Sodium Gluconate: A Deep Dive into Its Development, Properties, and Future
Historical Development
Sodium gluconate doesn’t burst onto the scene out of nowhere. Early uses of gluconic acid came from folks fermenting glucose-rich fluids, sometimes linked to food spoilage. By the early 20th century, chemical researchers figured out that sodium gluconate, the salt form, could be sourced by fermenting natural feedstocks like corn syrup or sugar. As the world’s appetite for safer, more sustainable chelating agents grew, industries in Europe, North America, and China redirected attention toward sodium gluconate. Cement companies, urban planners, water treatment operators, even food processors, started to see value in something as simple as a five-carbon sugar acid paired with a basic sodium ion. Over decades, companies invested in large-scale fermenters, purification routines, and built technical expertise, not just for producing, but for tailoring its use across dozens of applications.
Product Overview
On paper, sodium gluconate looks unassuming—a white, odorless, crystalline powder or sometimes a granular substance. It dissolves well in water, resists caking if kept dry, and doesn’t clump up the way some salts do. What really sets it apart? That carboxyl group and chelating backbone do some heavy lifting: binding metal ions with surprising strength, inhibiting scaling, and stabilizing formulations even in harsh conditions. Food manufacturers, construction managers, and cleaning product scientists didn’t choose this compound by accident; they tried dozens of others, but came back to sodium gluconate thanks to its predictable behavior and reliable sourcing.
Physical & Chemical Properties
Sodium gluconate’s chemical formula is C6H11NaO7, with a molecular weight of around 218.14 g/mol. This compound scores high marks for solubility—it jumps into solution fast, up to about 60g in 100ml of water at room temperature, no fussing with special solvents. Its pH drifts a bit alkaline, which fit nicely for cement additives and detergent use. It melts at relatively high temperatures, above 200°C, and degrades cleanly without hazardous by-products. The gluconate backbone binds with calcium, magnesium, iron, and other problem ions, fending off scale in pipes, keeping concrete smooth, and protecting industrial boilers from mineral build-up. Its ability to shut down metal-catalyzed reactions gives it a safety edge in complex mixes—the same thing that helps it keep foods fresher, longer.
Technical Specifications & Labeling
Manufacturers spell out purity levels north of 98% for food or pharmaceutical grades, with strict controls on moisture, heavy metals, and insoluble residue. Each batch ships with a certificate listing appearance, pH range, heavy metal content, loss on drying, and sometimes microbial load. Bags, cartons, or drums feature clear labeling with grade, batch date, and country of origin. Supply contracts often dictate non-GMO, allergen, or vegan status, especially for food or cosmetic use. Customers in construction or water treatment tend to focus more on solubility, shelf life, and compatibility with other chemicals.
Preparation Method
The backbone of modern sodium gluconate production comes from aerated fermentation of glucose-rich syrup—derived mostly from corn. Using specialized strains of Aspergillus niger or similar molds, processors feed in glucose and manage airflow, temperature, and pH to get the maximum conversion to gluconic acid. Filtration removes the fungus, and caustic soda neutralizes the acid, instantly generating the sodium salt. Filtration, granulation, and drying steps follow, each aiming for a pure, stable product. Compared to older chemical oxidation routes, fermentation puts less strain on the environment. Operators, myself included, favor this approach, since it produces less hazardous waste, uses renewable feedstocks, and can be scaled up or down as market demands shift.
Chemical Reactions & Modifications
Sodium gluconate acts as more than a simple chelator or pH adjuster. In concrete, it slows setting time by keeping calcium ions out of action a bit longer, giving workers breathing room on large pours. In detergents, it latches onto dissolved metals so they don’t ruin cleaning action. Some newer research turns up ways to tweak its backbone—adding hydrophobic tails for specialty surfactants, linking with polymers to form biodegradable gels, or fusing it with zinc or iron for nutrition and medicine. Sodium gluconate also finds friends in the lab: it pairs nicely with citric, tartaric, and lactic acids, sometimes acting as a stabilizer or additive for rare chemistries. Its mildness and ease of reaction encourage all kinds of experimentation, which keeps labs busy with new ideas for green chemistry.
Synonyms & Product Names
In catalogs and supply sheets, you might see sodium gluconate labeled as D-gluconic acid sodium salt, sodium salt of gluconic acid, or E576 (when added to foods). Other suppliers promote it under registered trademark names, especially for construction-grade or pharma-grade lines, each vying for a slice of the global market. Knowing these synonyms gets buyers past confusion, covering naming differences in Europe, North America, and Asia. One universal: suppliers push traceability, listing CAS number 527-07-1 for all grade levels.
