Sodium Lactate: An In-Depth Commentary
Historical Development
The story of sodium lactate tracks back to the roots of organic chemistry and the quest for reliable food preservation methods. Early chemists saw that lactic acid formed in sour milk, and with the growth of industrial food processing in the 20th century, sodium lactate emerged as a crucial ingredient. Producers started turning to fermentation as the most dependable way to get large quantities, banking on microbes like Lactobacillus for efficiency and consistency. As sodium lactate moved into the mainstream, it found a place in medicine as an intravenous electrolyte solution, adding another layer to its history. Over time, the shift from artisanal food preservation to clinical-grade pharmaceuticals marks how sodium lactate grew up with modern science.
Product Overview
In a typical setting, sodium lactate appears as a colorless to slightly yellow, nearly odorless liquid. It's widely used for its role as a humectant and preservative in foods such as deli meats, dressings, and bakery products. Beyond food, hospitals stock sodium lactate for fluid therapy when dehydration threatens patients or blood chemistry needs a quick fix. Every day, it pops up in skin care, working to retain moisture and bump up the skin's natural barrier. Thanks to its multi-faced value, sodium lactate has become almost invisible but essential, touching industries without drawing attention.
Physical & Chemical Properties
Sodium lactate, with the formula C3H5NaO3, typically boasts a high solubility in water and a mildly alkaline pH, which plays well with products that must stay stable over time. Its density hovers around 1.33 g/cm³ for the liquid form, and what matters in practice is how this property lets professionals add it to liquid formulations without compatibility worries. Since it resists crystallization at standard storage temperatures, companies working with delicate solutions avoid headaches commonly caused by less stable chemicals. The boiling point lands over 300°C, which keeps sodium lactate from evaporating in regular processing environments, making it reliable during pasteurization and high-heat handling.
Technical Specifications & Labeling
Consumers and manufacturers alike need clear information. Regulatory bodies, including the FDA and EFSA, mandate that food-grade sodium lactate must contain 60% active ingredient in aqueous solution by mass. Labels call out E325 for European packaging, letting customers track whether food safety standards have been met. In medical contexts, every vial or IV bag states concentration, sterility status, and expiration to ensure patient safety. Pharmacists and food technologists both check for certificates of analysis to prove absence of heavy metals or unlisted contaminants. Every batch tracked, every shipment traced, these basics help keep trust front and center in production lines and clinics.
Preparation Method
Fermentation stands out as the main method to get sodium lactate. The process begins by sending sugars—glucose from corn or potatoes, most often—through a fermentation tank loaded with Lactobacillus cultures. The bugs chew through sugars, spitting out lactic acid in the process. After reaching the needed acidity, producers neutralize the liquid with sodium hydroxide, resulting in sodium lactate. This crude product then goes through filtration and evaporation to reach food- or pharma-grade purity. The knack lies in controlling temperature and pH at every step, picking up small swings in production to head off problems before they snowball.
Chemical Reactions & Modifications
Sodium lactate sits at a low rung on the chemical reactivity ladder, but don't mistake it for a pushover. On rare occasions, mixing it with strong acids can tip the sodium lactate back to lactic acid and sodium salt, shifting flavors or shelf life in foods. Under high heat, sodium lactate breaks down slowly, which limits its use in very high-temperature cooking or industrial settings where residue must stay minimal. Formulators sometimes team sodium lactate with other salts—such as sodium diacetate or citrates—to improve bacteriostatic action or play with sodium reduction. These tweaks let manufacturers hit taste or shelf life targets without introducing harsh chemicals.
Synonyms & Product Names
Depending on context, sodium lactate hides behind a handful of names. In ingredient decks, you’ll see “lactic acid, sodium salt,” “E325,” or just “sodium lactate solution.” Pharmacists often refer to “Ringer's lactate solution” for a specific IV fluid mix. In European supermarket aisles, “E325” signals regulatory approval, while technical data sheets from chemical suppliers may offer “racemic sodium lactate” or “DL-Sodium lactate” to describe specific optical isomers. These names help buyers and scientists navigate the fine differences between product grades and legal labeling requirements.
Safety & Operational Standards
Sodium lactate has a long record of safe use when handled with reasonable care. In food, concentrations below 4% rarely trigger any gastrointestinal upset, even in sensitive adults or children. Hospitals follow published IV administration guidelines, carefully balancing sodium intake to avoid making patients hypernatremic. Factories working with sodium lactate insist on using gloves and goggles to head off mild skin irritation. Wastewater containing sodium lactate breaks down readily in municipal treatment plants, sidestepping the chronic toxicity or persistence linked with more exotic chemicals. As always, workers need to check the latest safety data sheets and training to stay ahead of accidents or misuse.
