Sodium Hydrogen Carbonate: A Deep-Dive Into an Ubiquitous Compound
Historical Development
Sodium hydrogen carbonate, known to many as baking soda, has woven itself into countless aspects of daily life, science, and industry. People in ancient Egypt mixed natural deposits of natron, which contains sodium bicarbonate, into cleaning and preservation rituals thousands of years ago. Fast forward to the late 18th century, French chemist Nicolas Leblanc found a reliable way to synthesize sodium carbonate, setting the stage for the commercial production of sodium hydrogen carbonate by the Solvay process in the 1860s. Over time, the accessibility and affordability of this compound allowed it to become an essential household and industrial staple, embedded in global food, cleaning, and healthcare practices.
Product Overview
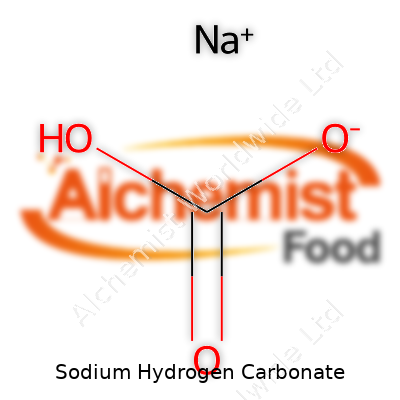
Most kitchens and laboratories carry sodium hydrogen carbonate as a white crystalline or powdery solid. Recognized by the formula NaHCO3, it can be found under different names, depending on the field—bicarbonate of soda, baking soda, or simply sodium bicarb. Chemists, bakers, and medics depend on reliable supplies, which must meet strict industry standards for purity. Manufacturers calibrate their processes to deliver specific particle sizes for baking, tablet formation, and fire suppression. Packaging usually highlights its sodium content and presence of trace metals, as these details can impact food safety and chemical reactions.
Physical & Chemical Properties
Sodium hydrogen carbonate forms a gritty, odorless, white powder, slightly soluble in water but not in alcohol. The compound decomposes above 50°C, releasing carbon dioxide, water, and sodium carbonate. It reacts to both acids and bases, making it a gentle buffer. Its mild alkalinity gives it a pH near 8.3 in solution. Thanks to its stability in dry conditions and safe handling properties, it can be blended and stored without special equipment. It won’t catch fire, won’t poison users on contact, and holds up well in humid climates unless it absorbs too much moisture. These qualities help maintain shelf life in commercial and domestic settings alike.
Technical Specifications & Labeling
Regulatory bodies set precise benchmarks to let buyers judge quality—ASH content, loss on drying, heavy metal limits, and sodium percentage. For food-processing, purity usually must clear 99% with strict microbiological profiles. Industrial grades may tolerate minor impurities depending on the application; pharmaceutical standards permit even less deviation. Suppliers break down specifications—particle size range, bulk density, and labeling requirements must meet FDA and EU food codex rules, including allergen statements and country of origin. For import or export, customs inspections scrutinize batch numbers, expiry dates, and packaging seals.
Preparation Method
Industrial-scale sodium hydrogen carbonate production typically starts with the Solvay process, a method that combines ammonia, carbon dioxide, and sodium chloride (salt) in water. This yields sodium hydrogen carbonate, which is then filtered, dried, and milled to desired consistency. Home brewers sometimes use ‘baking’ methods, where sodium carbonate is exposed to carbon dioxide and water under gentle conditions. While natural sources still exist, most commercial product comes straight from this controlled chemical reaction pathway. Waste is rigorously managed, especially ammonia emissions, since environmental controls now enforce much tighter standards than those in the compound’s early industrial history.
Chemical Reactions & Modifications
This compound delivers robust chemical versatility, serving as a mild alkali and an acid-neutralizer. It reacts with acids such as vinegar or lemon juice with visible fizzing as carbon dioxide leaves the mixture, and forms sodium salts plus water. Adding strong heat converts it to sodium carbonate, letting bakers produce lighter textures in cakes and breads. The compound plays a central role in buffer solutions to help maintain stable pH environments. Scientists often tweak the particle size, add anti-caking agents, or combine it with other chemicals to tailor its release rate or solubility for specific industrial tasks.