Safety & Operational Standards
Sodium gluconate stands out for a strong record on human and environmental safety. Regulatory agencies including the US Food and Drug Administration and the European Food Safety Authority permit it for food use up to certain levels without special restrictions. Proper storage—dry, cool, ventilated—keeps it safe from caking or contamination. Operators should avoid unnecessary dust exposure; particle control and masks matter in bulk handling, just like with any fine powder. Incineration or landfill disposal gets approved in most regions, but wastewater plants prefer advanced treatment—its breakdown products feed bacteria but can tip the balance if dumped in large amounts. Regular batch testing, clean-room operations in pharma or food settings, and compliance with ISO quality standards form the backbone of responsible production.
Application Area
Looking around cities or grocery stores, sodium gluconate’s fingerprints are everywhere. Cement and concrete get smoother pours and fewer cracks thanks to its setting-delaying action—ask any builder pouring a highway or bridge deck on a hot summer afternoon. In detergents and cleaners, it keeps water minerals from fouling up surfactants, so you end up with spotless surfaces and fewer residues. Food companies rely on it as a sequestrant, stabilizer, or acidity regulator, showing up in processed cheese, leavened baked goods, and preserved vegetables. Water treatment operators add it to stop scale and keep metal pipes clear, especially in drinking water plants and industrial cooling towers. More recent developments include pharmaceuticals—where it carries magnesium or calcium ions—or even cosmetics, boosting shelf life and texture. In my own work, switching to sodium gluconate in cleaning products took headaches out of complying with phosphate bans, because it’s gentle on waterways and aquatic life.
Research & Development
Teams in university and industry labs keep pushing sodium gluconate into new territory. Microbial fermentation methods get frequent upgrades, including gene-edited strains for bigger yields or faster runs. Polymer chemists blend it into bioplastics, looking for stronger, safer, and greener materials. Green concrete researchers tweak dosages to lower CO2 emissions without weakening the end result. Ecotoxicology researchers track its fate in soil and water, while food scientists test its ability to protect nutrients and extend shelf life without off-flavors. Some expansion comes from regulatory drivers—tightening rules around phosphates, nitrates, and synthetic chelators create market pull for safe alternatives. Collaboration across sectors—food, cleaning, construction, healthcare—brings down barriers, building better products with fewer compromises.
Toxicity Research
Most toxicity studies back up what you see from practical use: sodium gluconate rates low for skin, inhalation, and ingestion hazards. Regulatory filings and independent studies report high LD50 values in animals, no evidence of mutagenicity or carcinogenicity, and minimal effects on wildlife when used responsibly. Researchers track its breakdown and biocompatibility, confirming rapid microbe-led decomposition in soil and water. Still, as with all industrial salts, chronic high-dose exposure or improper disposal brings risks—so responsible operators follow best practices and ensure proper disposal. Data transparency, open publication of toxicity findings, and early reporting of adverse effects help keep industry and consumers on safe ground.
Future Prospects
If current patterns hold, demand for sodium gluconate looks set to keep rising. Urbanization, stricter environmental controls, booming packaged foods, and growth in water reuse all drive greater uptake. Researchers probe new uses in green energy—battery electrolytes, eco-friendly coatings, even as biostimulants for sustainable farming. Digital twins and process automation optimize fermentation and scale-up, lowering costs and carbon footprints even more. There’s space for smarter sourcing too: companies turning to non-corn, non-GMO feedstocks bring new players to the table. All the while, regulatory pushes against old, dirty, or persistent chemicals keep sodium gluconate attractive for manufacturers, policymakers, and end users alike. Even after a century of use, this workhorse salt keeps offering practical answers to both old and new challenges.
What is Sodium Gluconate used for?
Everyday Life and Industry Meet in the Same Powder
You probably spot sodium gluconate on food labels, but its reach goes far beyond a grocery cart. This powder, derived from glucose, helps people out in ways that don’t always stand out. In my own time working around commercial kitchens, hospital supply rooms, and even construction sites, I kept running into sodium gluconate in places that surprised me.
The Food Connection
Most people know it as a food additive. It stabilizes flavors and helps preserve food by slowing down spoilage. Restaurants and packaged food makers rely on it to keep meats fresh and dressings from separating. The FDA and European Food Safety Authority both consider it generally safe under standard guidelines. It doesn’t add its own taste, so you don’t notice it’s there making things more stable. That behind-the-scenes role actually does a lot for food safety and storage around the world.