Application Area
Every time you buy a rotisserie chicken, slices of ham, or smoky beef jerky, sodium lactate probably played a hand in keeping it safe and juicy. Chefs rely on it as a flavor fixer and a moisture savior, since it limits bacterial spoilage without hammering taste. Personal care formulators slip sodium lactate into lotions and soaps, counting on its water-binding powers to outdo cheaper humectants. Clinics and field hospitals lean on sodium lactate as a quick way to restore lost body fluids, especially in trauma cases or post-surgical settings. Even breweries and construction supply companies put sodium lactate to work—one as a yeast nutrient in low pH worts, the other as a workhorse anti-freeze in concrete mixes during freezing weather.
Research & Development
The world of R&D never stands still, and sodium lactate continues to spur innovation. Chemists keep searching for ways to tweak its antimicrobial properties, pairing it with clean-label mold inhibitors to cut down on artificial preservatives. Researchers look into using lactate in sports medicine, following how it helps buffer lactic acid buildup in muscle tissue and promote faster recovery. Studies in sustainable packaging focus on sodium lactate's power to regulate moisture transfer, letting food stay fresh longer without sealed plastic. Teams in synthetic biology tinker with custom Lactobacillus strains to boost yield or drop unwanted byproducts, helping both cost structure and product purity.
Toxicity Research
Decades of toxicology data have built a clear picture. Animal studies and human trials agree: sodium lactate poses little risk at doses used in foods or medicines. Cases of toxicity almost always trace back to massive overdoses or scenarios with severe underlying kidney trouble where waste builds up. No evidence links long-term, low-level intake of sodium lactate to cancer, developmental defects, or allergy. Food safety authorities routinely review these numbers, requiring ongoing surveillance and random testing to confirm their conclusions. Regulatory limits stand not because sodium lactate invites trouble, but because confidence rests on continuous vigilance.
Future Prospects
With pressure rising for lower sodium content in processed foods, sodium lactate brings a unique angle as a salt substitute. By delivering saltiness with less elemental sodium, it helps food companies meet health targets without cutting out flavor or shelf life. Clean-label and plant-based trends continue to fuel demand, as formulators seek alternatives to nitrites and the like. Therapies using sodium lactate show promise in intensive care, helping to treat some metabolic imbalances in liver and kidney patients. The push for greener chemistry endears sodium lactate to scientists aiming for biodegradable, nontoxic household cleaners and lubricants. The future likely holds more applications, more scrutiny, and more ways to turn a modest ingredient into a quiet powerhouse.
What is sodium lactate used for?
Sodium lactate doesn’t show up on the dinner table, but you can spot it in your fridge and medicine cabinet. I started seeing it on food labels while working in a restaurant kitchen, and the chef just called it “the magic stuff that keeps deli meats looking fresh.” That’s not just kitchen folklore; sodium lactate gets used a lot to help food hold onto moisture, look better for longer, and stop bacteria from growing. Pick up a pack of sliced turkey or ham, and chances are it helped keep that meat juicy and safe.
Food Preservation and Taste
Sodium lactate comes from a simple process of fermenting sugars—usually from corn or beets. Food makers like it because it has a mild, salty flavor and helps keep things moist. Chefs sometimes use it in sausage because it helps keep fat from separating and shrinking during the cooking process. In barbecue joints, brisket gets injected with sodium lactate to lock in juices all the way through a long smoking session. That keeps the meat from drying out and cuts down on the risk of nasty bacteria like listeria, which thrives in ready-to-eat foods. The Centers for Disease Control and Prevention points to listeria as a top foodborne threat. Adding sodium lactate is one effective measure the industry relies on to reduce that risk.
Healthcare Applications
In hospitals, sodium lactate steps into a very different role. Doctors turn to it for intravenous fluids, especially in formulas like Ringer’s lactate. Patients with dehydration, burns, or serious trauma need more than plain water—they need their electrolytes balanced and their blood pH stable. Sodium lactate helps doctors fix this quickly without overwhelming the body. During my own hospital stay following surgery, I remember nurses checking my IV bags and mentioning lactate. Turns out, that fluid helped keep my body from slipping into dangerous acidosis as I recovered. The World Health Organization lists lactate solutions as an essential medicine, crucial for stabilizing patients across the world.