Synonyms & Product Names
Across regions and applications, sodium hydrogen carbonate appears under a range of names: baking soda, sodium bicarbonate, bicarb soda, and even E500 (as a food additive code). Medical fields refer to it as sodium bicarb, where it appears in intravenous formulations for acid-base correction. In cleaning aisles, it shows up as a key ingredient in denture cleansers or multipurpose powders.
Safety & Operational Standards
Authorities such as the Occupational Safety and Health Administration (OSHA) set rules that keep workers safe when handling bulk sodium hydrogen carbonate. The dust can irritate lungs or eyes in high concentrations, so proper ventilation and dust masks save headaches and health claims. Food standards agencies limit heavy metal contamination, and drinking water codes set allowable levels downstream from processing plants. Strict batch testing prevents any substandard lots from entering shops or kitchens, and tertiary packaging reduces the risk of accidental contact with incompatible chemicals. The compound has a practically non-existent flammability, and environmental risk remains limited—proper disposal in wastewater rarely poses regulatory headaches except in massive spills.
Application Area
Bakers prize sodium hydrogen carbonate for its leavening abilities, giving rise to countless breads, cookies, and cakes. In households, it acts as a deodorizer in fridges and trash cans, and as a gentle cleaning scrub for surfaces and teeth. Medical teams use it to correct certain types of metabolic acidosis, and aquarists maintain water pH with careful dosing. Fire departments carry sodium hydrogen carbonate to knock down small grease and electrical fires. Scientists and water treatment experts add it to balance chemical reactions or buffer acid spills in the lab or field. Its versatility finds new outlets every year, from sustainable packaging to animal husbandry, with manufacturers keen to exploit its benign safety profile and broad compatibility.
Research & Development
Researchers dig deeper into sodium hydrogen carbonate’s ability to capture and sequester carbon dioxide, with newer processes looking to address climate change. Advances in encapsulation technologies aim to better control how and when the compound reacts to acidic triggers—especially in pharmaceutical or agricultural settings, where precise dosing cuts costs and boosts reliability. Material engineers explore new uses for sodium hydrogen carbonate in biodegradable foam or as a gentle abrasive in consumer goods, while agriculture scientists refine its utility as a fungicide and soil conditioner. These efforts often draw on cross-disciplinary partnerships, combining chemical engineering know-how with real-world data from field trials or clinic audits.
Toxicity Research
Despite a reputation for safety, sodium hydrogen carbonate attracts periodic scrutiny from public health officials. Ingesting moderate amounts rarely causes harm for most people, though chronic overuse can disturb electrolytes in at-risk populations. Accidental inhalation of fine powder, especially in occupational settings, can trigger respiratory irritation or short-term discomfort. Medical literature documents complications at high intravenous doses, but these arise mainly from misuse or dosing errors. Ongoing animal studies explore the compound’s safety margins in food and water at levels well above usual dietary exposure. Environmental studies show it breaks down harmlessly over time, with limited effects on aquatic life. By staying aware of updated toxicity thresholds and refining guidelines, health and safety experts help ensure continued confidence in this familiar ingredient.
Future Prospects
Demand for sodium hydrogen carbonate looks set to climb as industries search for greener, safer alternatives to harsher chemicals. Growth in plant-based foods and clean-label processed products fuels a steady thirst for well-proven leaveners and acid reducers. Efforts to slash greenhouse gases bring attention to sodium hydrogen carbonate’s role in carbon dioxide capture. Medical research continues to probe its buffering effects, targeting conditions linked to acid build-up. Manufacturing improvements may unlock new grades with ultra-low contaminants for advanced applications in electronics and pharmaceuticals. Changes in consumer habits—more interest in natural cleaning agents and less use of synthetic fragrances—may push households to rediscover many old uses. Because regulatory and environmental requirements always evolve, manufacturers and regulators will keep honing safety and quality controls to keep pace with global markets and changing public priorities.
