Sodium Dihydrogen Phosphate: More Than Just a Common Chemical
Historical Development
People have relied on sodium dihydrogen phosphate for over a hundred years, with early roots in the industrial revolution when chemistry first touched everyday life beyond the laboratory. Phosphate salts like this one made their way into farms, bakeries, and medicine cabinets. Chemical plants started producing it on a large scale in the early 20th century, responding to demand across agriculture, medicine, and food. Steadily, it transformed from a niche laboratory reagent to an item recognized in public health efforts, food processing, water treatment, and even everyday cleaning supplies. The growth matched a broader understanding of how important phosphates became in everything from treating water to keeping food shelf-stable.
Product Overview
Sodium dihydrogen phosphate, often labeled as monosodium phosphate or simply MSP, is a water-soluble white solid. People encounter it in powder or granular form, sometimes as a crystal loaded with water molecules. The formula is NaH2PO4. This simple salt stands out because it helps keep things acidic at the right pH range and does a lot of the behind-the-scenes heavy lifting in industries requiring precision chemistry. Factories put it in fertilizer blends, food additives, detergents, buffering solutions, and even some pharmaceutical products.
Physical & Chemical Properties
Sodium dihydrogen phosphate usually appears as colorless crystals or a fine white powder. It melts at around 60°C when hydrated, and anhydrous forms push past 200°C before breaking down. It pulls water from the air, absorbing moisture until it resembles damp sugar, and dissolves in water easily, making it great for fast, predictable reactions. Acidity shows up in its pH, with clear acid–base behavior—solutions made from it sit around pH 4.5. This makes it popular for keeping conditions stable in chemistry and food science. The salt tastes mildly salty and sour, which plays a role in flavor modification.
Technical Specifications & Labeling
Product labels in the industry typically state the chemical formula (NaH2PO4), purity levels, molecular weight (119.98 g/mol for the anhydrous form), water content (some forms hold one or two water molecules), and the grade (food, pharmaceutical, or technical). In my own lab work, suppliers often provide a certificate of analysis, listing not just sodium dihydrogen phosphate content, but also possible impurities, like heavy metals or other phosphate salts. To satisfy regulators and buyers alike, packaging includes storage tips to keep the product dry and uncontaminated, details about batch numbers, and production dates.
Preparation Method
Manufacturers turn phosphoric acid and sodium carbonate or sodium hydroxide into sodium dihydrogen phosphate by straightforward acid-base chemistry. They add the right amount of sodium base to phosphoric acid, heat the mix, and keep an eye on pH to lock in the monosodium version. The liquid cools, crystals appear, and the solution concentrates as water evaporates. Factories filter, wash, and dry the final product, typically choosing conditions that give them the desired hydrate or anhydrous form.
Chemical Reactions & Modifications
This salt shows its versatility in reaction tanks. Mix it with strong acid, get phosphoric acid again. Heat it up with another phosphate salt, and you step up to disodium or trisodium phosphate. Combine it with calcium salts, and it gives dicalcium phosphate, which lands in toothpaste and dietary supplements. In buffer systems, sodium dihydrogen phosphate partners with disodium phosphate or sodium hydroxide to help scientists and engineers control pH with precision. Food technologists use it to modify texture and color through chemical adjustments that involve metals or more complex phosphate networks. Wastewater engineers also mix it with iron or aluminum salts for precipitation reactions, cleaning water before it returns to rivers.
Synonyms & Product Names
If you scan ingredient labels—or chemical supply catalogs—you’ll see sodium dihydrogen phosphate under names like monosodium phosphate, MSP, or sodium phosphate monobasic. In the European Union and beyond, food-grade material often appears as E339(i). In laboratory work, it can be sold as sodium acid phosphate, primary sodium phosphate, or simply 'monosodium phosphate anhydrous/monohydrate'. These terms all refer to the same basic substance, with minor tweaks in water content or crystal structure.
Safety & Operational Standards
Regular handling of sodium dihydrogen phosphate means reading safety data sheets and double checking compatibility with other ingredients. Touching or breathing in the dust carries some risks, especially for sensitive groups; protective gloves, safety glasses, and dust masks keep workers safer. Food and pharmaceutical grades follow heavy regulations—the European Food Safety Authority, the US FDA, and others check for heavy metal contamination, microbial purity, and clear labeling. Storage needs dry conditions to keep clumping away, as the compound absorbs moisture. On big sites, spill control plans and emergency showers form part of a responsible chemical operation.
Application Area
This compound turns up in a long list of products most folks take for granted. Bakeries add it to flour mixes to help bread rise and look appealing. Cheese makers count on it as an emulsifier, giving processed cheeses that smooth, spreadable feel. Water treatment plants use it to block dangerous metals and soften water supplies for households. Farms add it to feed and fertilizers to boost phosphorus for stronger crops and livestock. Buffering capacity matters in pharmaceutical preparations and oral medicines—especially those that need a consistent pH. Print shops, cleaning product makers, and even fire control all tap its chemistry in subtle ways.