Skincare and Cosmetics
Take a stroll down the lotion aisle and you’ll see sodium lactate listed on bottles. It works as a humectant—a water magnet that draws moisture from the air and helps keep skin hydrated. People struggling with dry skin or eczema can benefit, since sodium lactate absorbs water into the outermost skin layer. It’s not flashy, but it works better than plain glycerin. Some dermatology studies show it actually improves the skin’s ability to hold onto water over time, making it more than just a temporary fix.
Safe Usage and What to Watch For
Scientists and doctors trust sodium lactate in these roles because they’ve tested it for years. The U.S. Food and Drug Administration labels it as “generally recognized as safe.” At home, it’s wise to read ingredient labels—especially if you have sensitive kidneys or are on a low-salt diet. In rare cases, people with certain metabolic issues shouldn’t take in extra lactate. For most people, though, eating or using products with sodium lactate brings no serious risks.
Why Paying Attention to Ingredients Matters
Lots of folks just skip the ingredient list, assuming everything sold in stores must be harmless. That’s not always true. Knowing about sodium lactate’s benefits can help people pick foods with better shelf life or choose lotions that really hydrate. On the other hand, reading labels helps people avoid anything that could interact with their health needs. Food manufacturers can go further by clearly highlighting sodium lactate’s purpose—whether it’s preserving flavor or improving safety—so consumers don’t have to guess at what’s inside their food or products.
Is sodium lactate safe for skin?
What Is Sodium Lactate?
Sodium lactate shows up on plenty of ingredient lists, especially in moisturizers and soaps. It comes from the fermentation of natural sugars like corn or beets, and chemists combine that lactate with salt to create the finished ingredient. Some folks might think “lactate” means there’s dairy involved, but sodium lactate does not contain milk, so people with sensitivities can breathe easier. What’s striking is how sodium lactate pulls water into the skin, acting as a humectant — a fancy word for a moisture-grabber. I’ve seen handmade and commercial brands use it to keep lotions smooth and bars of soap a little harder.
Safety of Sodium Lactate on Skin
Most dermatologists and cosmetic chemists say sodium lactate rates as safe for skin in the amounts used in personal care products. Large-scale safety reviews, including those published by the Cosmetic Ingredient Review (CIR), show reactions are rare. Researchers found sodium lactate doesn’t usually bother healthy skin. It’s even present in our own skin as part of the natural moisturizing factor (NMF). This NMF group helps keep the outer layer of skin from drying out. You might notice sodium lactate in medical settings, too. Some IV solutions use it because the body handles lactate in a familiar way.
Concerns for Sensitive Skin
It’s never possible to promise zero irritation for every person. People with extremely sensitive or compromised skin, such as those recovering from cosmetic procedures, may sometimes notice mild stinging from humectants, including sodium lactate. My experience as someone with eczema—where my skin barrier often feels raw—shows that low concentrations in a lotion never bother me, but higher percentages can bring a little tingle. Most commercial moisturizers keep levels around 1% to 5%, which matches up with what studies say is comfortable. Homemade soap makers sometimes use higher levels, which can throw off the pH and lead to discomfort. Anyone with allergies should still read the entire ingredient list because other components might be the real culprit.
Myths and Facts
Sodium lactate sometimes gets confused with lactic acid. Both help attract water, but lactic acid also exfoliates. That exfoliating property can cause redness for those with delicate skin. Sodium lactate usually skips that issue, providing gentle hydration without peeling. There’s also a misconception that sodium lactate “dries out” the skin. Genuine clinical studies do not support the idea that well-formulated products containing this ingredient worsen dryness — reports point in the opposite direction. In fact, the NMF in our own bodies includes sodium lactate precisely to help seal in moisture.
Potential Solutions for Real-World Concerns
People want more transparency about personal care products. More brands offer full ingredient breakdowns and clear percentage disclosures. Patch testing at home remains a smart first step with any unfamiliar product. Whether buying from big stores or small-batch hobbyists on Etsy, checking how much sodium lactate they use pays off. Cutting out unnecessary fragrances, dyes, and harsh surfactants creates skin care routines less likely to trigger a reaction. Formulators could offer more fragrance-free versions for those with sensitivities. Anyone worried about safety can talk with a dermatologist—especially if they’ve struggled with reactions in the past—to find options that work in real life, not just on paper.
Can sodium lactate be ingested?