What is Sodium Hydrogen Carbonate used for?
Everyday Uses Beyond the Kitchen
Sodium hydrogen carbonate, usually called baking soda, hides in most kitchen cupboards, but its reach goes well beyond baking cookies and cakes. I keep a box under the sink not just for cookies, but to tackle funky smells in the fridge, or sprinkle a dash to scrub the stubborn stains in sinks and tubs. Years ago, after a bad oven spill, a baking soda paste was the only thing that lifted the burnt mess. It’s a natural cleaner, washes away easily, and doesn’t fill the home with harsh chemical scents.
Health and First-Aid Go-To
Anyone who’s ever suffered a bout of heartburn knows about antacids. Baking soda can settle an upset stomach. Doctors have recommended it to temporarily relieve acid indigestion, and while it isn’t a cure-all, it gets people out of discomfort in a pinch. A mixture with water can also calm insect bites and soothe mild sunburn.
Helping Out in the Laundry Room
Baking soda finds more work in the laundry basket than one might guess. Socks come out fresher, stained shirts look brighter, and towels get a second life. In my own household, we fight summer grass stains and muddy paw prints with it—straight into the wash, and it lifts odors that regular detergent can’t touch. Research from the American Cleaning Institute shows that baking soda boosts detergent performance and softens water, giving better results with less chemical load in every load.
Gardens and Green Spaces
Gardeners talk about baking soda as a gentle way to keep fungal growth off roses and vegetables. Dusting a bit around tomato plants keeps mildew in check without harming the soil. With so many families growing a few herbs or tomatoes on balconies and patios since lockdowns, baking soda keeps backyard gardening practical and less reliant on synthetic pesticides.
A Backstage Star in Industry
Few people know that this kitchen champion plays a huge part in firefighting. It helps put out small grease and electrical fires; a giant box sits near my stovetop for that reason. In larger quantities, industries use it to prevent explosions in coal mines, clean machinery, and control emissions at power plants. Food processing plants depend on baking soda for pH control and cleaning; the U.S. Food and Drug Administration lists it as a “generally recognized as safe” ingredient.
Environmental Solutions
Urban wastewater treatment uses baking soda to neutralize acids, improve water quality, and help microorganisms clean up pollution. Even NASA uses it, treating astronaut waste on the International Space Station. With more people speaking up about chemical run-off and water contamination, baking soda stands out as a mild, biodegradable alternative. Studies, like those published in Environmental Science and Technology, have shown it works without spiking harmful byproducts.
Connecting Tradition and Science
Sodium hydrogen carbonate might seem simple, but it stands right at the intersection of old traditions and modern science. Whether cleaning a countertop, calming an upset stomach, or making the energy sector cleaner, it’s an ingredient that demands respect for its versatility and proven track record. More folks could take advantage of its friendly power, cutting their chemical use, and maybe finding a bit of homegrown magic along the way.
Is Sodium Hydrogen Carbonate safe for consumption?
Understanding Sodium Hydrogen Carbonate
Sodium hydrogen carbonate, better known as baking soda, shows up in most kitchens across the world. It looks like a basic white powder, but it’s been a staple in home baking, cleaning, and even in health remedies for ages. I’ve kept a box tucked away for everything from sour stomachs to scrubbing my sink. Someone who has tried to put out a grease fire or whip up a batch of cookies probably knows the value of baking soda.
Baking Soda in Food and Medicine
Baking soda earns its way into recipes, especially those needing a leavening agent. Chefs and home bakers use it because it helps doughs and batters get that light, fluffy texture. The U.S. Food and Drug Administration lists sodium hydrogen carbonate as “generally recognized as safe” (GRAS) when used as intended in food. The body handles small amounts pretty well. It works by neutralizing acids, which is why it calms heartburn. Pharmacists and doctors have recommended it for many years to ease mild digestive discomfort.
