Sodium Dihydrogen Pyrophosphate: A Closer Look at Its Journey and Role
Historical Development
Some stories in chemistry start from necessity rather than accident. Food preservation in the twentieth century demanded better solutions, fast. Around that time, sodium dihydrogen pyrophosphate (Na2H2P2O7) found footing as a food additive. Manufacturers looked for leavening agents that could improve flavor, shelf life, and texture without adding unwanted tastes. Chemical companies responded with methods to synthesize this compound reliably. Looking at patent filings and publications from the mid-1900s, there’s no mistaking the push to meet consumer demand with science. The use of sodium pyrophosphates quickly expanded into baked goods, processed meats, and even detergents. Not every story in industrial chemistry turns into a household name, but nearly everyone has eaten or used products containing this additive.
Product Overview
Sodium dihydrogen pyrophosphate wears a lot of hats in industrial and food chemistry. As a white, crystalline powder, it’s not flashy. Its value lies in how it controls chemical reactions in foods — especially doughs and meats. Bakers rely on it for the precise release of gases during baking, which gives cakes rise and keeps muffins from turning flat. Meat processors add it to improve color and water-holding ability. Anyone who has mixed water with pancakes made from a boxed mix has likely seen the invisible hand of this compound at work. In cleaning products, it works to soften water and improve action in detergents. The reach of this compound crosses from bakeries to industrial cleaning plants.
Physical & Chemical Properties
Sodium dihydrogen pyrophosphate appears as an odorless, white powder with a slight tart taste. Its solubility in water makes it favored in many formulations, dissolving easily even in cool environments. The pH of a 1% solution hovers between 4.0 and 5.0, useful for products that need acidity without being harsh on the palate or the skin. Chemists appreciate its stability under dry conditions, though it can absorb moisture from the air over time. Moisture fiddles with its structure and performance; improper storage can mean caked powder or loss of function in recipes. Unlike many phosphate salts, it does not leave strong flavor notes, which is another reason for its widespread use in subtle food environments. Its melting point sits high — above 200°C — marking it as sturdy during heat processing steps.
Technical Specifications & Labeling
Inspect a label on a food additive bag, and sodium dihydrogen pyrophosphate will often appear as E450(i) or simply SAPP. Industrial-grade products get sorted by purity; some batches suit sensitive food needs, others target cleaning or industrial uses. Food codes ask for clear labeling, usually by its International Numbering System, such as INS 450(i). Safety sheets break down handling and storage: keep it sealed, avoid breathing the dust, and wash after use. Producers may label the mesh size to suit dough mixes or liquid processing. Accurate technical sheets list assay, bulk density, and moisture content — crucial factors for producers aiming for consistent product performance. Certifications address kosher, halal, and allergen status, reflecting the increasingly global reach of food supply chains.
Preparation Method
Crafting sodium dihydrogen pyrophosphate begins with sodium carbonate and phosphoric acid — both staples in the world of industrial chemistry. Heat triggers a reaction, producing monosodium phosphate, which then undergoes further heating to dehydrate and condense into the desired pyrophosphate. Large-scale facilities rely on closed reactors to control water loss and minimize exposure. Those working with the process watch temperatures closely; drift above recommended levels, and unwanted byproducts emerge, changing taste and performance. After cooling, the product gets ground and sieved according to purpose. This preparation route produces consistent material in both food and technical grades, which allows businesses to trust the stability in their product lines. Some manufacturers tweak the process for specialized uses, like finer particles for rapid hydration in baking.
Chemical Reactions & Modifications
Sodium dihydrogen pyrophosphate readily dissolves in water and can act as a buffer, stabilizing pH in food and technical systems. Its primary chemistry involves donating sodium ions and pyrophosphate anions, which can bind water, chelate metal ions, or participate in redox balance. In baked goods, it reacts with sodium bicarbonate to form carbon dioxide that helps dough rise. The compound can hydrolyze under acidic or prolonged moist conditions, breaking back down into orthophosphates. This reversibility means shelf life can suffer in humid environments, so careful packaging stays important. Research teams have also looked at modifying the base molecule, replacing sodium with potassium for specific dietary uses, or blending with other phosphates for more even leavening in baked products. The adaptability of its core chemistry, together with well-established manufacturing techniques, keeps it an industry favorite despite new trends in food technology.