What Sodium Lactate Actually Is
Sodium lactate often shows up on ingredient lists for processed food and sports drinks, but not everyone knows what it actually is. Despite the technical-sounding name, it’s just the sodium salt of lactic acid, which comes from fermenting sugar sources like corn or beets. Lactic acid gets its fame from yogurt and fermented foods, but the “sodium” in sodium lactate comes in when it's neutralized to make it shelf-stable and easy to use. Bakers, food scientists, and even some chefs rely on it to bump up flavor and keep food moist.
What Science and Regulations Say About Consuming Sodium Lactate
Food safety isn’t something to leave to chance. Organizations such as the U.S. Food and Drug Administration (FDA) keep sodium lactate on the Generally Recognized as Safe (GRAS) list for use in food. In Europe, food makers rely on it too, under the additive code E325. It helps to control acidity and keeps cooked meats and cheeses fresh for longer. In hospitals, sodium lactate plays a role in IV fluids, especially in what’s known as “lactated Ringer’s solution.” Medical teams use this to balance minerals during surgery or after trauma. So whether as an ingredient or in clinical settings, sodium lactate has history and oversight.
Uses and Daily Exposure
People eating cured meat, pre-cooked seafood, or sliced cheese probably get a little sodium lactate in their meals. Makers use it to retain moisture and help meat last longer in the fridge. The taste doesn’t jump out, so most folks don’t realize it’s in their favorite deli ham or chicken breast. Endurance athletes know it well too, since sodium lactate sometimes slips into electrolyte drinks to help rehydrate and fight cramping. From a nutrition perspective, sodium lactate contains less sodium per gram than table salt, though it’s still worth watching total sodium intake for blood pressure health.
Risks and Who Should Avoid It
Most healthy people process sodium lactate without trouble in normal food amounts. Things can get complicated for those with kidney or liver issues, though. These organs handle the job of filtering metabolites, and a buildup of extra lactate (from any source, not just sodium lactate) might create problems. Folks with heart failure or severe dehydration might also want to steer clear. While rare, lactic acidosis—a buildup of lactic acid in the blood—can happen if people can’t clear lactate well. Those with sensitivities or underlying conditions should talk to a doctor if they spot sodium lactate on labels frequently.
Commonsense Approaches for Everyday Eating
Anyone reading labels will see plenty of unfamiliar additives, but sodium lactate isn’t one to cause much worry in regular meals. Moderation matters, as with anything containing sodium. For people with hypertension, trimming back on all forms of sodium, including sodium lactate, supports long-term health. For everyone else, eating fresh, minimally processed food and balancing it with convenience items helps keeps things in check. Science backs up sodium lactate’s safety in typical diets, and real-world use supports this as well.
Navigating a World Filled with Food Additives
Learning what’s in packaged food helps people feel confident in their choices. Rather than panicking about unfamiliar names, a glance at credible sources—like the FDA, the European Food Safety Authority, or major nutrition clinics—shows that sodium lactate has earned its spot at the table for good reason. Being a mindful shopper gives anyone the skills to manage their health and keep food both flavorful and safe.
Is sodium lactate natural or synthetic?
Understanding What Sodium Lactate Actually Is
Supermarkets stock plenty of ingredients that raise eyebrows, and sodium lactate pops up on more and more labels. This colorless liquid or solid salt comes from lactic acid. Lactic acid itself turns up in fermented foods like yogurt, kimchi, and sourdough. Sodium lactate, on the other hand, goes through an extra step: manufacturers take lactic acid, usually made by fermenting sugar from cornstarch or beets with bacteria, and shake it up with sodium compounds to make the salty form we see in foods and cosmetics.
The Nature vs. Synthetic Debate
People often worry about where their food comes from and whether what they eat is something found in the natural world. Sodium lactate occupies a gray area. Its building blocks—sugar, bacteria, and salt—are about as earthy as things get. Left alone, these ingredients still make lactic acid as part of nature’s cycles. The twist comes from the processing: pulling out pure lactic acid, purifying it, and turning it into sodium lactate in a controlled setting. So, in the strictest sense, the powder sprinkled in a bakery batch was made by people, not scraped from a rock or plant.
Sometimes, people draw hard lines between what counts as "natural" and "synthetic." Things made by living organisms seem less suspicious to most of us. Sodium lactate taken directly out of fermented vegetables would probably pass any farmer’s test, but commercial sodium lactate almost never comes straight from sauerkraut juice. Industry relies on fermentation tanks, not cabbage barrels, for consistent quality and large volume.
Why Do We Care?