The Facts Behind Safety
Baking soda has a good safety record if used responsibly. That’s important, because excess sodium can cause trouble for people with certain health issues. The sodium in baking soda pulls in water and can raise blood pressure, making it risky for folks with heart disease, kidney problems, or high blood pressure. The Centers for Disease Control and Prevention echoes this concern — most adults in America eat too much sodium, not just from baking soda, but from lots of processed foods. Still, using it to bake a cake or settle an upset stomach poses little harm for healthy people. Trouble mostly comes from large or repeated doses, especially if taken without checking with a health professional.
Potential Risks and Overuse
Some social media posts and home remedy websites suggest occasional “cleanses” or self-treatments using baking soda. From my own experience, advice from a doctor always beats something picked up from an online post. Swallowing large or repeated amounts of sodium hydrogen carbonate can mess with your body’s acid-base balance. Symptoms like nausea, cramps, and even more dangerous conditions can develop. The National Capital Poison Center receives about 140 calls every year about baking soda overdoses, usually from well-meaning people who wanted a quick fix for stomach troubles.
Solutions and Smart Use
People deserve clear information so they can use household items safely. Companies now print suggested serving sizes and medical warnings on labels. This helps, but sometimes the language gets buried in fine print. More outreach from health organizations about safe uses for baking soda would help. Most people use it safely as a leavening agent or a mild antacid. If someone needs it for a chronic condition or wants to use it regularly for heartburn, it pays to talk with a doctor or pharmacist first.
Trusted Resources Matter
Trust in science-backed information keeps consumers safe. The U.S. FDA, World Health Organization, and many national health services review household chemicals for safety. Their advice comes from research, not rumor. As with all things, a little is good, but more doesn’t mean better. Using sodium hydrogen carbonate as part of normal cooking or for occasional heartburn relief stays in the safe zone, as long as you respect common-sense limits.
What are the storage requirements for Sodium Hydrogen Carbonate?
Everyday Chemistry, Real-World Challenges
Anyone who’s opened a box of baking soda to freshen the fridge or clean a coffee carafe knows sodium hydrogen carbonate by a simpler name: baking soda. It’s hard to find a household or a laboratory without it. Some see it purely as a cooking essential, others recognize its value for fire suppression or as a gentle cleaning agent. Despite the familiarity, storing it right takes practical know-how and a bit of care. Even something as common as baking soda can cause trouble when storage takes a back seat to convenience.
Why Conditions Matter
Sodium hydrogen carbonate absorbs moisture with ease. Clumps, reduced reaction in recipes, and diminished fire suppression power all trail from humidity, not to mention the chance it could cake so badly it’s unusable. Folks who keep boxes of baking soda under kitchen sinks or laboratory shelves know that all it takes is a bit of humidity to turn powdery goodness into a chalky mass. To keep the powder flowing, keep it dry. Storing it in an airtight container beats the cardboard box the grocery store provides every time. Glass, metal, or heavy-duty plastic cans do the trick in kitchens and classrooms. Keeping it off the floor and away from drains reduces risks nobody wants to deal with, especially at scale in manufacturing or chemical storage.
Heat Brings a Different Set of Problems
Leave sodium hydrogen carbonate near radiators or heating vents, it will break down before you need it. When it gets too warm, the powder loses water and carbon dioxide, leaving behind sodium carbonate. That chalky residue won’t do a thing for batter or absorb those smells in the fridge. Industry sees this play out in storage warehouses where temperatures swing up and down through the seasons. Storing baking soda where the temperature stays steady, ideally below 25°C (77°F), preserves its usefulness. In our increasingly connected world, measured storage saves both money and resources, especially when costs of spoilage add up fast. Firefighters and restaurant owners who stock up for emergencies bet on those stable conditions to keep their gear and pantries ready at a moment’s notice.