Research & Development
Universities and corporate R&D labs keep exploring what sodium dihydrogen phosphate can do beyond the basics. Researchers search for new uses in food safety and preservation, testing how it might slow spoilage or fight off bacteria. Environmental science teams experiment with different blends to clean water polluted with lead, chromium, or other heavy metals. Medical researchers evaluate its value in controlled-release drugs and specialty oral medications, using its solubility and acid-base response to deliver active ingredients at the right spot in the body. The push for greener and more sustainable phosphates sparks ongoing interest—especially as phosphate resources tighten globally.
Toxicity Research
Researchers keep probing how safe sodium dihydrogen phosphate is for people and the planet. Acute toxicity for humans is quite low; it won’t harm healthy adults in the amounts seen in food or medicine, as confirmed by repeated studies and food safety authorities. Problems mostly show up when large amounts enter ecosystems repeatedly, tipping the balance in lakes and rivers toward harmful algal blooms. Animal studies track possible impacts from long-term exposure, especially in kidney function or bone health, but typical consumer use falls well below worrisome levels. Regulators set strict thresholds for food, water, and medicine, guided by ongoing rat and cell culture testing.
Future Prospects
Sodium dihydrogen phosphate won’t fade from labs, farms, or kitchens any time soon, but pressure builds around environmental impact and resource scarcity. The hunt for substitutes in food and cleaning products picks up as regulations tighten, especially in places where phosphate pollution causes trouble. Phosphate recycling grows in importance as natural mines run low. Researchers explore biotechnology approaches to recover used phosphate and think about new, phosphorus-sparing additives in food and water treatment. Efficiency upgrades in manufacturing, lower-impact mining, and smarter waste handling all play a role in keeping the compound useful for years to come.
What are the main uses of Sodium Dihydrogen Phosphate?
Making Food Safer and Tastier
Sodium dihydrogen phosphate quietly shapes the food you see on grocery shelves and in restaurant kitchens. It takes on a simple but vital job as a leavening agent in baking powder. Breads and cakes rely on the evenness sodium dihydrogen phosphate brings, letting them rise on cue and hold just the right texture.
You’ll also spot it in processed meats and cheeses. It binds to water and salts, holding everything together without falling apart during cooking. In studies published by Food Chemistry, researchers found that adding it to dairy helps keep cheese from clumping, staying smooth on your pizza or sandwich. In meat processing, it prevents the juices from running out, keeping products moist even after cooking. Sodium dihydrogen phosphate plays a role in adjusting pH in foods. This isn’t about chemistry trivia—it keeps canned and packaged food from growing the wrong kind of bacteria, reducing foodborne illness risks. This function matters in both small kitchens and massive food-production lines.
Chemistry and Pharmaceuticals Rely On It
Many labs and hospitals use sodium dihydrogen phosphate in buffer solutions. These solutions stabilize the environment for sensitive chemical reactions. For clinical labs, accuracy can mean the difference between catching a disease early and missing it. Buffer quality underpins every blood and urine test that passes through diagnostics, according to studies in the Journal of Clinical Pathology.
Doctors trust prepared oral sodium phosphate solutions as a “clean-out” before colonoscopies. It works because the body reacts by drawing water into the gut and flushing it through quickly. That helps doctors spot problems without anything clouding their view. The World Health Organization includes it as a safe medicinal ingredient, reminding users to follow dose rules. In the wrong hands or at the wrong dose, it can upset your body’s electrolyte balance. Professionals keep a sharp eye on that when using it for patients.
Boosting Crops and Water Treatment
Farmers include sodium dihydrogen phosphate in fertilizers, aiming for strong crop growth. It offers an efficient way to deliver both sodium and phosphate. Phosphorus shortage leads to weak roots and low yields. According to recent reports from the International Fertilizer Association, modern agriculture in many countries counts on these types of additives to keep up with demand. They do not use it alone, but as part of a mix tailored by the soil’s needs.
City water systems and swimming pool operators add sodium dihydrogen phosphate to help keep pipes and pools clear. Phosphate controls mineral build-up and helps prevent corrosion. By managing scaling, water keeps flowing, and repairs stay rare. Environmental engineers point to customer health and long-term savings as reasons to keep using it, based on case studies available from the American Water Works Association. The safeguards tied to its use keep toxin risks low, as long as operators respect limits.
Looking Ahead: Responsible Use Matters
No matter the industry, responsible use weighs heavy. Strong regulatory oversight exists in food, medicine, agriculture, and water management. Clear labeling, transparent supply chains, and limits set by experts support safety. Research and hands-on experience both tell us sodium dihydrogen phosphate does its job well—when professionals respect the boundaries and follow the best practices set by regulators and scientists alike.
Is Sodium Dihydrogen Phosphate safe for consumption?
Understanding What’s in Your Food
Ingredients show up in grocery items that read more like science experiments than lunch options. Sodium dihydrogen phosphate comes up a lot—on labels for processed foods, in baking powders, in sports drinks. Many people probably see the name and wonder if this food additive should raise alarms.
I’ve spent time reading ingredient lists and researching food safety—not just as a writer, but as a parent trying to figure out if things I give to my family pass a common-sense test. Food safety authorities approve its use, but that doesn’t always reassure folks, so it helps to walk through some facts and practical experiences.
What Exactly Is Sodium Dihydrogen Phosphate?
