Sodium Ferrocyanide: An Industry Staple with Diverse Roles
Historical Roots of Sodium Ferrocyanide Use
Long before laboratory syntheses refined our chemical landscape, people stumbled upon potassium ferrocyanide as a bright blue pigment, Prussian blue, in the 18th century. From that discovery came sodium ferrocyanide, a compound that pioneers managed to produce by adjusting the salts involved. Chemists soon realized it could be formed in bulk from cyanide wastes, especially as the textile and iron industries boomed throughout the 19th century. Over the decades, its technical uses expanded, and health agencies weighed in as its presence in food and water entered public view. Most policies about sodium ferrocyanide trace their roots back to this early industrial period when large-scale chemical plants first got established in Europe and North America.
Product Overview
Sodium ferrocyanide, often found as pale yellow crystals, pops up in sectors ranging from food processing to pigment production, and even plays a role in some analytic labs. You might recognize it under different trade names, but all roads point back to its chemical core. Common labeling reveals this salt as a food additive—more specifically as E535—in many countries. If you’ve ever checked the fine print on packaged salt and spotted E-numbers, you’ve already crossed paths with sodium ferrocyanide. End users expect bulk shipments of this compound to meet strict technical specs, often in granular or crystalline form, which factors directly into purity and safety testing.
Physical and Chemical Properties
Talking chemistry means looking at sodium ferrocyanide’s crystalline structure and stark yellow tint. This solid melts above 70°C and dissolves well in water, less so in alcohols. Chemists pin its chemical formula as Na4[Fe(CN)6]·10H2O, indicating four sodiums liganded to a hexacyanoferrate core, rounded out by ten water molecules. The salt remains stable in air, yet breaks down under intense heating or acidic conditions, releasing not just cyanide ions but also tricky gasses like hydrogen cyanide—an obvious safety concern. On the pH scale, its water solution leans slightly alkaline, typical for sodium-based salts.
Technical Specifications and Labeling
Procurement departments sort through mountains of paperwork for materials like sodium ferrocyanide simply because technical standards matter. Certified lots rarely allow more than traces of calcium, magnesium, or other similar ions. Most producers outline heavy metals content—lead, arsenic, and the like—well below various regional thresholds. Labels on bulk drums or bags typically list water of crystallization and minimum purity rates around 99%. Batch numbers, manufacture date, and food additive codes appear front and center, allowing for easy tracing across supply chains. Most safety datasheets warn of dust inhalation and suggest standard chemical storage: keep it dry, isolated from strong acids, and sealed tightly.
Manufacturing and Preparation Methods
Factories produce sodium ferrocyanide chiefly via process streams that involve reacting sodium carbonate with iron sulfate and a cyanide source, such as calcium cyanide or even scrap cyanides from other industries. One process soaks iron filings in sodium cyanide, then oxidizes the orange solution to precipitate out iron compounds before neutralizing with sodium salts. Operators closely monitor reaction conditions because a swing in temperature or pH changes more than just yield—it may liberate toxic hydrocyanic acid. Filtration, crystallization, and repeated washing follow, ensuring commercial product keeps impurities to a minimum. As with any cyanide chemistry, plant workers handle slightly outdated equipment and real human risk, not just theoretical lab hazards.
Chemical Reactions and Modifications
Sodium ferrocyanide makes its mark in coordination chemistry. Its 6 cyanide ligands on iron grant it stability, yet chemical tinkerers can break apart or swap components under the right conditions. Strong acids tip the balance, releasing hydrogen cyanide gas—a recipe for trouble in confined or unventilated spaces. On the flip side, reactions with iron(III) salts create Prussian blue pigment, still seen in dye and ink shops today. More than just legacy chemistry, sodium ferrocyanide’s reactivity opens avenues for newer, greener chemical syntheses, especially in electronics and specialty pigment fields where compound purity shapes product viability.