Chemical Compatibility Counts
A pile of open chemical bags, jugs, and powders—most folks who’ve worked in a chemistry lab or garage have seen it. Mixing the wrong items means corrosion or even outright danger. Sodium hydrogen carbonate won’t mix well with acids. Reactions throw off carbon dioxide gas, and that gas can cause pressure to build in sealed spaces or even pop open containers if the fit isn’t tight. Safe storage calls for separation from acids, ammonium salts, and strong oxidizers. Anyone who’s juggled lab supplies or cleaned up after a leaky bottle knows how cross-contamination happens easily when proper shelves or marked bins don’t exist.
Building Habits for Good Storage
Good storage habits build on practical steps. Marking expiration dates, labeling containers, and regular checks for lumps or discoloration matter. Problems like unknown spills or swapped labels crop up fast in shared spaces. Training new employees, students, or even family members to spot trouble early keeps accidents off the record and out of the news. In industries and homes where baking soda serves dozens of uses, these habits prevent waste and keep everyone a little safer. Real experience proves: getting storage right never happens by accident.
Can Sodium Hydrogen Carbonate be used for cleaning purposes?
What Makes Sodium Hydrogen Carbonate Stand Out for Cleaning?
Sodium hydrogen carbonate, more familiar as baking soda, sits in almost every kitchen. Many people grew up seeing a box of it in the fridge, under the sink, or tucked near the laundry. My grandmother swore by it, and years of real-world use keep proving her instincts right. Chemical structure aside, its real value shines through grime—and it’s not about being fancy or expensive.
This white powder tackles odor, scours out stains, and brightens up corners that other cleaners sometimes miss. Unlike harsh bleach or ammonia, sodium hydrogen carbonate offers a gentle touch. The science matches the experience: its slightly alkaline nature helps to break up dirt and grease while not damaging common household surfaces. Food-safe status means people don't risk dangerous fumes or residues that worry families with kids or pets.
Where It Earns Its Stripes: Real Uses at Home
Coffee stains in the mug? Add some baking soda, a splash of water, and a quick scrub erases the brown ring without much elbow grease. Kitchen counters that pick up the smell of onions or fish freshen up fast with a sprinkle and a wipe-down. Even old towels, stuck with musty smells, bounce back after a soak with baking soda in the wash.
Some folks deal with scuffed shoes or stained grout. A little paste of baking soda and water brings both back to life, paying off for sneakerheads and home renovators equally. I once used it to zap away a foul smell in my car after a spill. Just a cup tucked under the seat, and the odor lost the fight in a day or two.
Science Supports the Approach
Research doesn’t leave sodium hydrogen carbonate in the dark. The U.S. Environmental Protection Agency lists it among recommended mild abrasion cleaners. Studies published in journals like the Journal of Food Science outline its effectiveness in breaking down certain food-based messes. Hospitals even turn to baking soda for low-risk surface cleaning where infections matter most.
The safety profile also outperforms popular household chemicals. Ingesting a bit by accident rarely turns into a crisis—a far cry from bleach-based products, which lead to thousands of calls to poison control every year. People who battle allergies often look to baking soda as a fragrance-free solution that won’t aggravate symptoms or leave strong chemical traces.
Challenges and Smarter Solutions
Still, sodium hydrogen carbonate can’t clean everything. It struggles with greasy buildup on stove hoods or baked-on oven mess. For those jobs, many combine it with a shot of vinegar, letting the fizz do part of the lifting. This combination sometimes helps, though it doesn’t replace tough degreasers when heavy-duty cleaning calls.
Folks interested in greener living turn to baking soda as an alternative to single-use cleaning wipes and aggressive sprays. Even so, the commercial world could push for better education on safe mixes—baking soda paired with the wrong acid can create unwanted reactions. Product labels and outreach from cleaning brands may help families pick up the basics, reducing accidents caused by well-meaning but misinformed use.