As someone who spends time reading ingredient lists and tries to avoid artificial additives, I get the confusion. The word “lactate” alone can sound intimidating if you don’t understand the science. But everything boils down to what’s safe, what’s real food, and what’s clever chemistry. On one hand, sodium lactate’s origins in fermentation look pretty natural. Its production just helps keep our food safer for longer and brings out certain flavors, especially in cured meats and baked goods.
Some folks need reassurance: multiple studies confirm sodium lactate’s safety when used as directed. As a bonus, it helps control bacteria that can make us sick. The ingredient also shows up in medical uses—think IV fluids—because it’s gentle on the body.
What Shapes Public Trust?
Trust grows with clear, honest labeling. If food packagers described how sodium lactate starts with plant sugar and ends up as a salt through fermentation and a touch of chemistry, public worries might soften. There’s a truth gap when vague language hides what really happens behind the factory doors.
Shoppers deserve options. Brands can spotlight sodium lactate from organic, non-GMO sources for customers who focus on clean eating. Regulators could encourage stricter guidelines about how companies talk about “natural” versus “synthetic” ingredients. That way, no one leaves the grocery aisle guessing what’s behind a name—or whether they’re ingesting something made solely by science or given a little help by nature.
Moving Forward
Food culture shifts all the time. People are curious, skeptical, and sometimes overwhelmed by science on their plates. With sodium lactate, the line between natural and synthetic blurs, but safety and transparency should always win out. The best story of any ingredient comes from understanding where it came from and how it ended up in the foods and products we use every day.
How should sodium lactate be stored?
Looking After a Common Ingredient
Sodium lactate turns up in many routines—working in food production, health care, or even homemade soap. It keeps bread softer for longer, helps balance acidity, and nurses use it every day in drips. Some people think any old shelf will do, but getting storage wrong can cost more than a ruined batch. Safe handling matters for your team, your inventory, and the end user relying on the quality of your work.
Watch the Basics: Dry, Cool, Clean Space
Moisture messes with sodium lactate. Even though it draws in water, letting too much humidity get in will change the consistency and shortens shelf life. Store containers in a dry environment. If the air feels damp, that’s a red flag. I remember a time in a shared kitchen where a leaky roof left caps stuck and the solution turned cloudy. That set us back days because we tossed whole cases to avoid a chain reaction.
Keep the temperature steady, not too cold or hot. Most labels recommend something similar to general pantry or storeroom temperatures—about 15°C to 25°C (59°F to 77°F). Baking heat warps bottles, and cold snaps can force crystals to form. Once, in a storage room that doubled as a back porch, we lost inventory one winter—as sodium lactate clumped up and took ages to redissolve.
Original Containers: Not Just a Suggestion
Always return sodium lactate to the original container with a good seal. Recycled bottles or jugs sometimes react, especially if they’ve held cleaning agents or acids before. In my early days making soap, I reused a vinegar container and ended up dealing with odd smells and streaks. Avoid cross-contamination by labeling everything and checking lids after every use.
Keep Out of Sunlight and Away from Chemicals
Direct sunlight gradually breaks down even tough packaging and triggers chemical changes. Leave bottles in a closed cupboard or on shaded shelves. Sunswept windows are for tomatoes, not sodium lactate.
Strong-smelling chemicals nearby can cause problems. Fumes slip past loosened lids, changing the taste or smell of sodium lactate. In commercial kitchens, I once saw flavor taint in packed goods when open bottles sat near bleach under the sink.
Track Expiry and Practice Good Rotation
Every bottle comes stamped with a best-before. Don’t treat this like a guess—expired sodium lactate grows murky, loses potency, and can spoil recipes or medical applications. Rotate stock so older supplies get used ahead of new deliveries. I always keep a log, and checking dates before opening a fresh jug is now a habit.
Safe Handling and Spillage Response
Wear gloves if handling big quantities. Even if a splash isn’t considered dangerous, repeated skin contact dries hands out fast. For spills, wipe up with lots of water, then dry the area so no sticky residue attracts dust, bugs, or mold.
Simple Storage Really Pays Off
Most quality concerns come from tiny lapses—leaving a lid off, forgetting to rotate stock, or storing next to incompatible supplies. Training everyone to respect the basics pays off. Keeping sodium lactate fresh calls for simple routines and a bit of attention, but those habits save both money and headaches for anyone who depends on this ingredient.