Synonyms and Other Product Names
Walk into a chemical supply warehouse and you’ll see sodium ferrocyanide listed under names like ‘yellow prussiate of soda’, ‘sodium hexacyanoferrate(II) decahydrate’, and even by food additive code ‘E535’. Each name ties to its historical or chemical backstory. Some suppliers favor local translations, but most technical export documents stick with the IUPAC-approved title. In the past, some confusion arose in customs or transshipment scenarios, thanks to inconsistent labeling, which drove the current push for standardized nomenclature and clear hazard icons at every stop.
Safety Protocols and Operational Standards
Anyone handling sodium ferrocyanide on a regular basis keeps safety goggles close by and maintains proper air extraction in their workspace. Cyanide chemistry, even in relatively stable forms, scares regulators for good reason. Direct ingestion or high-temp decomposition can generate acute poisoning risk. Long-term exposure policies set limits on airborne particles, so big users constantly monitor both workspaces and wastewater streams. International standards, including those pushed by the EU and the U.S. EPA, force regular review and audit of storage practices, spill response plans, and even personnel training. Labeling regulations require that shipping containers and secondary packaging indicate toxicological risk, emergency measures, and—more recently—application sphere, especially when destined for food processing plants.
Application Areas Across Industries
Table salt producers sprinkle sodium ferrocyanide as an anticaking agent to block clumping during humid months—without it, salt forms sticky lumps and gums up production lines. Photographic developers once used it to stabilize silver images, though that use faded with the rise of digital media. Pigment manufacturers still rely on its iron chemistry to produce blues that last in both artistic and industrial coatings. In water treatment, some municipalities investigate controlled use for heavy metal precipitation and as part of advanced analytic testing. Almost every major chemical distributor counts sodium ferrocyanide among its default stockpile, pointing to a broad acceptance but also to regulatory scrutiny.
Research, Development, and Emerging Uses
Academic groups still dig into sodium ferrocyanide’s unique ability to coordinate metal ions, offering a launching pad for cutting-edge sensor technologies. Recent efforts in battery R&D chase new ways to use its structure in sodium-ion battery systems, where affordability and cycling efficiency matter far more than in traditional lithium chemistries. In the world of green chemistry, researchers look to tweak this compound for safer, less toxic analogs—especially since the global regulatory trend clamps down hard on potential cyanide release. My own time collaborating on water analysis projects saw us reach for sodium ferrocyanide not just for baseline testing, but also in research on novel remediation strategies for mining runoff.
Insights from Toxicity Research
Long-term animal studies show that sodium ferrocyanide packs less acute toxicity than its infamous parent, sodium cyanide, largely due to tight cyanide binding within its crystal lattice. Under normal conditions, biological systems break down sodium ferrocyanide far less easily, so accidental ingestion or inhalation proves less dangerous than many other cyanide sources. Problems erupt if the compound hits strong acids—think spilled car battery acid on a shipment—or undergoes intense heating, both of which can drive off free hydrogen cyanide, prompting evacuation protocols. Regulatory agencies draw food residue and workplace limit lines well below established thresholds, a stance built off decades of practical toxicology research and accidental exposure case records.
Future Prospects and Industry Trends
Technological shifts toward sustainable energy open new doors for sodium ferrocyanide, especially as battery innovators seek alternatives to rare or expensive metal salts. Demand spikes keep up with new environmental regulations, pushing suppliers to streamline manufacturing and limit effluent cyanide emissions. In the food sector, stricter consumer scrutiny has driven some companies to swap out traditional anticaking agents, but global market data still shows strong usage figures where national regulations allow. As environmental scientists hammer out best practices for handling metal-cyanide complexes in wastewater, sodium ferrocyanide stands as both a legacy tool and a likely building block for the next generation of chemical solutions aimed at balancing industry needs, human health, and ecological safety.
What is sodium ferrocyanide used for?