It works as a preservative, acidity regulator, and helps processed cheese keep its creamy texture. It belongs to a group of food phosphates used everywhere from breakfast cereals to canned soup. The U.S. Food and Drug Administration (FDA) lists it as Generally Recognized as Safe (GRAS) for consumption. Trusted authorities in Europe and other regions do the same.
These agencies don’t make those calls lightly. Food safety scientists run long-term studies, looking at how additives like this affect health, digestion, and nutrient balance at the levels people typically eat.
Getting the Facts Straight
Phosphates, including sodium dihydrogen phosphate, show up in some surprising places. They pop up in milk powder formulas, help keep baked goods from falling flat, and keep beverages shelf-stable for longer stretches. The body actually needs phosphorus, a key part of bones and muscles. The challenge comes with balance—too much phosphate from food additives paired with underlying kidney trouble can sometimes create issues, especially for folks with diagnosed kidney disease.
For most healthy adults and kids eating a usual diet, research shows that sodium dihydrogen phosphate at standard levels doesn’t push phosphorus beyond what the body can handle. Over the years, I’ve listened to dietitians and doctors talk at parent groups about the real risks of excess salt, sugar, or fat, but food phosphates almost never draw warnings unless specific medical problems exist.
Who Should Watch Their Intake?
People with kidney disease often hear specific directions to avoid foods with extra phosphates. The kidneys have to work harder to get rid of phosphorus the body doesn’t use. If you fall into that group or care for someone who does, your doctor probably already discusses ingredients like sodium dihydrogen phosphate. For most others, food safety experts emphasize moderation and a varied diet, rather than avoiding every ingredient with a long name.
Finding Solutions Through Better Choices
The biggest step toward safer eating comes from understanding labels and making choices rooted in whole foods. I’ve seen many families worry over the occasional boxed macaroni and cheese, but spend fewer hours reading up on vegetables, fruits, or nuts. If trust in processed foods hits a wall, sticking with basic recipes using fresh ingredients removes most food additive concerns.
Still, not everyone has the time or resources to do all their own cooking. That’s where clear labeling, honest science communication, and public health education make a big difference. Companies and regulators both share responsibility to keep dangerous levels of any additive—including sodium dihydrogen phosphate—out of the food system. Community groups, schools, and health professionals all have roles in teaching how to make sense of ingredient lists, so families aren’t left guessing.
It helps to check sources, ask questions, and seek advice from nutrition experts who base their answers on real science instead of online rumors. We can push for transparency in how food ingredients are explained, without stirring up unnecessary fear or missing out on convenience foods that serve a purpose in many households.
What is the chemical formula of Sodium Dihydrogen Phosphate?
Understanding Sodium Dihydrogen Phosphate
Growing up in a small town, I remember seeing large bags of white powder stacked behind the shelves at the local hardware store, labeled for farm use. Years later, in chemistry class, I learned those bags sometimes contained sodium dihydrogen phosphate, a compound with real impact far beyond laboratories. On paper, its chemical formula reads NaH2PO4, translating to sodium (Na), two hydrogens (H2), one phosphorus (P), and four oxygens (O4). That string of elements provides a foundation for some major roles in food processing, agriculture, even medicine.
Sodium Dihydrogen Phosphate: Beyond the Formula
Let’s talk about why this compound matters. Despite the industrial name, NaH2PO4 pops up in kitchens, cleaning cupboards, and farm fields. Food companies use it as an emulsifier and a leavening agent, helping baked goods rise or processed cheeses keep their smooth texture. I remember my first job in a bakery—no one glanced at ingredient labels, but without reliable leavening agents, our bread would have turned dense.
Not just for food, sodium dihydrogen phosphate plays a part in controlling pH levels. Hospitals and clinics rely on it for making buffer solutions in blood tests or IV fluids. Every time doctors use these buffers, patients benefit from carefully managed chemistry. In agriculture, it boosts soil phosphorus, supporting healthy plant roots. Phosphorus is a building block of life, shaping DNA and cell energy. I’ve talked with local farmers who swear that regular use of phosphorus fertilizers supports better crop yields.
Concerns and Considerations
Chemicals like NaH2PO4 help modern life, but there’s a bigger environmental story. Overapplication in farming runs the risk of runoff, driving excess phosphorus into waterways. That causes algae blooms and dead zones downstream. The impact isn't theoretical—fisheries and drinking water supplies often pay the price. Studies by the US Environmental Protection Agency consistently point to phosphate runoff as a leading cause of freshwater pollution. Urban use compounds the problem, as wastewater treatment plants sometimes struggle to filter out every trace of phosphate from detergents and cleaners.
The food supply brings another aspect. Regulators like the US Food and Drug Administration set strict limits for sodium phosphate use in foods. Excessive consumption of phosphate additives may stress kidneys or cause imbalances in people with poor renal health. Nutritionists raise concerns about the growing list of processed items that contain sodium-based additives, pushing for better labeling and more public awareness. At home, reading ingredients becomes a quiet but important act of consumer caution.
Seeking Smarter Use and Transparent Practices
Balancing the benefits of sodium dihydrogen phosphate calls for smarter application techniques in agriculture, improved water treatment, and clear food labeling. Technologies for precision farming help minimize fertilizer waste, delivering nutrients only where crops really need them. Municipal water managers continue to invest in better filtration and monitoring to reduce phosphate leakage. In supermarkets, clearer packaging and informed shopping habits—like comparing ingredients and choosing less-processed foods—make a difference too.