Synonyms & Product Names
In international trade, sodium dihydrogen pyrophosphate goes by several names. SAPP leads most ingredient lists, followed by E450(i), an assigned code for food applications in Europe. In technical contexts or regulatory filings, sodium acid pyrophosphate or disodium dihydrogen pyrophosphate also appears, reflecting its chemical composition. Business deals often see it sold under brand names unique to large chemical suppliers. Packaging might display the full chemical formula, Na2H2P2O7, for clarity. Differences across jurisdictions mean regulatory paperwork sometimes needs alternate synonyms listed to cover all legal bases.
Safety & Operational Standards
Workplace safety matters with chemicals that are powdered and handled in bulk. Inhalation of dust isn't pleasant and can irritate the nose and lungs, so personal protective equipment such as masks or respirators, gloves, and protective work clothing see regular use in production areas. Food-grade facilities check product for heavy metals, purity, and microbial contamination, looking to comply with standards from the Joint FAO/WHO Expert Committee on Food Additives, US Food and Drug Administration, and the European Food Safety Authority. Data sheets outline proper first aid in case of accidental exposure. Facilities store the material in cool, dry areas away from moisture and incompatible substances, such as strong acids. Handling standards have grown stricter over recent years due to greater scrutiny of workplace exposure and contamination risks. Regular audits and batch testing assure that what goes out the door matches label claims both in safety and chemistry.
Application Area
Food applications anchor the demand for sodium dihydrogen pyrophosphate. Bakers count on it as a leavening acid in cake, pancake, and batter mixes. Meat processors appreciate its water-binding and color-retaining abilities, especially in cured ham and sausages. The additive proves reliable in ready-to-eat meals, tortillas, and even canned seafood. In laundry detergents and cleaning agents, it acts as a water softener and dispersant, helping break down tough stains. The ceramics world uses it as a dispersing agent, and in oil drilling, it finds a role in controlling mud viscosity. The breadth of its uses reveals just how interconnected our food and industrial systems have grown. Many people don’t realize how a single compound threads through their breakfast, cleaning cabinet, and infrastructure projects all in a single week.
Research & Development
Recent years have seen a surge in studies on alternative leavening systems and dietary impact. Researchers explore blends with other phosphate and non-phosphate acids to reduce sodium intake or enhance functional properties in gluten-free baking. Academic labs dive into the molecule’s behavior in water-rich sauces, analyzing its ability to keep proteins and minerals stable. Analytical chemists look for better ways to detect trace contaminants in the compound itself or in finished food. With concerns about phosphate consumption and environmental runoff growing, research often focuses on more sustainable sources, better recovery methods from industrial waste, and greener production processes. Most research outcomes find their way into commercial applications within just a few years thanks to strong collaboration between academia and food technology companies.
Toxicity Research
Extensive toxicological studies assess sodium dihydrogen pyrophosphate as both a general chemical and a food additive. Animal studies conducted in the latter half of the last century show low acute toxicity, making it safe to use in regulated amounts within food products. Chronic exposure studies examined its effect on kidney function and mineral absorption. Regulatory agencies have set acceptable daily intake levels based on the latest metabolic and epidemiological findings. Overuse in food formulations can push dietary phosphate levels above recommendations, prompting industry review and formulation tweaks, especially for vulnerable groups such as children and those with kidney or heart conditions. Environmental toxicity centers mostly on aquatic life, as phosphates run off into water systems and contribute to eutrophication. Upcoming research looks at both the nanostructure interactions inside the human body and large-scale aquatic ecosystem impacts, looking to balance function, safety, and environmental stewardship.