Looking Ahead with an Old Standby
People value sodium hydrogen carbonate for its price, safety, and time-tested results. Families and professionals both stand to gain from exploring its simple power. Cleaners will keep evolving, but sometimes, the best solution is already on the pantry shelf, waiting to handle today’s mess with no drama and a lot of heart.
What is the shelf life of Sodium Hydrogen Carbonate?
Understanding Freshness in Everyday Ingredients
Sodium hydrogen carbonate—known as baking soda to anyone who cooks, cleans, or enjoys a simple science experiment with kids—sits in kitchen and laundry cupboards across the world. Most people buy a box, use a spoonful, and forget about the rest for months, if not years. This white powder seems almost invincible, but even something as basic as sodium hydrogen carbonate won’t last forever.
The Actual Shelf Life
Unopened containers can keep for years. Most manufacturers stamp a “best by” date on baking soda cartons, usually two or three years out. The FDA classifies sodium hydrogen carbonate as “generally recognized as safe” (GRAS), and that safety doesn’t fade much over time. Once moisture or contaminants enter the picture, things change.
Opened packages slowly lose their punch for leavening. Most home bakers notice cookies stop rising, pancakes lose their fluff, muffins turn out somewhat flat. This loss in performance has nothing to do with science fiction or invisible spoilage. Moisture and air do the dirty work, drawing out carbon dioxide from the powder long before it meets any vinegar or lemon juice.
A box sitting in the refrigerator keeps odors away but also soaks up humidity much faster. After about six months to a year, the effectiveness drops off. For anyone using it for household cleaning or odor control, that might not seem like a problem—but those looking for full strength in recipes should care. Once the reaction with acid—the magic behind baking soda—starts to fade, cakes no longer rise, and crunchy cookie edges go missing.
What Experience Teaches
My grandma would keep a single box “for everything.” She never worried about expiration dates. She used her nose and eyes. At holiday time, we’d bake gingerbread. If the dough looked flat or the house didn’t fill with that puffy yeast smell, she’d hand me the box, tell me to add a squirt of lemon juice, and watch for bubbles. No foam meant it was time for a new box.
This old kitchen trick—mixing some powder with vinegar or lemon juice and watching for fizz—works as well as any fancy test. If it doesn’t bubble, the powder’s spent. This also saves people from tossing out still-active soda just because of an arbitrary date printed on the box.
Why All This Matters
In food service or labs, predictable outcomes build trust and safety. An off batch of dough or a weak cleaning solution can cost money or, worse, health. Reliable shelf life information protects time, reputation, and taste. In homes, families want to keep costs down and waste low. A little knowledge about how sodium hydrogen carbonate degrades helps everyone.
Extending Shelf Life and Making Better Choices
Storing sodium hydrogen carbonate in a cool, dry place without strong odors pays off. Airtight containers slow down the process, keeping moisture out. Mark the date you opened it, use the fizz test from time to time, and you’ll always know if it’s good for tomorrow’s baking or ready for scrubbing the sink.
Companies and educators can help by providing clearer guidelines on packaging. Best-by dates work, but nothing beats teaching a child how chemical reactions work—and letting them test it for themselves.