The chemistry behind NaH2PO4 quietly shapes modern industry, agriculture, and health care. Understanding the science, respecting the risks, and applying best practices open the door for both practical progress and long-term environmental care.
How should Sodium Dihydrogen Phosphate be stored?
Looking Beyond the Label
Sodium dihydrogen phosphate doesn’t look like much at a glance. White, grainy, easy to overlook if you aren’t used to the tools of the lab or workshop. But the way you store it shapes how it performs in your daily tasks—everything from water treatment to fertilizers can run smoother or spiral into waste, depending on what you do the moment you crack open that big container.
The Moisture Problem
Let’s talk about clumping. Few things frustrate more than prying a shovel into a rock-solid heap of what just yesterday looked like bakery sugar. Sodium dihydrogen phosphate pulls in moisture from the air. Leave it out, and you might return to find a lump glued to the canister wall. The stuff crumbles under humidity, breaks down, and sometimes even changes chemically if left unchecked long enough. I learned this the hard way after storing a bag in a humid storeroom, then finding the batch ruined after just a couple weeks.
A tight seal makes all the difference. Glass jars with screw-on lids work best on a small scale. For larger amounts, high-density polyethylene drums with gasketed lids hold up well, keeping the crystals free-flowing and ready to use.
Temperature and Sunlight
Some folks stash chemicals in basements or sheds, thinking, “It’s out of the way, what’s the harm?” A few degrees here and there seem harmless, but direct sunlight and high heat kick up chemical breakdown and may yellow the powder or even risk unwanted reactions—especially if the product is mixed with traces of other things. Sunbaked workbenches and warm attic spaces put the material at risk.
A shelf kept out of the sun, in a room where the temperature doesn’t swing too wildly, gives your supply the best shot at long life. Cool and dry wins every time.
Avoid Mixing Dangers
Sodium dihydrogen phosphate plays well with most materials by itself, but care gets tricky when chemicals share space. Corrosives and strong oxidizers do not belong nearby. I once saw someone store it next to bleach, a mix-up that could put more inexperienced staff at risk. A mistake like that calls for an immediate fix—chemicals each deserve their own space, labeled and spaced apart, so nothing dangerous happens by accident.
Schools and businesses do well to follow rules from OSHA and the National Fire Protection Association. They offer practical direction and help avoid problems by spelling out proper shelving, labeling, and maximum allowed volumes.
Practical Steps for Everyday Use
I keep a scoop handy that never leaves the sodium dihydrogen phosphate jar. Mixing utensils borrowed from other containers drag in traces of moisture or contaminants, which speeds up spoilage. I write the date I opened the container right on the lid. If the stuff sits too long, or if the environment feels off, the date tells me it’s time to check for clumping or color changes.
Keeping personal and workspace safety at the top of the list helps prevent medical emergencies. Even though sodium dihydrogen phosphate isn’t the most hazardous chemical in the shed, a set of gloves, safety glasses, and handwashing never goes to waste.
In my experience, small changes—labeling shelves clearly, using solid containers, storing in cool and dry places—prevent bigger headaches. It’s not only about following rules, but about respecting the materials and the people working alongside them.
What are the potential hazards or side effects of Sodium Dihydrogen Phosphate?
Why Everyday Chemicals Like This Matter
Sodium dihydrogen phosphate pops up everywhere: in packaged foods, water treatment, medicine cabinets. I’ve seen it used to keep cheese creamy and help baked goods rise. None of that sounds threatening, but regular exposure to food additives can sneak up on us. That’s why it makes sense to look at the risks as well as the reasons companies keep it on the ingredients list.
Health Effects That Can Creep In
Eating or drinking a little sodium dihydrogen phosphate doesn’t ruin your day, but side effects start appearing if the amount goes up. I remember patients on phosphate solutions before colonoscopies, and a handful always mentioned muscle cramps or nausea. If someone doesn’t have healthy kidneys, the body can’t flush out extra phosphate very well. That leads to high blood phosphate levels, confusing the natural balance of calcium and phosphorus in your blood. Bones grow weaker over time. Blood vessels stiffen up.
People with normal kidney function handle a moderate dose without trouble. Trouble walks in with high or frequent intake. Reports from the FDA show that overdosing can cause irregular heartbeat, low blood pressure, and dehydration. It’s easy to dismiss warnings on packaging, but the body pays the price for carelessness.
Risks Beyond the Obvious
Even in small quantities, combining sodium dihydrogen phosphate with other drugs demands attention. If someone takes drugs for hypertension or uses non-steroidal anti-inflammatories, unexpected interactions follow. A lip-smacking soda or a snack stuffed with phosphates might not say much about these combos on the label.
I learned from pharmacists that patients undergoing treatment for heart or kidney disease need to keep their phosphate and sodium levels steady. Too much can slow recovery, raise blood pressure, or require even more medication changes. I’ve heard stories from dietitians about how kids, who love convenience foods, end up with more phosphate than their bodies need—for no other reason than food manufacturers love its texture-preserving qualities.