Future Prospects
Consumer trends toward cleaner labels and lower sodium prompt both challenge and opportunity for this veteran additive. Companies investigate ways to reduce use, substitute with minerals like potassium, or blend with organic acids to achieve the same culinary results. Regulatory bodies revisit phosphate limits in food, which encourages chemical firms to innovate new versions and improved labeling to keep consumer trust strong. Environmental regulations targeting phosphate runoff put pressure on industrial users to treat waste streams more effectively and seek alternatives in cleaning and water treatment. As people look for functional foods that balance texture, taste, and nutrition, sodium dihydrogen pyrophosphate will face new rounds of research and scrutiny. Its adaptability, along with ongoing product improvements, suggests it will keep a presence in global supply chains, so long as industry and regulators find ways to use it responsibly and transparently.
What is Sodium Dihydrogen Pyrophosphate used for?
The Role in Food
Most folks probably haven’t heard of sodium dihydrogen pyrophosphate unless they spend a lot of time reading food ingredient labels. It’s got a complicated name, but you’ll run across it mainly in processed foods. Bakers mix it into self-rising flour to help bread and cakes puff up. It creates the reaction in leavening that gets baked goods fluffy without yeast. For someone who has struggled with fallen pancakes or dry muffins, this stuff keeps the texture soft and breaks up any lumps. I’ve even talked with a chef at a local bakery who swears by it for keeping biscuits light when he’s baking at scale for breakfast rushes.
It goes beyond just bread. Potato products like frozen hash browns use sodium dihydrogen pyrophosphate to keep them looking fresh instead of turning gray or brown. Nobody wants soggy, gray fries, and that’s one reason you taste the same fresh potato flavor whether you’re eating at home or in a fast-food drive-thru. This preservative action helps reduce waste and maintain food appeal, which means less food gets thrown away
Other Everyday Uses
Food isn’t the only place where you’ll spot this ingredient. I once bought a bag of shrimp at the market and checked the label out of curiosity. There it was again. Seafood processors depend on it to help keep shellfish firm and prevent them from becoming mushy. Cooked shrimp take on a bouncy texture and color stays inviting because this additive manages the water content and chemical reactions that lead to spoilage.
It’s even found in some toothpaste varieties. Some toothpaste brands use sodium dihydrogen pyrophosphate as a tartar control agent. According to the American Dental Association, it works by slowing the hardening of dental plaque into tartar, which can keep teeth cleaner for longer between professional cleanings. I remember switching toothpastes after my hygienist pointed out tartar buildup around my lower front teeth. That small change on the label made a noticeable difference over six months.
Health and Safety Considerations
People have growing concerns about food additives. That’s fair—trust in what we eat matters. U.S. Food and Drug Administration researchers have approved sodium dihydrogen pyrophosphate as safe in set amounts. Like many food ingredients, it only becomes an issue when consumed at levels far beyond what is typical in a balanced diet. Some studies show that excess phosphates from food additives could add up, especially for people with kidney trouble. Groups like the Mayo Clinic caution folks with chronic kidney disease to pay special attention and discuss these additives with a medical professional.
Bakers, manufacturers, and dentists all balance performance with safety. They rely on ongoing research and food safety monitoring. For people concerned about processed foods, the best move remains the same: prepare more meals from scratch and check labels closely. If something feels off or unfamiliar, a quick call to a dietitian or a little digging in reputable nutrition sources can help make sense of what’s safe and what’s not. That simple habit of reading food packages goes a long way toward building confidence in what goes on your plate and in your mouth.
Finding the Balance
Sodium dihydrogen pyrophosphate won’t disappear from store shelves any time soon. It helps keep food fresh and appealing, and its use in products like toothpaste shows its value across more than one industry. As shoppers, the power to make food choices that fit our own health goals and comfort levels stays in our hands. I’ve found that small decisions—like comparing ingredient lists or choosing products with fewer additives—can add up to healthier eating without sacrificing convenience or flavor.
Is Sodium Dihydrogen Pyrophosphate safe to eat?
