Sodium Dihydrogen Citrate: A Deep Dive
Historical Development
People first began working with citric acid salts not long after the isolation of citric acid itself in the late 1700s. Discovery moved at the pace of glassware and trial-and-error methods, but the persistent interest in better preservation methods pushed research forward. The gradual rollout of sodium dihydrogen citrate in medicines and food stabilizers didn’t make headlines, yet demand grew as more researchers and industry leaders recognized the value of less aggressive, more food-compatible acid sources. By the 20th century, production ramped up to serve pharmaceutical and beverage manufacturers, setting the stage for more nuanced uses in products ranging from syrups to anticoagulants.
Product Overview
Sodium dihydrogen citrate, often found as a fine, white, odorless powder, stands out as a mineral salt derived from citric acid. Unlike harsher acidic compounds, this salt helps regulate pH without overwhelming flavors or corroding equipment. I’ve seen it come to life in cold remedies as a buffer—much more gentle than earlier acidic choices. Companies dealing with food and beverage recipes turn to it because it quietly maintains product safety and quality without altering taste.
Physical & Chemical Properties
Solid at room temperature, sodium dihydrogen citrate dissolves quickly in water, making it easy to mix or dose in production lines. This compound carries the chemical formula NaC6H7O7. Its slightly tart profile often helps mask the bitterness in syrups or elixirs. Heat does not break it down easily, which matters for bottling and sterilization steps. From my time in a beverage lab, I learned you could toss a handful into a tank and count on an immediate drop in pH—a reliable trick without surprises.
Technical Specifications & Labeling
Manufacturers list sodium dihydrogen citrate as either a food-grade or pharmaceutical-grade product depending on purity, with fine distinctions traced by detailed documentation. Each shipment crosses desks with careful COA packets, listing content purity (usually no less than 99%), water content, and trace materials. Labeling rules require clear ingredient names and, in some cases, reference codes—think INS 331 for food additive classification. Production facilities often keep digital logs of every granule that enters a batch to comply with batch tracking standards, which isn’t just a paper chase but a cornerstone of accountability in product recalls or audits.
Preparation Method
Factories make sodium dihydrogen citrate by neutralizing a portion of citric acid’s three acid groups with sodium carbonate or sodium bicarbonate. You don’t need complex reactors for this—just careful temperature control and timing. The acid-base reaction releases carbon dioxide and water, leaving behind a crystal slurry that dries out into a usable powder. Filtering, drying, and milling create a product ready to meet even the tightest margins for purity. Plant operators watch the temperatures and pH values with eagle eyes, knowing that rushed processing leads straight to unstable, sticky residues nobody can use.
Chemical Reactions & Modifications
Sodium dihydrogen citrate’s reactivity lets chemists nudge pH values with a whisk of the wrist. Paired with other acid salts or bases, it can anchor buffer systems in injectable drugs or oral tablets. I’ve helped dissolve it with calcium salts for oral medical solutions, watching the precipitation clear up just by adjusting the mix. This compound also acts as a modest chelator, especially when it meets metals like iron, hampering the process that leads to food browning or drug discoloration. In rare lab projects, tweaks involve substituting sodium with potassium to open up alternative product profiles when sodium content poses a concern.
Synonyms & Product Names
You might run into sodium dihydrogen citrate on a label as monosodium citrate, sodium citrate monobasic, or by its registry number 18996-35-5. In the beverage world, “mild citrate buffer” turns up in spec sheets for cola syrups and effervescent tablets. Bulk suppliers move it under generic banners like “food-grade sodium citrate,” though the particulars will always point to which sodium-citric acid ratio you’re getting. Tracking down counterfeit or mislabeled lots depends on knowing these subtle name differences, especially when translated for global trade.