Environmental and Occupational Exposure
Factories pumping out sodium dihydrogen phosphate dust pose a different type of problem. Workers sweeping or bagging dry powder can breathe in dust, irritating their throats and lungs. Direct skin contact sometimes brings on redness or itching. After years of experience, plant managers learned that masks and gloves matter a lot more than fancy ventilation. Exposure guidelines from organizations like OSHA help, but corners sometimes get cut if nobody’s watching.
Practical Steps to Lower Hazards
Label reading remains the first defense. You don’t need a lab to spot “sodium dihydrogen phosphate” right in the ingredients list. Food and pharmaceutical companies could swap in alternative, safer emulsifiers or leaveners—or at least use smaller amounts. I’ve seen local bakeries and food producers listen when regular customers ask for fewer food additives. It’s simple: the more folks request change, the more industry listens.
Doctors and pharmacists can steer patients toward safe usage, especially when prepping for medical procedures. Routine blood tests become crucial for people with kidney or heart issues, picking up subtle phosphate imbalances before they turn into real problems. For workplaces, investing in decent personal protective equipment isn’t just a box-checking exercise; it keeps people healthy and saves on liability down the line.
Long story short, sodium dihydrogen phosphate brings convenience, but not without a cost. Paying attention to labels and speaking up about concerns can push the food and pharmaceutical world to offer better, safer choices.
What is Sodium Dihydrogen Phosphate used for?
What Is This Compound?
Sodium dihydrogen phosphate doesn’t grab headlines, but it sneaks into more places than most folks realize. This white, water-soluble powder packs a quiet punch, bringing real value to kitchens, labs, and even medical clinics. In science talk, it’s NaH2PO4, which sounds more complicated than the jobs it handles every day.
The Role in Food Processing
Take a stroll through the grocery store, and odds are you’ll pick up more than one item that owes something to sodium dihydrogen phosphate. This stuff lands in everything from baked goods to processed cheese. In baking, it helps control the acidity of dough and batter, balancing the pH to support yeast activity and give bread a better rise and texture. In cheese-making, manufacturers rely on it to stabilize the fats and keep the cheese smooth — nobody wants gritty cheddar on their sandwich. It also taps into the meat industry as a curing agent and moisture holder. With pre-cooked meats, moisture can disappear fast, so sodium dihydrogen phosphate steps in to keep things juicy and tender.
Supporting Health and Medicine
Switching lanes to medicine, this same compound steps into far weightier shoes. In oral electrolyte solutions, doctors use it to help prepare patients for procedures like colonoscopies. Nobody loves prep day, but sodium dihydrogen phosphate clears the way for safer, easier diagnostics. Beyond the hospital, you’ll find it in some over-the-counter laxatives. If someone struggles with constipation, a well-formulated dose can give the relief modern life sometimes demands. These uses show its importance extends far beyond the dinner table.
On the Chemistry Bench
Chemists lean on sodium dihydrogen phosphate for all sorts of laboratory work. It’s a key player in buffer solutions, keeping pH steady during research, which is crucial for reliable results. As someone who has worked in a college chemistry lab, I saw firsthand how a small mistake in pH can ruin an experiment and waste hours of work. In schools and advanced research, this chemical’s reliability saves time and sharpens results.
Environmental Uses and Water Treatment
In environmental engineering, this compound pops up again. Water treatment plants use sodium dihydrogen phosphate to adjust minerals and manage hardness. Hard water causes limescale, which can wreck pipes and heating systems. By helping control minerals like calcium and magnesium, this chemical protects equipment and improves water for households and industries. Cleaner water means better health for people and more efficient machines.
Concerns and Practical Solutions
With so many uses, attention to safety matters. Consuming small amounts through food or water is generally considered safe, backed by regulatory bodies like the FDA. But ongoing exposure to excessive phosphates, whether through processed food or supplements, can upset the balance in the body or the environment. Communities can lower risk by encouraging balanced diets and supporting clean manufacturing practices. Factories can invest in updated waste treatment systems. Food companies can explore replacing phosphates where possible — more natural alternatives keep showing up on grocery shelves, driven by health-conscious shoppers.
The Takeaway
Looking at sodium dihydrogen phosphate’s full range of roles shows why a simple chemical can deserve a spot in thoughtful discussion. Its track record in food, medicine, research, and industry points to a substance that works quietly behind the scenes, shaping routines most people take for granted. Responsible use and ongoing innovation will keep it useful — and safe — in years to come.
Is Sodium Dihydrogen Phosphate safe for human consumption?
The Role of Food Additives in Everyday Life
A walk through any grocery store highlights how often food ingredients twist our tongues, and sodium dihydrogen phosphate lands squarely among those. You’ll spot it in powdered products, processed cheese, baked goods, instant puddings, and many shelf-stable items. What’s it doing there? Mostly, it regulates acidity and acts as a preservative. It also stabilizes food textures and supports the rise in baked goods, which is a bonus for anyone obsessed with pancakes rising just right.
What Actually Goes into Our Bodies?
Chemical names on food labels spark concern. Once I picked up a bag of shredded cheese, read “sodium dihydrogen phosphate,” and hesitated. Curiosity took over and sent me on a search through research journals and reports from agencies with skin in the food safety game, such as the US Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA).