A Secret Helper in Cleaning
Sodium gluconate takes on much more in the cleaning world. I learned this firsthand in my own kitchen, watching stubborn stains and hard water residue on glassware. This compound acts as a chelating agent, which means it grabs hold of minerals like calcium and magnesium that show up in hard water. Instead of letting these minerals stain or interfere with detergents, sodium gluconate locks them away, so cleaning products work better. Industrial dishwashers, laundries, and janitorial services go through tons of it every year. In countries with hard tap water, it’s considered a quiet essential—without it, dishes get streaky and washing machines get grimy a lot faster.
Role in Construction Zones
I’ve spent some time volunteering for Habitat for Humanity, mixing concrete in chilly weather, and noticed contractors tossing packets into the cement mix. Turns out sodium gluconate helps control how quickly concrete sets. Builders value that kind of flexibility, especially under tricky weather. It slows down the chemical reactions in cement so crews have more time to pour, spread, and shape. Bridges, tunnels, and city sidewalks often come together with its help, especially when a quick set wouldn’t give workers enough time to get things right.
Focus on Health and Pharmaceuticals
Hospitals and pharma companies lean on sodium gluconate too. It stabilizes some medicines by helping dissolve active ingredients that would otherwise settle out or lose effectiveness. Dialysis relies on solutions with sodium gluconate to keep minerals in the right balance for patient safety. Its track record in patient care builds trust, and that’s something you can’t put a price on.
Why It Matters: Trust and Transparency
Sodium gluconate’s wide range of uses makes it a crucial ingredient in places people don’t usually notice. Regulation keeps standards high, which protects both workers and consumers. Anyone buying imported foods, using heavy detergents, or working in construction should feel confident someone checks on the safety and sourcing. The more companies share about the origins and handling of this ingredient, the better. In my experience, transparency helps people make safer choices, whether they cook, clean, build, or care for others.
Is Sodium Gluconate safe for consumption?
Understanding Sodium Gluconate
Food labels can get confusing with their long lists of ingredients, many of which look like they've popped out of a chemistry textbook. Sodium gluconate is one of those names that leaves people scratching their heads and wondering if this is just one more food additive to worry about. As someone who tries to keep an eye on what goes into food, I dug into what sodium gluconate actually does and whether it’s risky or not.
Where You Find Sodium Gluconate
Sodium gluconate turns up as a preservative and a stabilizer. Manufacturers use it because it keeps products shelf-stable, and it helps certain foods hold their texture and taste. You’ll find it listed in baked goods, dairy products, pickles, and sometimes even in personal care products. The food industry values its chelating property—it grabs hold of minerals that could discolor food or make it spoil faster.
The Science Behind the Ingredient
Plenty of food additives face real scrutiny. With sodium gluconate, health authorities studied it before giving the green light. Both the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) have reviewed its safety. The FDA considers sodium gluconate “Generally Recognized As Safe” (GRAS) when used as intended in food. EFSA’s scientific panel also found no concerns at the approved levels for human consumption.
It’s worth pointing out that our bodies break down sodium gluconate into gluconic acid and sodium ions, which occur naturally and run through normal metabolic pathways. Gluconic acid actually shows up in fruits and honey. It passes through the digestive tract and leaves the body without lingering or building up.
Looking at the Numbers
For the health-conscious, facts matter. Research so far shows that sodium gluconate doesn't cause harm at the levels typically seen in food. It hasn’t been linked with cancer, genetic mutation, or developmental issues based on animal and cell studies reviewed by scientists in North America and Europe. Toxicology studies set safe exposure limits well above what anybody would get from a typical diet.
What About Sensitive Groups?
Some folks need to keep a closer eye on sodium for blood pressure reasons, and that’s where a big difference lies. Sodium gluconate adds some sodium to food, though the actual amount in a serving is fairly low compared to table salt. If you’re managing hypertension, it helps to take note of all sources of sodium. Swapping out highly processed foods for fresh options brings bigger benefits than worrying about sodium gluconate by itself.
Practical Tips and Personal Approach
After looking at the science and real-world intake, it makes sense to treat sodium gluconate as a low-risk ingredient. Like any food additive, it works best in moderation. If the ingredient list seems overwhelmingly long or filled with unfamiliar names, cooking from scratch or choosing whole foods offers better peace of mind. Tracking total sodium matters more than getting hung up on this one stabilizer.