Sodium Dihydrogen Pyrophosphate: What We’re Talking About
Turn around almost any bag of frozen potato wedges or box of cake mix, and there’s a good shot you’ll spot something called sodium dihydrogen pyrophosphate. This mouthful of a chemical name tends to raise alarm bells for a lot of shoppers. Looks like something straight out of a lab, not a kitchen. That’s a fair worry. Anything with a name you can’t pronounce usually gets a side-eye. But food science isn’t always about tongue-twisters. Sometimes, it’s there for a real reason.
‘Why’s This in My Food?’
From my own time in a small commercial bakery, I remember the challenge of keeping dough the right texture and color. Potatoes can go brown faster than you can blink. Left untreated, a French fry before cooking turns gray and unappetizing, no matter how fresh. Sodium dihydrogen pyrophosphate helps. It holds off that browning, keeping frozen foods looking the way you expect. Cooks and manufacturers want their final product to match the picture on the box. I learned quickly that people buy with their eyes as much as their hunger.
Looking at the Science
Let’s get past instincts and look at the evidence. The US Food and Drug Administration, the European Food Safety Authority, and Canada’s food regulators all label sodium dihydrogen pyrophosphate as “generally recognized as safe.” That label means research teams took a long look at its effects in the amounts typically found in food. They didn’t find a risk for healthy folks. Used at the levels seen in bread, pancake mix, and fried potatoes, it doesn’t pose harm to the body. The compound breaks down into phosphate and sodium, both nutrients naturally present in foods like milk and meat.
Too Much Phosphate, Too Much Sodium
Scratching beneath the surface, health matters do pop up. Our daily diets today already bring in more sodium and phosphate than nutritionists would recommend. Folks eating a lot of processed, salty foods add up their sodium just through sandwiches and snacks. Excess phosphate might sneak in, too. Health authorities point at potential links between high phosphorus intake and risks for kidney health or bone problems, especially for people already managing kidney disease. These issues don’t come from sodium dihydrogen pyrophosphate alone—it’s more about the sum total from all processed foods.
Staying Safe Without Fearmongering
Nobody should see an ingredient and panic. From years of watching food trends, I can say most worry comes from not knowing why something’s there. Food labels like “contains sodium dihydrogen pyrophosphate” often just mean the company wants to give you fries that don’t look like old apples. If you want to eat less added phosphate and sodium, spend more on home-cooked meals from raw ingredients. Plenty of home cooks get great results without using additives, mixing flour, salt, leaveners, and fresh produce their way. Those of us who rely on convenience foods once in a while don’t need to stress out—the occasional cake mix or bag of fries fits into most diets safely.
Looking Ahead
Shoppers deserve honest labels and informed choices. If food makers keep up with science, stay under recommended usage levels, and switch to alternatives where they make sense, safety stays at the center. At the end of the day, the ingredient list tells its story—one snapshot of a larger way we eat. The best step forward sits with balance, not fear. Seek out information, talk to health professionals, and keep variety in your diet. For most, sodium dihydrogen pyrophosphate won’t be a problem if the rest of the plate stays mostly whole, fresh, and real.
What are the common applications of Sodium Dihydrogen Pyrophosphate in food processing?
What Drives It Into Our Foods?
Sodium dihydrogen pyrophosphate, usually seen on labels as E450 or SAPP, pops up in way more foods than most folks realize. I started checking the ingredient lists after a baking project with my niece, and there it was, right at home with the flour and sugar. In the world of commercial baking, SAPP plays a big role in making baked goods rise evenly. That is the kind of chemical backup every home baker secretly wishes for, especially if you’ve wrestled with dense biscuits or sad pancakes.
Baking and Leavening with a Purpose
My first batch of homemade muffins never quite puffed up the way I wanted. The struggle was not mine alone. Commercial bakeries add SAPP to batters as a leavening acid, so CO2 gets released on cue, turning gooey mixtures into tall, fluffy goods. It regulates how quickly baked goods rise; that’s a huge deal for consistency and quality. In large bakeries, this kind of control saves a lot of money and keeps every batch looking the same — customers notice if their English muffin duds the size of a hockey puck.