What It Does in Food and Industry
Sodium ferrocyanide shows up in places many people don’t expect. You’ll often find it listed on food packaging, tucked in with salt, especially table salt. Its main job here is to keep salt from clumping up. Salt acts up as soon as there’s a bit of moisture, and nobody likes digging into a box of rock-solid salt. By bonding with moisture, sodium ferrocyanide helps keep the grains loose and easy to use. Most consumers don’t give it a second thought, but there’s real value in having salt that stays pourable, especially in kitchens or food factories running at full tilt.
Most governments and food safety authorities, including the European Food Safety Authority and the U.S. Food and Drug Administration, have weighed in on sodium ferrocyanide’s safety. Animal studies and decades of use show that at the low amounts found in food, it doesn’t break down into anything harmful inside the body. Regulators set pretty strict limits, so the risk stays in check. For anyone worried about cyanide—it’s a scary word—ferrocyanides are stable, water-soluble, and don’t release free cyanide under normal conditions.
Heavy Industries Lean on It Too
Food isn’t the only place where sodium ferrocyanide matters. It’s a workhorse in industrial corners people rarely see. Water treatment plants use it to help separate heavy metals, making cleanup easier and safer. The chemical does a good job binding with iron and copper, which can show up in water from old pipes or industrial runoff. Some uses reach into photography, where it plays a role in fixing and developing film.
Pigment manufacturing also leans on sodium ferrocyanide, especially when making Prussian blue—a pigment found in paints, inks, and even medical countermeasures for certain types of heavy metal poisoning. That blue pigment has a long history, reaching back centuries, and sodium ferrocyanide is central to its modern creation.
People Care Because It Touches Daily Life
Years ago, I worked in a bakery. We churned out mountains of bread and pastries every week, and salt storage was always on my mind. During humid months, bags would get sticky, granules fused together, and measuring became a headache. Salt blended with sodium ferrocyanide handled those challenges with ease, letting us focus more on baking and less on hammering apart clumps. Small tweaks like this shape how food businesses keep things running smoothly and safely.
Weighing the Benefits and Risks
There’s always a debate about chemicals in food. People look for names they recognize and wonder about anything that sounds like it belongs in a lab instead of a kitchen. For sodium ferrocyanide, clear research supports its safety at currently approved levels. Consumer trust hinges on transparency, so labeling matters. Companies and regulators should keep making data public, sharing updates as new science emerges.
The push for “clean label” ingredients means some brands explore mineral salts or rice flour as alternatives, but none match the performance of sodium ferrocyanide pound-for-pound. Cost and shelf life matter for producers and consumers alike, so choices have to balance safety, practicality, and price. Listening to customer concerns, answering questions, and staying current with research go a long way in building confidence.
Looking Ahead
Science doesn’t stand still. If future studies point to lower thresholds or better options for salt or water treatment, companies and regulators must adjust. For now, sodium ferrocyanide serves an everyday, often invisible purpose, making life easier from kitchen tables to huge factory floors. By keeping the discussion honest and informed, everyone can feel more comfortable with the food and products they use every day.
Is sodium ferrocyanide safe for consumption?
The Food Additive on Our Tables
Sodium ferrocyanide isn’t a name you spot on a menu, but it hides in plain sight—often in table salt. Many brands add this compound to stop clumps, keeping salt easy to pour even after weeks in a humid cupboard. The idea of “cyanide” in something edible alarms plenty of folks, especially because many know cyanide as a deadly poison. But there’s more to the story.
Chemistry and Context Matter
Chemists and regulators agree: not all compounds with “cyanide” behave the same. Sodium ferrocyanide locks cyanide up in a stable complex, making it almost impossible for the body to break apart and release actual cyanide. Food safety authorities like the European Food Safety Authority and US Food and Drug Administration have eyed the research, reviewed lab tests, and concluded that under proper usage levels, sodium ferrocyanide doesn’t pose a risk to people. According to the European Union, salt sold for regular eating can contain it within strict thresholds—typically just a few milligrams per kilogram.