To sum it up, sodium gluconate shows up on labels, but evidence puts it in the safe column. It’s smart to stay informed, read labels, and keep nutrition balanced. Trusting food safety experts and peer-reviewed science goes a long way for building confidence in what you eat.
What industries commonly use Sodium Gluconate?
Concrete and Construction
Anyone who has watched a big building go up knows the importance of timing and strength. Concrete needs time to set right, especially on large projects or in hot weather. Sodium gluconate works well here. It holds back the setting, giving crews longer to work, pour, and shape what they need. Concrete comes out smoother and stronger, not rushed or cracked. Builders rely on this compound to protect work from heat and speed, and ultimately to avoid costly mistakes.
Water Treatment
City homes and big factories count on clean water. Sodium gluconate helps pull out old minerals and metals, especially iron and manganese. Water treatment plants rely on this cleaning action to keep pipes clear and extend the life of their machines. Without it, scale builds up, breaking down filters and clogging systems. Anyone who’s had a rusty or clogged tap at home can see why water companies look for ways to manage minerals—sodium gluconate is a steady helper in that fight.
Detergents and Cleaning Products
Laundry day doesn’t get much attention until things go wrong. Faded colors, rough clothes, or stubborn stains often come from hard water. Sodium gluconate steps in to soften water, letting detergents do their job. It grabs on to the metals that make water “hard,” keeping them from reacting with soap. People use less detergent this way. Companies like the cost savings, and so do families dealing with stubborn stains.
Food Industry
Food factories and kitchens use sodium gluconate for more reasons than most would guess. It keeps canned vegetables firm, often shows up in dairy products, and controls acidity in soft drinks. This matters for taste, shelf life, and safety. Sodium gluconate is considered safe by most food safety watchdogs, which carries real weight in a world where food recalls are never far from the news. Producers like it for its dependability and the level of control it offers during big batch production.
Pharmaceuticals and Medicine
Hospitals and pharmacies don’t just worry about treating disease; they look at how medicine holds together and absorbs in the body. Sodium gluconate helps balance minerals in IV fluids, which shows up in patient care every day. Calcium, magnesium, and iron all need tight control to avoid side effects. By binding with these metals, sodium gluconate supports both safety and healing, giving doctors more options in complex treatments.
Personal Experience and Looking Forward
Having worked in water treatment, I know first-hand how scale buildup can wreck expensive equipment and ruin flow. Sodium gluconate made maintenance a lot less stressful. Colleagues in food processing see it as an insurance policy—one small change in acidity or texture can spoil a whole batch. While few outside these fields talk about this chemical, its absence would cause a lot of headaches. As industries look for greener and safer ways to solve old problems, the track record of sodium gluconate gives it a lasting place in the toolkit. Companies keep searching for safe, smart ways to keep things clean, strong, and stable, and this humble compound fits that goal.
What are the benefits of using Sodium Gluconate in cleaning products?
Unlocking Real Cleaning Power
Walking through any store aisle packed with cleaning products, it's hard to spot what's actually doing the work. After spending years cleaning up after grimy projects and family messes, I've come to notice that some products simply cut through tough stains and hard water spots better than others. Looking at labels and talking with folks in the cleaning business, sodium gluconate kept popping up as a secret weapon.
Hard Water Doesn’t Stand a Chance
Homeowners battle water spots and stubborn limescale in showers, kitchens, and even washing machines. Most of that trouble traces back to minerals in hard water—calcium and magnesium showing up as crusty deposits. Sodium gluconate steps in by grabbing onto those minerals before they settle, stopping scale in its tracks. That means fewer repeat scrubs and less wear on appliances. I’ve seen dishwashers last longer simply because the detergent included this ingredient.
Safe for Health and Planet
Every parent, landlord, or pet owner wants to know what's left behind after a deep clean. A big selling point for sodium gluconate is its profile—derived from glucose and totally biodegradable. It skips the harsh fumes, avoids skin irritation, and doesn’t leave behind nasty residues. Splashes and spills become less of a headache, a relief in homes with crawling kids or curious pets. After years spent outdoors and in messy garages, using cleaners without harsh side effects sure pays off.
No Tarnish, No Worries
Cleaning old sinks, car parts, or hand tools made from metal is a regular job for many. Some strong cleaning products strip away grime but take the shine with them, especially on softer metals like aluminum. Sodium gluconate plays it differently—it cleans without attacking the surface. Even in industrial shops or commercial kitchens, it's prized for keeping metals looking new. This saves money on replacement parts and keeps things working smoothly.