Maintaining the Look: Color and Freshness
Anyone who has tried to cut fresh potatoes and left them out knows they start browning fast. Adding SAPP stops that. It knocks out the enzymes that turn cut potatoes or pre-made dough crusts brown and unappetizing. I’ve seen firsthand how french fries in a fast-food kitchen keep their just-peeled glow. Processors lean on this ingredient to keep their products bright and appealing, which keeps customers coming back.
Keeping Meats Moist and Tasty
It’s no secret that processed meats lean on science to keep texture and moisture. SAPP helps trap water inside hot dogs, canned hams, and chicken nuggets. Meat processors use it to bind water and protein so products stay juicy after cooking. Nobody wants a rubbery turkey slice on their sandwich. This process helps with sodium control too, since SAPP can reduce the need for higher salt loads in cured meats; with less sodium, that means a healthier finished product for folks watching their diet.
Stabilizing Dairy and Cheese
I spent a summer at a cheese plant and watched how tricky it can be to slice processed cheese neatly. SAPP stabilizes the cheese texture, keeping it from turning hard or crumbly in the package. This makes sandwich cheese slices stack cleanly and melt the same way, every time. In processed cheese spread, SAPP keeps everything smooth, even if the cheese gets cold or sits out at a picnic. Consumers appreciate it, even if most don’t give it a second thought.
Safety and Oversight
SAPP sits under a regulatory spotlight. The U.S. Food and Drug Administration recognizes it as safe when used within set limits. Food scientists monitor how much goes into any product, partly because excessive phosphates could tick up risks tied to kidney or bone health. Groups like the FDA and EFSA regularly comb through new research, updating guidelines for producers. As more people include processed foods in their diets, that layer of scrutiny only grows more important.
Looking Ahead: Finding Balance
Companies now experiment with natural leavening agents or alternative preservatives, but SAPP delivers consistent results that keep it in demand for everything from snacks to ready-to-eat meals. Calls for cleaner labels have companies rethinking which additives really matter. Seeing SAPP on an ingredient list means someone has weighed those options, aiming for better texture, longer shelf life, and food that looks and tastes the way people expect.
Are there any health risks associated with Sodium Dihydrogen Pyrophosphate?
What’s in Your Food Adds Up
People often overlook the ingredient list on packaged foods. Over the years, I’ve noticed many foods on my table—from pancakes to canned fish—sharing a common additive: sodium dihydrogen pyrophosphate (SDPP). This mouthful of a name hides behind E450 in food labels, quietly improving texture, color, and shelf-life. Grocery shoppers may wonder what happens once SDPP lands in their stomachs. Let’s clear the air about what science says, and what matters for everyday health.
The Science Behind SDPP
SDPP helps raise dough, balance acidity, and reduce discoloration—jobs that hardly sound sinister. Phosphates like SDPP break down in the body, helping maintain energy and bone health. Most health agencies—including the European Food Safety Authority and FDA—mark SDPP as generally recognized as safe (GRAS), provided consumption stays within set boundaries.
Problems start when phosphate intake runs high. The modern diet already pours in plenty of phosphates from cheese, processed meat, sodas, and baked snacks. This can mean phosphate levels rise far beyond requirements. Too much phosphate strains the kidneys. Health studies link excessive phosphate intake to heart disease and weakened bones, especially for those managing chronic kidney issues.
Kids and At-risk Groups
I remember the stress of reading food labels when my own family member developed kidney problems. People with reduced kidney function need to keep an eagle eye on phosphate intake, since the kidneys filter out extra phosphorus. SDPP acts fast—it bumps blood phosphate levels quickly. Children are also more sensitive to phosphate build-up because their bodies are still growing, and they eat more food in proportion to their size. Higher blood phosphate may disturb mineral balance, raising chances of bone issues later in life.
How Much Is Too Much?
Official bodies limit how much phosphate makes it into food. For example, EFSA’s safety threshold for daily phosphorus hovers at about 40 milligrams per kilogram of body weight. Still, with processed food on the daily menu, people can overshoot this limit. A research snapshot published in the journal Advances in Nutrition revealed most people in the United States eat double the phosphorus their bodies call for. The bulk of that load sneaks in through additives like SDPP, not whole foods.