Putting Safety to the Test
Regulators don’t just take the chemical company’s word for it. Risk assessments and toxicology studies run over years, with scientists tracking what happens after exposure at different doses. Large quantities—hundreds of times above the level found in your kitchen—can stress the kidneys of lab animals. In people, the real trouble comes only at doses you won’t ever reach through normal diets. Even cooking with salt enriched with ferrocyanide, or eating salty processed foods, falls way below what safety experts call the “acceptable daily intake.”
Transparency and Trust
Still, most people want more information about the items they eat every day. Labels in Europe mark sodium ferrocyanide as E535, and food manufacturers have to stick to limits set by law. Recent reviews continue to uphold these regulations as safe. But trust relies on honest enforcement; shortcuts or lack of oversight would be a recipe for trouble. In countries with less regulation, quality control gaps raise the risk of someone adding too much or an unscrupulous supplier cutting corners. My own look into recall reports turns up few incidents linked to this compound, suggesting that manufacturers mostly handle it well.
Natural Alternatives and Consumer Choice
Some people skip conventionally treated salt to avoid any risk, aiming for sea salt or brands selling products “additive-free.” The verdict on benefits depends on personal priorities. Sea salt costs more and can clump in damp weather, but for those allergic or extra cautious, it’s a trade-off worth making. In my own kitchen, I keep both on hand—regular salt for cooking, sea salt for adding at the table.
Room for Clearer Labeling
Food safety comes down to science plus transparency. Current studies and decades of everyday use back up sodium ferrocyanide’s safety at regulated levels. Companies and watchdogs should keep the data open, labels readable, and limits enforced. If more people demand clear ingredient lists, the industry has a chance to prove it acts in the open, not behind closed doors. Every bag and box deserves honesty, no matter how small an additive—so everyone gets to make a choice they trust.
What are the main properties of sodium ferrocyanide?
The Yellow Salt with a Surprising Role
Most folks hear the word “cyanide” and picture danger. Yet sodium ferrocyanide plays an important role in daily life, and it would be a mistake to mix it up with those highly toxic cyanide compounds from spy movies. Proudly yellow, sodium ferrocyanide’s real power comes from its tightly bound structure—not likely to fall apart and release cyanide under regular conditions.
Stability and Safe Use
Sodium ferrocyanide stands firm against breakdown under normal temperatures, whether stored in a warehouse or packed into table salt. Scientific tests have shown its stability. Food regulators in the US and EU gave it the green light as an anti-caking agent. Used the right way, it does not split to release dangerous free cyanide. This approval only came after careful vetting and decades of research tracking human exposure.
Water Soluble and Ready to Work
Drop a pinch into water, and it dissolves easily—no clumps, no fuss. That solubility makes it practical for industry. In salt manufacturing, a tiny amount sprinkled in during production keeps the grains free—no messy salt shakers or rock-hard lumps. Dockworkers, food producers, and pretty much anyone who deals with bulk salt have relied on this “yellow prussiate” to make handling easier.
Industrial and Laboratory Ally
Chemists appreciate sodium ferrocyanide for its reliability. In the lab, it helps test for metals like iron, turning a giveaway blue when iron’s present. This test isn’t just for classrooms; it pops up in water quality labs and old-school analytical work. Chemists also use it to separate out metals from mixtures, acting as a key ingredient in pigment making. If you’ve seen “Prussian blue,” you’ve seen its signature hue, rooted in metal chemistry that shapes both art and detection science.
My Own Take on Food Additives
Like many others, I hesitated the first time I noticed the name sodium ferrocyanide on a salt packet. Years of study—the kind that meant long hours in the lab—helped me understand the huge difference between true hazards and misunderstood names. Sodium ferrocyanide has a track record. Toxicologists have put it to the test. As a consumer, safety always matters to me. I keep an eye on what science says, and so far, the evidence on this compound points to very low risk at the amounts used in food.