Sodium dihydrogen phosphate stands out as part of the phosphate family—compounds naturally present in both animal and plant foods. Our bodies handle phosphates every day since they’re fundamental for bones, cell membranes, and energy use. Both the FDA and EFSA view sodium dihydrogen phosphate as “generally recognized as safe” when used in reasonable amounts. This assessment comes from large toxicology studies and decades of real-world food use without serious population-level side effects.
Possible Health Concerns
Not everything is smooth sailing. Processed foods pile up the phosphate load, and research shows that overdoing phosphate can stress kidneys and potentially weaken bones. Most healthy adults do fine, but people with advanced kidney disease should limit phosphate since their bodies have trouble getting rid of it. For those with this condition, a pharmacy shelf overflowing with phosphate-based additives just makes life harder. Rates of phosphate additives in processed foods jumped in the last 50 years, and studies connect high phosphate intake with some heart problems in vulnerable groups. These connections push many to check food labels and trim processed food intake.
The World Health Organization and other expert groups suggest that keeping total daily phosphate intake under 3,750 milligrams helps prevent problems. An ordinary diet based mostly on homemade meals makes this mark easy to stay under. The trouble usually starts with meals that rely heavily on packaged or convenience foods.
Transparency, Choice, and Solutions
I aim for transparency when I shop and cook. For those who want to keep phosphate intake in check, reading ingredient lists still works well. Cutting back on heavily processed snacks shifts the balance, since fruits, vegetables, grains, and meats rarely carry extra phosphates. Some brands now advertise “phosphate free” cheese slices or deli meat, and smaller businesses keep ingredients lists short so shoppers can feel more in control.
Education plays a big role, as many people never realized how common phosphate additives like sodium dihydrogen phosphate became. Nutrition labels now list phosphorus in some parts of the world, which gives more tools to families managing kidney trouble or other conditions.
It takes more than personal vigilance for real change. Governments update dietary guidelines and food safety rules by weighing new science against the realities of food production. Doctors and nutritionists can spread the word for those most at risk, so medical advice meets people where they are.
Summing Up the Evidence
Experience as both a parent and food label detective tells me: sodium dihydrogen phosphate, used within recommended amounts, poses no threat to the average healthy person. Caution makes sense for anyone with kidney disease or for those who eat lots of processed convenience foods. Keeping meals fresh, preparing more foods from scratch, and asking questions about ingredients go a long way toward safer choices and better long-term health.
What are the storage and handling requirements for Sodium Dihydrogen Phosphate?
Real-World Risks Often Lurk Behind Simple White Crystals
Sodium dihydrogen phosphate may look like an unremarkable bag of salt. Still, its journey from production to use takes careful attention to storage and handling. Take it from anyone who’s seen what a little moisture or a careless spill can do. Keep this material dry, and you stop clumping, corrosion, and loss of function right from the start. Humidity sneaks up, and before long you’ll have solid masses that jam up dispensers or damage bag liners. Apharmacy or food processor won’t accept product that looks off, and who can blame them?
Even basic chemistry tells us this powder draws water from the air. Soggy phosphate can break down, lower shelf life, and threaten accuracy in lab or food recipes. A simple airtight drum or bag with a good seal makes all the difference. Quality managers who cut corners here invite financial loss and safety audits. Opening a drum only as needed, then resealing it, ranks right up there with storing it off the damp floor. These are habits learned after one too many ruined batches.
Don’t Ignore the Skin or Eyes
Some folks may downplay the hazards of these white crystals, but skin and eyes still complain if you skip gloves or goggles. Anyone who’s mixed dry chemicals knows that dust gets airborne fast. One careless move, and you’re dealing with red eyes or irritation all afternoon. In crowded workspaces or during bulk transfers, run a fan or open a window. If you skip the dust mask, you risk a cough or worse after a busy day. Sensible protection like gloves, closed shoes, and safety glasses protect workers and lead to fewer interruptions or sick days.
Labeling and Spill Response Aren’t Optional
Accidental mix-ups waste money and threaten compliance. Labels keep everyone honest. Clear, durable labels for each drum and a record for the shelf or rack save time and cut the risk of using the wrong chemical. If a spill happens, dry phosphate on the floor picks up fast with a broom and dustpan. Still, cleaning crews keep bags handy just in case. Any residue needs water and then regular housekeeping to avoid slippery spots or chemical build-up. Getting lazy about this never ends well and upsets both inspectors and coworkers alike.
Long-Term Storage Builds Trust with Buyers
Warehouse space isn’t cheap and not every site offers climate-controlled storage. Still, stacking pallets up off the floor, avoiding direct sunlight, and rotating stock as it arrives all help preserve product quality. Older bags should get used first to dodge hard lumps or reduced performance. Customers trust suppliers who deliver fresh, clean, reliable bags every order. Once a shipment gets rejected, it takes ages to restore that reputation. Even small shortcuts in storage can snowball into lost contracts.
Continuous Learning, Fewer Accidents
Some manufacturers offer regular staff training, and old-timers pass down their own tips. Stories of failed batches or workplace accidents still teach more than generic rules tacked up on a wall. Taking shortcuts on storage and handling never pays. Checking up on bags, watching humidity, and giving workers proper training ensure sodium dihydrogen phosphate does its job safely, batch after batch. Quality grows out of habits, not just manuals—something I’ve learned time and again from years working with industrial chemicals.