Possible Ways Forward
Awareness lights the path to better choices. Cooking at home gives control over what goes on the plate. Swapping processed options for whole foods—building a meal with fresh potatoes rather than grabbing instant mashed potato mix—shrinks unwanted phosphate. Asking food producers for transparent labeling would give shoppers the knowledge to pick smart. Doctors and dietitians can pitch in, flagging high-phosphate additives for those who need to cut back.
It helps to read up and ask questions. If a food ingredient sounds unfamiliar, it pays to check where it comes from, why it lands in the product, and what science shows about its effects. Making decisions armed with facts and experience protects long-term health much better than ignoring an ingredient like SDPP until problems show up.
Is Sodium Dihydrogen Pyrophosphate gluten-free and suitable for vegans?
What’s Inside That Food Additive?
Sodium dihydrogen pyrophosphate doesn’t spark much recognition for most shoppers, but scan the back of a bag of frozen french fries, pancake mix, or canned potatoes, and there it is. Modern food manufacturing uses this additive to keep potatoes white, leaven baked goods, and adjust acid levels in processed items. Big words on a label can raise questions, especially for folks who’ve managed gluten intolerance, celiac disease, or stick to a vegan way of eating.
Understanding Gluten Concerns
Celiac disease and gluten sensitivity push many people to look closely at food labels. Gluten itself only pops up in wheat, barley, rye, and their cousins. Chemically, sodium dihydrogen pyrophosphate starts from minerals and phosphoric acid—nothing grain-based. In my experience combing through labels for a family member with celiac disease, I’ve learned to look for hidden forms of wheat or cross-contamination rather than worry about chemicals from the phosphate family.
Food safety bodies like the FDA and European Food Safety Authority don’t list sodium dihydrogen pyrophosphate as risky for gluten concerns. It’s considered gluten-free at the source, especially when produced in controlled facilities that follow allergen management standards. That’s a sigh of relief for shoppers who fear accidental exposure.
Is It Vegan-Friendly?
The question of whether an ingredient fits a vegan diet comes up more and more these days, with interest in plant-based lifestyles growing every year. Sodium dihydrogen pyrophosphate doesn’t rely on any animal products in its manufacture. It results from combining sodium carbonate (usually synthetic or from salt) with phosphoric acid (usually derived from minerals or manufactured using inorganic chemistry). No animal enzymes, no dairy origins, no bone char processing. Most ingredient suppliers confirm their certificate of analysis to show no animal byproducts involved.
Big food companies chase clear supply chains for their vegan-labeled foods. Brands know plenty of people care what goes into their granola bars or pancake mixes. So if a label says vegan and lists sodium dihydrogen pyrophosphate, that claim holds up.
Why Does It Matter in Everyday Life?
For years now, food labels have grown as important as taste or price. Allergies top the list. I’ve seen firsthand the anxiety spike during holiday meals or dinner at a friend’s place—nobody wants to risk an allergic reaction or break a personal value without warning. Hidden gluten lands people in the ER, and accidental animal-based ingredients can cause genuine hurt for devoted vegans.
Misinformation travels fast, especially online. Panic can get people to eliminate safe foods from their diets and miss out on convenience or nutrition. Instead, a little transparent science from trusted sources helps people eat confidently.
Solutions for Clearer Food Choices
Reliable ingredient sourcing and plain language labeling do the biggest favor for shoppers with food needs. Manufacturers can boost trust with ‘gluten-free’ and ‘vegan’ icons, backed by certification from groups like GFCO or Vegan Society. Food educators can clear up the confusion behind chemical names by breaking down where ingredients come from and what processes are involved. Community support, from gluten-free baking groups to vegan recipe forums, gives practical tips for navigating the grocery aisle.
Sodium dihydrogen pyrophosphate shows that not every long name on a label is worrying. Smart shopping comes down to checking credible sources, asking direct questions about sourcing, and looking for verification when uncertainty crops up. On food journeys rooted in health or ethics, staying informed matters just as much as a good meal.