References
- U.S. Food and Drug Administration GRAS Notices
- American Baking Association: Shelf Life Guidelines
- Personal culinary experience and family kitchen practice
| Names | |
| Preferred IUPAC name | Sodium hydrogencarbonate |
| Other names |
Sodium bicarbonate Baking soda Bicarbonate of soda Bread soda Cooking soda Saleratus Nahcolite |
| Pronunciation | /ˌsəʊ.di.əm haɪˈdrɒdʒən ˈkɑː.bə.neɪt/ |
| Preferred IUPAC name | Sodium hydrogen carbonate |
| Other names |
Baking Soda Sodium Bicarbonate Bicarbonate of Soda Carbonic Acid Monosodium Salt Sodium Acid Carbonate Monosodium Carbonate |
| Pronunciation | /ˌsəʊ.di.əm haɪˈdrɒ.dʒən kɑːˈbɒn.eɪt/ |
| Identifiers | |
| CAS Number | 144-55-8 |
| 3D model (JSmol) | `Na([O-])C(=O)O` |
| Beilstein Reference | '1713882' |
| ChEBI | CHEBI:32139 |
| ChEMBL | CHEMBL1359 |
| ChemSpider | 5749 |
| DrugBank | DB02238 |
| ECHA InfoCard | 03-2119483205-54-0000 |
| EC Number | 207-838-8 |
| Gmelin Reference | 37982 |
| KEGG | C01353 |
| MeSH | D017782 |
| PubChem CID | 516892 |
| RTECS number | VZ0950000 |
| UNII | OE875S8Z99 |
| UN number | UN3077 |
| CAS Number | 144-55-8 |
| 3D model (JSmol) | `Na([O-]C(=O)O)` |
| Beilstein Reference | 1713882 |
| ChEBI | CHEBI:32139 |
| ChEMBL | CHEMBL1357 |
| ChemSpider | 5758 |
| DrugBank | DB01390 |
| ECHA InfoCard | 100.011.004 |
| EC Number | 205-633-8 |
| Gmelin Reference | 784 |
| KEGG | C01353 |
| MeSH | D017782 |
| PubChem CID | 516892 |
| RTECS number | VZ0950000 |
| UNII | OEHBP1HLU6 |
| UN number | UN2808 |
| Properties | |
| Chemical formula | NaHCO3 |
| Molar mass | 84.01 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.16 g/cm³ |
| Solubility in water | 8.6 g/100 mL (20 °C) |
| log P | -7.68 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.3 |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Dipole moment | 1.87 D |
| Chemical formula | NaHCO3 |
| Molar mass | 84.01 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | D=2.16 g/cm3 |
| Solubility in water | 7.8 g/100 mL (20 °C) |
| log P | -3.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.3 |
| Basicity (pKb) | pKb = 7.7 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Dipole moment | 1.41 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 102.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −947.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -947.7 kJ/mol |
| Std molar entropy (S⦵298) | 102.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –947.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -947.7 kJ/mol |
| Pharmacology | |
| ATC code | A02AH01 |
| ATC code | A02AH01 |
| Hazards | |
| Main hazards | No significant hazard. |
| GHS labelling | Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| Pictograms | GHS07 |
| Signal word | No signal word |
| Hazard statements | Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| NFPA 704 (fire diamond) | 0-0-0-Special |
| Explosive limits | Non explosive |
| Lethal dose or concentration | LD50 (oral, rat): 4220 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 4220 mg/kg |
| NIOSH | SC 297 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 mg/kg |
| IDLH (Immediate danger) | Not listed |
| Main hazards | Not a hazardous substance or mixture. |
| GHS labelling | Not a hazardous substance or mixture according to GHS |
| Pictograms | GHS07 |
| Signal word | No Signal Word |
| Hazard statements | Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. |
| Precautionary statements | Precautionary statements: "Store in a dry place. Store in a closed container. Dispose of contents/container in accordance with local/regional/national/international regulations. |
| NFPA 704 (fire diamond) | 0-0-0 |
| Explosive limits | Non explosive |
| Lethal dose or concentration | LD50 oral rat: 4220 mg/kg |
| LD50 (median dose) | LD50 (median dose) Oral Rat: 4220 mg/kg |
| NIOSH | VZ0950000 |
| PEL (Permissible) | 10 mg/m³ (total dust) |
| REL (Recommended) | 500 mg/kg |
| IDLH (Immediate danger) | Not listed. |
| Related compounds | |
| Related compounds |
Sodium carbonate Potassium bicarbonate Ammonium bicarbonate Calcium bicarbonate Potassium carbonate Sodium hydroxide |
| Related compounds |
Sodium carbonate Sodium chloride Potassium bicarbonate Calcium carbonate Sodium hydroxide |