What is the chemical formula and molecular weight of Sodium Dihydrogen Phosphate?
Understanding the Chemical Formula
Sodium dihydrogen phosphate carries the chemical formula NaH2PO4. That simple combination speaks volumes in scientific and practical circles. I remember learning in a university chemistry lab how this formula helps prompt precise mixing in everything from buffer solutions to industrial settings. With sodium, two hydrogens, one phosphorus, and four oxygens, the structure itself shapes its properties—especially its behavior in water and its role as an acid salt.
Why the Molecular Weight Matters
The molecular weight lands at 119.98 g/mol. This isn’t just a trivia answer; it’s the kind of number that shapes everyday lab routines. I spent years watching colleagues use this exact weight while measuring out buffer solutions, where accuracy means reliable results. Even a simple miscalculation leads to skewed outcomes, wasted reagents, and sometimes dangerous reactions. Pharmacists, food technologists, and wastewater specialists all depend on this figure to balance formulas and keep products stable.
Why This Compound Shows Up Everywhere
Many people miss how sodium dihydrogen phosphate shows up in baked goods, lab kits, and even dialysis fluids. It doesn’t just slide into formulas for the sake of it—its acidifying qualities help regulate pH with impressive precision. In the food world, it acts as a leavening agent, working with baking soda to help dough rise evenly without a sour aftertaste. In medicine, hospitals rely on it to stabilize blood pH during treatments. When I worked with dieticians developing low-sodium food alternatives, balancing this compound’s benefits with sodium restrictions brought home just how influential it can be.
Trouble with Overuse and Misuse
Sodium intake sparks concern across nutrition and healthcare circles. While sodium dihydrogen phosphate offers key benefits, regular overuse contributes to the swelling health crisis of excessive dietary sodium. This raises blood pressure and pushes up the risk of cardiovascular diseases. The Centers for Disease Control states that most Americans consume well above recommended sodium limits, and processed additives like sodium dihydrogen phosphate play a part. Product reformulation takes real determination, but it brings better long-term outcomes.
Finding an Effective Middle Ground
Responsible manufacturing could make a real difference. Food producers can reformulate, choosing lower-sodium alternatives or reducing overall additive amounts. In the laboratory, tighter guidelines and digital calculators cut dosing inaccuracies. Public policy moves could push for clearer labeling, making it easier for consumers to identify sodium-based additives and adjust their intake. As a parent, I see the value of trustworthy ingredient lists, since it lets families decide what’s in their dinner.
Supporting Better Choices
Access to simple, clear information changes the way individuals and professionals engage with chemical additives. A well-founded decision relies on facts—not just numbers, but context about real-world use and the ripple effects of each choice. Sodium dihydrogen phosphate’s formula and molecular weight look like tiny pieces of a puzzle. In reality, they play a bigger role in supporting food safety, medical care, and public health efforts. By staying alert to how such compounds are used and monitoring intake, we keep science working for people, not just processes.
Is Sodium Dihydrogen Phosphate available in different grades or purity levels?
Grades: More Than Just a Label
Anyone who’s spent time with chemicals knows small details can take on big meaning. Sodium dihydrogen phosphate shows up in labs, food, and even water treatment. Each of these fields can’t just scoop from the same barrel. Why? Purity decides whether your bread rises, your fish survive, or your experiment tells the truth.
The Wide World of Purity Levels
So, let’s strip away the textbook language. You pick up a bag of sodium dihydrogen phosphate—what’s inside really depends on where it’s heading. Pharmaceutical and food industries gravitate to “food grade” or “pharmaceutical grade.” Here, limits on impurities stay tight. Standards like FCC or USP lay out exactly how pure it needs to be, including caps on heavy metals and moisture. The reason? Even a tiny contaminant can trigger allergic reactions or skew medicine dosages.
Then there’s the tech, industrial, or technical grade. These are fine for jobs like cleaning, fertilizer production, or wastewater treatment. Refining the stuff to ultra-high purity drives up costs with little benefit for such applications. Lower-grade products help manufacturers keep expenses down while still supporting soil health or keeping boilers running smoothly.
Why Bother? The High Stakes of Purity
Working in a research lab, I once saw how one batch of lower-grade sodium dihydrogen phosphate spoiled an experiment we’d repeated for weeks. That experience sticks because no one likes wasting time or money because a supplier swapped grades without warning. For food producers, it’s even tougher. Regulators inspect everything from bread to canned veggies. No company wants to see a recall over a tainted additive.
In water treatment, impurities could mean higher pollution coming out the other end. People trust something as basic as tap water. Sub-par chemicals risk breaking that trust.
Solutions That Actually Work
For anyone handling sodium dihydrogen phosphate, it pays to get nerdy about paperwork. Reliable suppliers provide certificates showing purity numbers for each lot shipped. Lab testing on receiving batches might sound excessive, but failures get expensive fast. Some companies have invested heavily in in-house testing labs, after getting burned by contaminated shipments.
Stronger partnerships across the chemical supply chain make all the difference. Encouraging transparency in how the stuff gets handled, shipped, and stored offers protection against nasty surprises. Industry groups can push for clear labeling and tighter tracking systems.