The Real Risks and Common Sense
Problems could happen if large spills reach livestock water—cattle are more sensitive than people—or if strong acids get mixed in and break its bonds. Care in handling and labeling remains important, but routine consumer contact just doesn't match up with scenarios for real danger. Consistent regulatory monitoring catches missteps early, long before shoppers would notice.
Smart Regulation and Solutions
With strong rules in place for how much goes into food, and with an army of inspectors and researchers staying alert, sodium ferrocyanide’s benefits far outweigh its limited risks. People working in big salt plants should get clear training, and industries using the compound need to keep up good standards. As research keeps pushing safety margins, regulators can continually reassess, update guidelines, and make sure this quiet workhorse keeps doing its job safely.
How is sodium ferrocyanide produced?
Straight Talk About a Yellow Crystal Everyone Uses
Sodium ferrocyanide often gets talked about with a certain kind of suspicion, just because of “cyanide” in its name. You’ll find it in table salt, for instance, to stop clumping. Yet this compound shows up all over—wine fining, pigment production, even metal processing. Rather than jump to conclusions, let’s walk through how it actually gets made, what goes on inside the factory, and why this recipe matters beyond just chemistry.
How the Stuff Gets Cooked Up
It starts with scraps from the coal and steel world—specifically iron filings and leftover cyanide from purifying coal gas. This waste used to sit around and threaten groundwater, but decades ago, chemists figured out how to pull the cyanide and use it for good. The process looks a little messy: workers add iron filings to cyanide solutions, along with sodium carbonate, in big tanks. The mix simmers under heat, with oxygen kept away. Iron binds with the cyanide to form the more stable ferrocyanide complex. Soda ash, or sodium carbonate, swings the pH to the right side, keeping the whole chemical soup in line.
Not every factory handles this the same way. Some tweak the recipe for batch size or speed; some recycle waste gas, others don’t. Yet the goal stays steady: get the sodium ferrocyanide to form. Once the crystals separate out, they filter and wash them, then dry the lot in rotary kilns. Final product looks like lemon-yellow salt, easy to spot and unlikely to go astray in the warehouse.
What’s to Worry About? Safety, Pollution, and Precision
As with any industrial chemical, risk lurks at every step. Cyanide earns a nasty reputation for a reason. In this context, it no longer exists in the truly dangerous form: iron binds the free cyanide so tightly the human body can’t break it loose under normal use. That said, factories must manage both air and water emissions. Spills of unreacted cyanide could still spell real danger for workers and local rivers.
Regulators keep a sharp eye on these production lines for that reason. The European Food Safety Authority and the US FDA both set limits on how much sodium ferrocyanide ends up in food salt. Studies confirm that the amounts used pose no health threat, but the rules offer a necessary backstop. What strikes me each time I check a plant? How simple slip-ups, like a misread pH meter, could send unreacted cyanide downriver. Accidents can—and do—happen, so investment in careful batch tracking and waste recycling offers a direct line to better safety.
Looking for Better Ways to Manufacture
More efficient, less wasteful processes begin with smarter raw material use. Research teams test catalysts that squeeze every drop of cyanide from waste streams and prototype reactors that recapture water and heat before it escapes. Closed loop systems get attention for slashing both risk and cost. Automated monitoring reduces human error and flags leaks early. Green chemistry methods, like using plant-based precursors instead of fossil-fuel-derived ones, could clean up this industry even further. For operators, tighter rules often drive investment in upgrades, but so does the simple hope of keeping their reputations clean and their staff safe.
Why It Matters—Beyond the Factory Fence
Everyday products depend on the stability sodium ferrocyanide provides. Yet its story shows how industrial leftovers can gain new lives, turning would-be pollution into something useful. With more transparency about chemical sourcing and firm commitment to safety, this humble compound continues to earn its keep in ways most people never notice, but would miss if it vanished overnight.