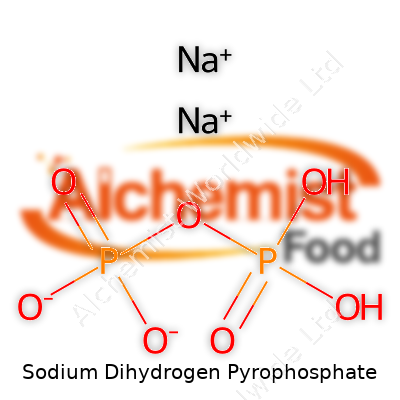
| Names | |
| Preferred IUPAC name | Sodium dihydrogen diphosphate |
| Other names |
Sodium acid pyrophosphate Disodium dihydrogen diphosphate SAPP |
| Pronunciation | /ˌsoʊdiəm daɪˈhaɪdrə.dʒən paɪ.rəˈfɒs.feɪt/ |
| Preferred IUPAC name | Sodium dihydrogen diphosphate |
| Other names |
Disodium pyrophosphate Disodium dihydrogen pyrophosphate Disodium diphosphate SAPP E450(i) |
| Pronunciation | /ˌsoʊdiəm daɪˈhaɪdrədʒən ˌpaɪroʊˈfɒsfeɪt/ |
| Identifiers | |
| CAS Number | 7792-85-6 |
| Beilstein Reference | 3497212 |
| ChEBI | CHEBI:63091 |
| ChEMBL | CHEMBL1201641 |
| ChemSpider | 86459 |
| DrugBank | DB09122 |
| ECHA InfoCard | 03-2119473200-54-XXXX |
| EC Number | 231-835-0 |
| Gmelin Reference | 6632 |
| KEGG | C14330 |
| MeSH | D000074272 |
| PubChem CID | 24856 |
| RTECS number | UX7350000 |
| UNII | XN3Z27R9F1 |
| UN number | UN3278 |
| CompTox Dashboard (EPA) | DTXSID6035983 |
| CAS Number | “7722-99-6” |
| Beilstein Reference | 3561846 |
| ChEBI | CHEBI:9129 |
| ChEMBL | CHEMBL1201081 |
| ChemSpider | 21169796 |
| DrugBank | DB11357 |
| ECHA InfoCard | 03-2119472284-47-0000 |
| EC Number | 231-835-0 |
| Gmelin Reference | 72487 |
| KEGG | C14428 |
| MeSH | D002611 |
| PubChem CID | 24857553 |
| RTECS number | TT8976000 |
| UNII | W47H9Z7FWH |
| UN number | UN3265 |
| CompTox Dashboard (EPA) | DTXSID4030362 |
| Properties | |
| Chemical formula | Na2H2P2O7 |
| Molar mass | 221.94 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.86 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 0.9 |
| Basicity (pKb) | 11.96 |
| Magnetic susceptibility (χ) | -51.0·10⁻⁶ cm³/mol |
| Viscosity | Viscous liquid |
| Dipole moment | 1.5 D |
| Chemical formula | Na2H2P2O7 |
| Molar mass | 221.940 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.86 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.4 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 1.0-2.0 |
| Basicity (pKb) | 1.0 |
| Magnetic susceptibility (χ) | -48.0e-6 cm³/mol |
| Viscosity | Viscous liquid |
| Dipole moment | 6.0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 225.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1511 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -247.8 kJ/mol |
| Std molar entropy (S⦵298) | 152.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1617 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1377 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A01AD11 |
| ATC code | A20AC02 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 oral rat 2,500 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2,000 mg/kg |
| NIOSH | WN3850000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 2500 mg/kg |
| IDLH (Immediate danger) | Not listed |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 2,200 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 3050 mg/kg |
| NIOSH | WH6650000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | Food Additives, 340(ii) |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Disodium pyrophosphate Tetrasodium pyrophosphate Sodium phosphate Monosodium phosphate |
| Related compounds |
Sodium acid pyrophosphate Disodium pyrophosphate Tetrasodium pyrophosphate |