Boosting Detergent Performance
Laundry days can drag on if dirt clings to fabric because soaps get tangled up with minerals. By locking away the minerals, sodium gluconate lets detergents stay focused on stains and dirt, not wrestling with hard water. That means cleaner clothes, brighter whites, and fewer repeat washes. After line-drying countless loads, the difference in softness stands out when this ingredient shows up in laundry solutions.
Backing Up the Science
Plenty of research backs these claims. Studies in major journals point out how sodium gluconate outperforms traditional builders in managing water hardness and improving cleaner results. Regulatory agencies note its safety profile and eco-friendliness. Big brands rely on it to meet rising sustainability targets and cut back on harsher chemicals. The Environmental Working Group (EWG) gives it a thumbs-up for low health hazards.
Finding Real Solutions at Home
Cleanliness at home or work depends on more than just elbow grease. Smart choices in cleaners mean easier maintenance, safer environments, and less waste. Ingredients like sodium gluconate deliver that edge, helping folks avoid the frustration that comes from trying to scrub away what water leaves behind. By switching to cleaners that use proven helpers, everyone wins: fewer headaches, cleaner spaces, longer-lasting tools and appliances, and a lighter footprint on the environment.
Does Sodium Gluconate have any side effects?
A Common Ingredient That Rarely Gets Attention
Sodium gluconate shows up on food labels, cleaning products, and even in cement mixes. In the kitchen, it works as a preservative and stabilizer. In medicine, it helps balance electrolytes. Grocery shoppers spot it in processed foods and beverages. Its widespread use mostly goes unnoticed.
Why Some Folks Worry About It
Curiosity usually kicks in for good reasons. Any chemical with sodium in the name often triggers worry, especially among people managing high blood pressure, kidney issues, or those who follow low-salt diets. Claims about “harmless additives” rarely reassure anyone. I’ve watched family members scan nutritional labels, searching for unfamiliar words, and wonder which ones will affect their health.
What Science Says About Safety
Most food safety agencies, including the FDA and EFSA, classify sodium gluconate as safe at levels found in foods. Human bodies process and eliminate it without much fuss. It passes through the kidneys and comes out in urine for most healthy people. No strong evidence ties sodium gluconate to toxic reactions, genetic harm, or cancer, according to several peer-reviewed studies.
Researchers have given it to rats and watched for changes. The animals tolerated far higher doses than anyone would ever get in a typical diet. Scientists didn’t find any lasting inflammation, organ damage, or odd behavior. Doctors use sodium gluconate in intravenous solutions during medical treatments, and patients seldom report unusual effects.
Potential Side Effects: Who Faces Risk
Most folks don’t feel anything from everyday levels found in food and drink. Stomach issues seem rare. An individual might get diarrhea or mild cramping after heavy consumption, but that requires more intake than most packaged foods deliver. People with preexisting kidney problems could see trouble if their bodies struggle to filter out extra sodium. In these cases, the additive contributes to an overall sodium load—not just as sodium gluconate, but from all sources combined. Elderly adults and infants can’t tolerate excess minerals as easily as healthy adults, so doctors still watch intake closely.
Topical or household uses, such as detergents or concrete treatments, almost never cause skin or eye problems. People with allergies to corn should read labels carefully, since manufacturers sometimes derive sodium gluconate from corn glucose.
Weighing Its Place In The Modern Diet
Processed food diets almost always carry more salt and food additives than home-cooked meals. When sodium gluconate appears on a label, it tells me to pause and review overall sodium intake for the day. The American Heart Association suggests keeping sodium well below 2,300 milligrams per day for adults. For someone with hypertension or kidney trouble, that number could drop by nearly half. If multiple products in the cart contain sodium gluconate, the numbers add up quickly.
Avoiding sodium gluconate altogether isn't practical for most families. I’ve seen that education works better. Reading every label empowers me to make smaller, smarter swaps. Whole foods—potatoes, beans, greens, unseasoned meats—keep additive levels low. Anyone worried about reactions ought to track symptoms and discuss label findings with their doctor. Knowledge often beats fear, at least in this kitchen.