Is sodium ferrocyanide the same as sodium cyanide?
Mixing Up Chemical Names Can Lead to Big Misunderstandings
The debate about sodium ferrocyanide and sodium cyanide keeps popping up online and in everyday conversation. At first glance, the names look alike, both contain "cyanide," and most folks assume they're interchangeable—or at least equally hazardous. This confusion isn’t just a matter of chemistry trivia; it can drive fear or mistrust around food safety, industrial safety, and regulations.
What Makes These Chemicals So Different?
Let’s look past the similar spelling. Sodium cyanide (NaCN) comes up in news stories mostly because of its high toxicity. It quickly releases hydrogen cyanide gas, blocking the ability of cells to use oxygen. Even small amounts can be fatal fast, so it shows up in headlines about gold mining spills or criminal accidents. The container usually comes with serious warnings and skull-and-crossbones stickers—nobody mistakes this stuff for something harmless at work.
Sodium ferrocyanide (Na4[Fe(CN)6]) is a whole different story. The iron ion at the center binds tightly with the cyanide groups, holding them together in a stable structure. This tight grip means the cyanide doesn’t just fall off and float free. Scientific studies consistently show that, under normal handling or even when you eat the trace amount that shows up in table salt as an anti-caking agent, your body doesn’t get exposed to free cyanide.
Food Safety Worries and Hard Facts
I remember a neighbor sending around a Facebook post warning everyone to toss out their table salt after seeing “E535” (the code for sodium ferrocyanide) on a label. Panic spread through the group chat. It took some digging and a calm head to explain the real risk. Major food safety agencies, including the European Food Safety Authority and the U.S. FDA, have run tests and reviewed the evidence. Their verdict: sodium ferrocyanide stays stable in salt, passes through your body without harm, and has no history of poisoning at the approved food levels.
This isn’t just about calming nerves—scientific transparency builds trust. The same trusted groups ban or restrict sodium cyanide because of its proven risk. That clear line of difference between the two isn’t a technicality. It’s something families and communities rely on for peace of mind. The lesson here: don’t just judge by a scary-sounding name. Doing your own research or listening to trusted science voices can save a lot of worry and wasted food.
Industry Use and Public Health Calls for Better Education
Sodium ferrocyanide lends a hand in real-world jobs. It stops salt from clumping and keeps certain pigments stable in paints. Without it, table salt on humid summer days would turn into a brick. Factories have used it safely for decades. Even so, misunderstandings pop up constantly, especially online or where chemical knowledge is thin.
To clear the air, schools and public health offices could teach basic chemical literacy from an early age. Talking with local scientists or nutritionists and inviting industry experts into classrooms might help people learn what labels really mean. Honest information from credible sources goes further than social media rumors or scary headlines.
We deal with chemicals every day—in food, cleaning, work, and the environment. Most are less dramatic than the stories suggest. Reading labels is smart, but panic doesn’t help. By learning how to interpret names and trust evidence, anyone can make safer, more informed choices.