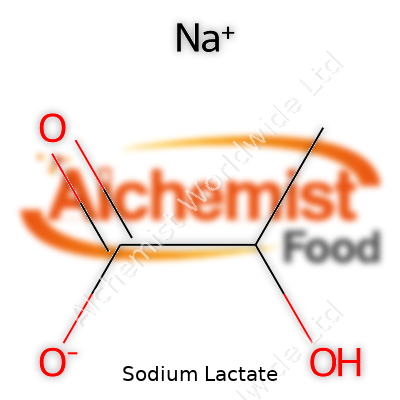

| Names | |
| Preferred IUPAC name | Sodium 2-hydroxypropanoate |
| Other names |
Dilactate E325 Sodium 2-hydroxypropanoate Sodium DL-lactate |
| Pronunciation | /ˌsəʊdiəm ˈlæk.teɪt/ |
| Preferred IUPAC name | sodium 2-hydroxypropanoate |
| Other names |
DL-Lactic acid sodium salt Sodium 2-hydroxypropanoate Sodium DL-lactate |
| Pronunciation | /ˈsəʊ.di.əm ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 72-17-3 |
| Beilstein Reference | 1720515 |
| ChEBI | CHEBI:6636 |
| ChEMBL | CHEMBL1357 |
| ChemSpider | 5370115 |
| DrugBank | DB09161 |
| ECHA InfoCard | 13b612e0-50a0-4186-8760-2d61eb2eb6fc |
| EC Number | 208-601-1 |
| Gmelin Reference | 72497 |
| KEGG | C01780 |
| MeSH | D017525 |
| PubChem CID | 23665598 |
| RTECS number | UE5608000 |
| UNII | “276DRX4QPE” |
| UN number | UNDLQ |
| CAS Number | 72-17-3 |
| Beilstein Reference | 3599144 |
| ChEBI | CHEBI:6636 |
| ChEMBL | CHEMBL1359 |
| ChemSpider | 5031 |
| DrugBank | DB09161 |
| ECHA InfoCard | '03c6795a-6b7e-4e12-aa32-8343137b3c87' |
| EC Number | EC 200-772-0 |
| Gmelin Reference | 9307 |
| KEGG | C01780 |
| MeSH | D017325 |
| PubChem CID | 23669752 |
| RTECS number | OE1225000 |
| UNII | 8P4116NN10 |
| UN number | UNDLQ |
| Properties | |
| Chemical formula | C3H5NaO3 |
| Molar mass | 112.06 g/mol |
| Appearance | Colorless or slightly yellowish, clear, viscous liquid |
| Odor | Odorless |
| Density | 1.33 g/cm³ |
| Solubility in water | Very soluble |
| log P | -3.8 |
| Acidity (pKa) | 15.3 |
| Basicity (pKb) | 12.4 |
| Magnetic susceptibility (χ) | `-12.0×10⁻⁶ cm³/mol` |
| Refractive index (nD) | 1.430 |
| Viscosity | Viscous liquid |
| Dipole moment | 5.585 D |
| Chemical formula | C3H5NaO3 |
| Molar mass | 112.06 g/mol |
| Appearance | Colorless or slightly yellowish, clear, viscous liquid |
| Odor | Odorless |
| Density | 1.33 g/cm³ |
| Solubility in water | Very soluble |
| log P | -3.8 |
| Acidity (pKa) | 12.8 |
| Basicity (pKb) | pKb ≈ 12.2 |
| Magnetic susceptibility (χ) | -13.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.420 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.88 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S⦵298 = 151.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1010.6 kJ/mol |
| Std molar entropy (S⦵298) | 236.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -686.2 kJ/mol |
| Pharmacology | |
| ATC code | B05BB01 |
| ATC code | B05BB01 |
| Hazards | |
| Main hazards | May cause mild skin and eye irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. Wash hands thoroughly after handling. |
| Autoignition temperature | Autoignition temperature: 400°C (752°F) |
| Lethal dose or concentration | LD50 (oral, rat): 2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2,000 mg/kg (oral, rat) |
| NIOSH | SNB130 |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 30% |
| IDLH (Immediate danger) | No IDLH established |
| Main hazards | May cause mild skin and eye irritation. |
| GHS labelling | GHS07, Warning |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Flash point | > 112.8 °C |
| Autoignition temperature | Autoignition temperature: 370°C (698°F) |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 (oral, rat): 2,000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2,000 mg/kg |
| NIOSH | SNB35000 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 2% |
| Related compounds | |
| Related compounds |
Lactic acid Potassium lactate Calcium lactate Magnesium lactate Sodium citrate |
| Related compounds |
Lactic acid Potassium lactate Calcium lactate Magnesium lactate |