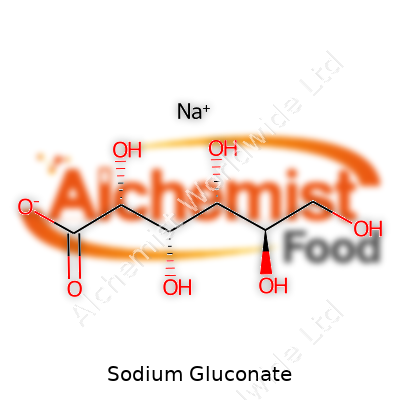
| Names | |
| Preferred IUPAC name | Sodium 2,3,4,5,6-pentahydroxyhexanoate |
| Other names |
Monosodium gluconate Gluconic acid sodium salt Sodium D-gluconate |
| Pronunciation | /ˈsəʊdiəm ɡluːˈkoʊneɪt/ |
| Preferred IUPAC name | Sodium 2,3,4,5,6-pentahydroxyhexanoate |
| Other names |
Monosodium gluconate Sodium salt of gluconic acid Gluconic acid sodium salt |
| Pronunciation | /ˌsəʊdiəm ɡluːˈkoʊneɪt/ |
| Identifiers | |
| CAS Number | 527-07-1 |
| Beilstein Reference | 1904746 |
| ChEBI | CHEBI:61343 |
| ChEMBL | CHEMBL1201781 |
| ChemSpider | 5716 |
| DrugBank | DB11165 |
| ECHA InfoCard | 100.016.320 |
| EC Number | EC 209-043-4 |
| Gmelin Reference | 23356 |
| KEGG | C00744 |
| MeSH | D018139 |
| PubChem CID | 23672374 |
| RTECS number | LJ5990000 |
| UNII | S00XD2D6SV |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID7020286 |
| CAS Number | 527-07-1 |
| Beilstein Reference | 1718739 |
| ChEBI | CHEBI:61343 |
| ChEMBL | CHEMBL1201543 |
| ChemSpider | 5310956 |
| DrugBank | DB15744 |
| ECHA InfoCard | '03bde1a7-844a-483a-bf2f-4eab83e98da6' |
| EC Number | 208-407-7 |
| Gmelin Reference | 119204 |
| KEGG | C01732 |
| MeSH | D019294 |
| PubChem CID | 23665761 |
| RTECS number | MB3435000 |
| UNII | F7LTH1S9LF |
| UN number | UN3077 |
| Properties | |
| Chemical formula | NaC6H11O7 |
| Molar mass | 218.14 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | Sodium Gluconate has a density of approximately 1.65 g/cm³. |
| Solubility in water | Easily soluble in water |
| log P | -3.7 |
| Acidity (pKa) | 13.7 |
| Basicity (pKb) | 11.98 |
| Magnetic susceptibility (χ) | Magnetic susceptibility (χ): -64.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.495 |
| Viscosity | 20~50 mPa·s (20°C) |
| Dipole moment | 4.51 D |
| Chemical formula | C6H11NaO7 |
| Molar mass | 218.14 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | Densitу: 0.86 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.7 |
| Vapor pressure | negligible |
| Acidity (pKa) | 13.6 |
| Basicity (pKb) | 13.6 |
| Magnetic susceptibility (χ) | −62 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.49 |
| Viscosity | 30-40 cps |
| Dipole moment | 3.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 200.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1610.08 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –3792 kJ/mol |
| Std molar entropy (S⦵298) | 165.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –1610 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2214 kJ/mol |
| Pharmacology | |
| ATC code | A11HA01 |
| ATC code | A11HA01 |
| Hazards | |
| Main hazards | May cause mild skin and eye irritation. |
| GHS labelling | GHS07, Warning, Causes serious eye irritation. |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 7,520 mg/kg (rat, oral) |
| NIOSH | Not Established |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 300 mg/kg bw |
| Main hazards | Not hazardous under normal conditions of use. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Not Hazardous |
| Hazard statements | Hazard statements: Not classified as hazardous according to GHS. |
| Precautionary statements | Keep container tightly closed. Store in a dry place. Avoid contact with eyes, skin, and clothing. Wash hands thoroughly after handling. Do not eat, drink or smoke when using this product. |
| Lethal dose or concentration | LD50 (oral, rat): 7,530 mg/kg |
| LD50 (median dose) | LD50 (median dose): 7,530 mg/kg (oral, rat) |
| NIOSH | Not Listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 500 mg/day |
| IDLH (Immediate danger) | Not listed. |
| Related compounds | |
| Related compounds |
Gluconic acid Calcium gluconate Potassium gluconate Iron(II) gluconate Magnesium gluconate Zinc gluconate Copper gluconate |
| Related compounds |
Gluconic acid Calcium gluconate Potassium gluconate Iron(II) gluconate Lithium gluconate |