Where It Goes Next
Looking at rising pressure from consumers and tighter government rules, expect a push toward better traceability and reporting. Digital tracking systems, like blockchain, could make it easier to prove the source and purity of every lot poured into food or pills. People want to know their food and water are safe. Quality control stops being a cost and starts to look like essential insurance.
Bottom line: purity isn’t just a detail—it's a dealbreaker for health, science, and trust. Those of us who’ve seen the fallout from a tiny impurity never forget. Keeping that standard high keeps everyone safe, from the lab bench to the kitchen table.
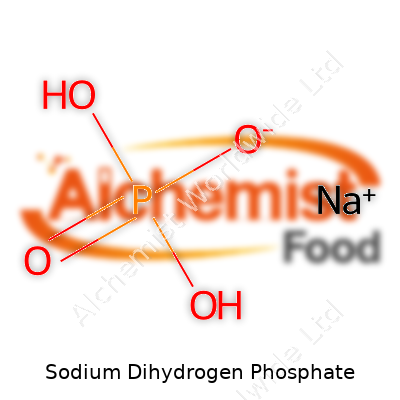
| Names | |
| Preferred IUPAC name | sodium dihydrogen phosphate |
| Other names |
Monosodium phosphate Sodium phosphate monobasic Monobasic sodium phosphate NaH2PO4 |
| Pronunciation | /ˌsəʊdiəm daɪˈhaɪdrədʒən fəˈsfeɪt/ |
| Preferred IUPAC name | sodium dihydrogen phosphate |
| Other names |
Monosodium phosphate Monobasic sodium phosphate Sodium phosphate monobasic NaH2PO4 |
| Pronunciation | /ˌsoʊ.di.əm daɪˈhaɪ.drə.dʒən ˈfɒs.feɪt/ |
| Identifiers | |
| CAS Number | 7558-80-7 |
| Beilstein Reference | 821873 |
| ChEBI | CHEBI:62955 |
| ChEMBL | CHEMBL1357 |
| ChemSpider | 5048 |
| DrugBank | DB09446 |
| ECHA InfoCard | ECHA InfoCard: 031f7b35-ce1e-4b4f-9c61-5fd00357fb44 |
| EC Number | 231-449-2 |
| Gmelin Reference | 67656 |
| KEGG | C01005 |
| MeSH | D006153 |
| PubChem CID | 24203 |
| RTECS number | WA1900000 |
| UNII | 7UED7186AP |
| UN number | UN9146 |
| CAS Number | 7558-80-7 |
| Beilstein Reference | 1721397 |
| ChEBI | CHEBI:86455 |
| ChEMBL | CHEMBL1377 |
| ChemSpider | 5959 |
| DrugBank | DB09449 |
| ECHA InfoCard | 05c35b74-d687-45fd-9c25-8841c1ec52b7 |
| EC Number | 231-449-2 |
| Gmelin Reference | 57248 |
| KEGG | C00161 |
| MeSH | Dihydrogen Phosphates |
| PubChem CID | 24203 |
| RTECS number | WB0400000 |
| UNII | 19F6RM814V |
| UN number | UN9146 |
| CompTox Dashboard (EPA) | DTXSID2021423 |
| Properties | |
| Chemical formula | NaH₂PO₄ |
| Molar mass | 119.98 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.04 g/cm³ |
| Solubility in water | Very soluble |
| log P | -3.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | 12.3 |
| Magnetic susceptibility (χ) | -46.6e-6 cm³/mol |
| Refractive index (nD) | 1.395 |
| Dipole moment | 6.0 D |
| Chemical formula | NaH2PO4 |
| Molar mass | 119.98 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.04 g/cm³ |
| Solubility in water | Easily soluble in water |
| log P | -4.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa1 = 2.15, pKa2 = 7.20 |
| Basicity (pKb) | 12.3 |
| Magnetic susceptibility (χ) | -41.6·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.395 |
| Dipole moment | NaH2PO4: 3.05 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 92.6 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1267 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1480 kJ·mol⁻¹ |
| Std molar entropy (S⦵298) | 92.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −1,277.0 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | −1277.4 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A06AA02 |
| ATC code | A06AA02 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, Warning, H319, P264, P280, P305+P351+P338, P337+P313 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "May cause respiratory irritation. |
| Precautionary statements | Keep only in original packaging. Wash hands thoroughly after handling. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 oral rat 8290 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 17,000 mg/kg |
| NIOSH | WH7400000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30-300 mg |
| IDLH (Immediate danger) | Not listed |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS07, Warning, H319, P264, P280, P305+P351+P338, P337+P313 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: May cause respiratory irritation. |
| Precautionary statements | Keep only in original packaging. Store in a dry place. Store in a closed container. Wash hands thoroughly after handling. Avoid release to the environment. |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Lethal dose or concentration | LD50 oral rat 8290 mg/kg |
| LD50 (median dose) | LD₅₀ (oral, rat): 17,800 mg/kg |
| NIOSH | WH7500000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 kg |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Disodium phosphate Trisodium phosphate Monopotassium phosphate |
| Related compounds |
Disodium phosphate Trisodium phosphate Phosphoric acid Monopotassium phosphate |

