out germs, parasites, or other threats in raw or untreated water. If someone tries using it as a primary disinfectant, they’re setting up the system for failure.
Science backs this up. Sulfite-based compounds don’t kill bacteria or viruses the way true disinfectants do. Sipping water treated only with sodium metabisulfite leaves people at risk. Disease outbreaks tie back to these shortcuts. The World Health Organization lists safe disinfectants, and sodium metabisulfite doesn’t make the cut. Using the right tool saves lives. The wrong one just gives a false sense of security.
Risks Too Big to Ignore
There’s another side people sometimes overlook. Sulfite sensitivity hits harder than most realize. Folks with asthma or certain allergies can react to small amounts of sodium metabisulfite. Add too much of it, and not only does it change the taste and smell, but it can also send vulnerable people to the hospital. Water treatment plants need to keep strict controls if they use it, including careful dosing and continuous monitoring. Any process letting residuals slip through the tap line risks public health. The United States Environmental Protection Agency warns against overuse, and for good reason.
Looking for Real Solutions
Clean water doesn't happen by accident. Every community relies on treatment methods proven to wipe out bacteria and viruses. Chlorine and UV treatment still stand out for their reliability. Sodium metabisulfite steps in as a helper, not a hero. It shines in targeted use—breaking down left-behind chlorine so finished water doesn’t taste off or corrode pipes. But nobody should expect it to do heavy lifting on pathogens. That job belongs to chemicals and technologies built for the task, like ozone, advanced filtration, or precisely controlled chlorine dosing.
People running treatment plants and plumbing operations need solid training. Audits help spot weak points, and public reporting keeps everyone honest. Research keeps pushing better ways to balance taste, safety, and sustainable operation. Whether it’s through stricter rules or updated public guidance, clear facts have to steer these choices. Investments in science-backed systems mean fewer emergencies and less public confusion.
Keeping Safety at the Center
The bottom line: sodium metabisulfite belongs in the toolkit, but not at the center of any water disinfection plan. It plays a narrow and important role in cleaning up after stronger chemicals have finished the real work. Trust in water systems grows when the science lines up with the results. This means leaning on time-tested disinfectants, not wishful thinking or half-measures that can’t deliver on public health and safety.
| Names | |
| Preferred IUPAC name | sodium oxidooxididosulfate(1-) |
| Other names |
Disodium disulfite Sodium pyrosulfite |
| Pronunciation | /ˌsəʊ.di.əm ˌmɛ.təˈbaɪ.sʌl.faɪt/ |
| Preferred IUPAC name | sodium oxidooxosulfate(2−) |
| Other names |
Disodium disulfite Sodium pyrosulfite |
| Pronunciation | /ˌsəʊdiəm ˌmɛtəˈbaɪsʌlfaɪt/ |
| Identifiers | |
| CAS Number | 7681-57-4 |
| Beilstein Reference | 1203743 |
| ChEBI | CHEBI:38101 |
| ChEMBL | CHEMBL1357 |
| ChemSpider | 5939 |
| DrugBank | DB14527 |
| ECHA InfoCard | 100.028.870 |
| EC Number | 231-673-0 |
| Gmelin Reference | 78070 |
| KEGG | C01438 |
| MeSH | D017616 |
| PubChem CID | 24414 |
| RTECS number | UX8225000 |
| UNII | 489X175EEZ |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID7020502 |
| CAS Number | 7681-57-4 |
| 3D model (JSmol) | `data/mol=Sodium%20Metabisulfite;model=JSmol` |
| Beilstein Reference | 3563788 |
| ChEBI | CHEBI:38102 |
| ChEMBL | CHEMBL1356 |
| ChemSpider | 52716 |
| DrugBank | DB09466 |
| ECHA InfoCard | 100.028.219 |
| EC Number | 231-673-0 |
| Gmelin Reference | 778 |
| KEGG | C18689 |
| MeSH | D013482 |
| PubChem CID | 24846 |
| RTECS number | UX8225000 |
| UNII | AGG2FN16EV |
| UN number | UN 3077 |
| CompTox Dashboard (EPA) | DTXSID3023492 |
| Properties | |
| Chemical formula | Na2S2O5 |
| Molar mass | 190.107 g/mol |
| Appearance | White or yellowish crystalline powder |
| Odor | Pungent, sulfurous |
| Density | 1.48 g/cm³ |
| Solubility in water | soluble |
| log P | -4 |
| Vapor pressure | <0.1 mmHg (at 20 °C) |
| Acidity (pKa) | 6.0 |
| Basicity (pKb) | 7.0 |
| Magnetic susceptibility (χ) | Magnetic susceptibility (χ): −53.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.448 |
| Dipole moment | 1.66 D |
| Chemical formula | Na2S2O5 |
| Molar mass | 190.10 g/mol |
| Appearance | White or yellowish crystalline powder |
| Odor | Slight sulfur odor |
| Density | 1.48 g/cm³ |
| Solubility in water | 65 g/100 mL (20 °C) |
| log P | -3.7 |
| Vapor pressure | Less than 0.1 hPa at 20 °C |
| Acidity (pKa) | pKa ≈ 1.2 |
| Basicity (pKb) | 6.6 |
| Magnetic susceptibility (χ) | -59.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.448 |
| Dipole moment | 4.13 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 149.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -941 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –935.6 kJ/mol |
| Std molar entropy (S⦵298) | 172.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -944 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -947 kJ/mol |
| Pharmacology | |
| ATC code | A16AX14 |
| ATC code | A12CE05 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye damage, may cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes serious eye damage. May cause respiratory irritation. |
| Precautionary statements | P264, P270, P271, P301+P330+P331, P304+P340, P305+P351+P338, P306+P360, P312, P337+P313, P403+P235, P501 |
| NFPA 704 (fire diamond) | 2-0-1-W |
| Autoignition temperature | 220°C |
| Lethal dose or concentration | LD50 oral rat 1,540 mg/kg |
| LD50 (median dose) | 2,300 mg/kg (rat, oral) |
| NIOSH | WI3850000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium Metabisulfite: 5 mg/m³ |
| REL (Recommended) | 30 mg/L |
| IDLH (Immediate danger) | 40 mg/m³ |
| Main hazards | Harmful if swallowed, causes severe skin burns and eye damage, may cause allergy or asthma symptoms or breathing difficulties if inhaled. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H319, H335 |
| Precautionary statements | P261, P271, P280, P301+P330+P331, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P233, P501 |
| NFPA 704 (fire diamond) | 2-0-1-W |
| Autoignition temperature | 220°C (428°F) |
| Lethal dose or concentration | LD50 oral rat 1132 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1132 mg/kg (oral, rat) |
| NIOSH | NIOSH: WS5600000 |
| PEL (Permissible) | 5 mg/m³ |
| REL (Recommended) | 30 mg/kg |
| IDLH (Immediate danger) | 40 mg/m3 |
| Related compounds | |
| Related compounds |
Sodium bisulfite Sodium sulfite Potassium metabisulfite Sulfur dioxide Sodium thiosulfate |
| Related compounds |
Potassium metabisulfite Sodium bisulfite Sodium sulfite Sodium thiosulfate Sulfur dioxide |