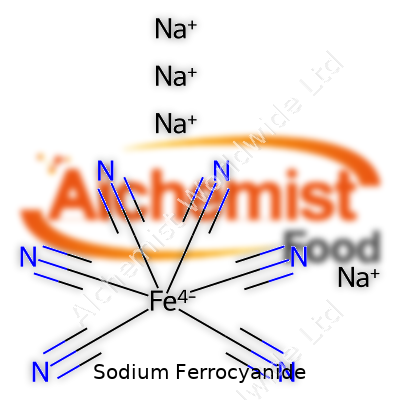
| Names | |
| Preferred IUPAC name | sodium hexacyanidoferrate(II) |
| Other names |
Yellow Prussiate of Soda Sodium hexacyanoferrate(II) E535 |
| Pronunciation | /ˌsoʊdiəm ˌfɛroʊˈsaɪənaɪd/ |
| Preferred IUPAC name | Sodium hexacyanoferrate(II) |
| Other names |
Yellow Prussiate of Soda Sodium hexacyanoferrate(II) E535 |
| Pronunciation | /ˌsəʊdiəm ˌfɛrəʊˈsaɪənaɪd/ |
| Identifiers | |
| CAS Number | 14434-22-1 |
| Beilstein Reference | 1718733 |
| ChEBI | CHEBI:61056 |
| ChEMBL | CHEMBL1352 |
| ChemSpider | 5707 |
| DrugBank | DB14004 |
| ECHA InfoCard | 100.101.238 |
| EC Number | 237-081-9 |
| Gmelin Reference | 604828 |
| KEGG | C14398 |
| MeSH | D015550 |
| PubChem CID | 10255 |
| RTECS number | WV9350000 |
| UNII | 195650M230 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DV974AQ27M |
| CAS Number | 14434-22-1 |
| Beilstein Reference | 3587159 |
| ChEBI | CHEBI:61049 |
| ChEMBL | CHEMBL1201644 |
| ChemSpider | 12654 |
| DrugBank | DB11093 |
| ECHA InfoCard | ECHA InfoCard: 03-2119471314-46-0000 |
| EC Number | 237-081-9 |
| Gmelin Reference | 13227 |
| KEGG | C18684 |
| MeSH | D018407 |
| PubChem CID | 10144052 |
| RTECS number | LX8300000 |
| UNII | 78W25F73EC |
| UN number | UN3077 |
| Properties | |
| Chemical formula | Na4Fe(CN)6 |
| Molar mass | 303.86 g/mol |
| Appearance | yellow crystalline powder |
| Odor | Odorless |
| Density | 1.89 g/cm³ |
| Solubility in water | soluble |
| log P | “-4.3” |
| Vapor pressure | Negligible |
| Basicity (pKb) | pKb = 4.0 |
| Magnetic susceptibility (χ) | -53.6e-6 cm³/mol |
| Refractive index (nD) | 1.460 |
| Viscosity | Viscosity: 1.2 cP (25 °C) |
| Dipole moment | 0 D |
| Chemical formula | Na4Fe(CN)6 |
| Molar mass | 303.86 g/mol |
| Appearance | Yellow crystalline powder |
| Odor | Odorless |
| Density | 1.68 g/cm³ |
| Solubility in water | Soluble |
| log P | “-4.3” |
| Vapor pressure | Negligible |
| Basicity (pKb) | pKb ≈ 4.3 |
| Magnetic susceptibility (χ) | -43.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.409 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 215.7 J⋅mol⁻¹⋅K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2112 kJ/mol |
| Std molar entropy (S⦵298) | 435.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –1876 kJ/mol |
| Pharmacology | |
| ATC code | S509 |
| ATC code | V03AB31 |
| Hazards | |
| Main hazards | May release toxic gases (hydrogen cyanide) on contact with acids; harmful if swallowed; may cause irritation to skin, eyes, and respiratory tract. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | No signal word |
| Precautionary statements | Wash thoroughly after handling. Do not eat, drink or smoke when using this product. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 Oral Rat 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1600 mg/kg (oral, rat) |
| NIOSH | WN3870000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 250 mg/kg bw |
| IDLH (Immediate danger) | IDLH: 15 mg/m3 |
| Main hazards | Harmful if swallowed; may cause irritation to skin, eyes, and respiratory tract. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07, GHS09 |
| Signal word | No signal word |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "P264, P270, P273, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | Autoignition temperature: 651°C |
| Lethal dose or concentration | LD50 (oral, rat): 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 1600 mg/kg |
| NIOSH | GR188 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 700 mg/kg |
| Related compounds | |
| Related compounds |
Sodium ferricyanide Potassium ferrocyanide Potassium ferricyanide Ammonium ferrocyanide |
| Related compounds |
Potassium ferrocyanide Sodium ferricyanide Potassium ferricyanide |