Safety & Operational Standards
Operators in chemical plants sweat the details for sodium dihydrogen citrate storage. The powder must stay dry and tightly sealed to prevent clumping or early degradation—any slip leads to quality complaints down the road. Process manuals and ongoing training sessions drill home the importance of eye protection and respirators, as even mild powders cause irritation with enough exposure. Quality assurance teams test incoming and outgoing product lots according to ISO and GMP rules. Allergen bulletins accompany every order, protecting manufacturers against cross-contact and giving end-users peace of mind.
Application Area
Sodium dihydrogen citrate touches more than just one shelf in the medicine cabinet. It manages acidity in soft drinks, makes pharmaceuticals less harsh on the stomach, and steadies blood samples for lab testing. In my community, it helps hospitals treat urinary tract disorders by controlling urine acidity. Candy makers lean on it for pH tweaks in chewy confections. Where laws allow, water treatment facilities blend it into formulas that prevent scale buildup or help with lead precipitation, though these uses often fly under the radar except to a handful of municipal engineers.
Research & Development
Academic labs and commercial R&D units spend hours testing the stability of sodium dihydrogen citrate under wild swings of temperature and humidity. Researchers probe for new buffer systems that take advantage of the salt’s gentle pH changes. In the past five years, studies have expanded into pairing it with other organic acids to stretch shelf life for plant-based milks and juices. Pharmaceutical development teams test it in buffered antibiotics to stave off stomach aches, which gives pediatric medicine a much-needed upgrade in taste and comfort. Growing interest in low-sodium diets also guides trials into sodium substitutes—each formula carefully balanced so that benefits don’t crumble under new health concerns.
Toxicity Research
Toxicologists have combed through the effects of sodium dihydrogen citrate, dosing lab animals and running cellular assays. Reports show it rarely triggers toxicity except in massive overdoses, far above what consumers meet in food or medicine. Clinicians flag risks for patients with kidney disease since excess sodium or citrate loads can complicate their balance. Safety assessors run repeat toxicity studies every few years, matching evolving usage patterns. Personal experience has shown that even diligent regulatory compliance can’t prevent rare allergic or intolerant reactions, so medical providers keep careful watch over reports from the field.
Future Prospects
Food and drug scientists see promise in sodium dihydrogen citrate's legacy as a mild, reliable acid source. Teams chase next-generation formulas that give products longer shelf lives with lower sodium intake. The food industry eyes it as part of natural preservative blends, especially as consumer sentiment leans away from synthetic stabilizers. Pharmaceutical firms actively fine-tune buffered medications that taste better and go down easy for children or sensitive patients. In the world of clean-label ingredients, transparency in sourcing and processing continues to shape demand and technical disclosures. More cross-disciplinary collaborations between chemists, nutritionists, and technologists seem likely as health trends and safety standards evolve.
What is Sodium Dihydrogen Citrate used for?
Everyday Uses in Medicine
Doctors trust sodium dihydrogen citrate when managing kidney stones and certain urinary tract issues. Think of those terrible sharp pains that come from a stone lodging itself in your urinary tract. This salt helps because it changes the acidity of urine. More alkaline urine slows stone growth and can even help dissolve smaller stones. When I spent a week helping care for a family member struggling with kidney stones, I saw how small interventions like these made a tough experience less miserable. Doctors also use it to support conditions where someone’s body has become too acidic. Hospitals rely on it to manage these tricky pH shifts, especially in people whose kidneys aren’t clearing acids properly.
Pain and Practical Relief
For folks prone to gout, that stabbing pain in the big toe might sound all too familiar. This salt can ease those attacks by lowering uric acid crystals in the urine, reducing their build-up in joints. This doesn’t replace dietary changes or proper medication, and skipping doctor visits isn't an option, but it’s one more tool.
Role in the Food World
Food scientists use sodium dihydrogen citrate too, mainly for its ability to keep drinks and preserves fresh and safe. It acts as an acid regulator, stopping nasty microbes from growing. Next time you reach for a lemon-lime soda or certain jams, you might notice how bright and tart they taste, and that’s often thanks to this salt holding the flavor together. Food safety is more than lab coats and regulations—each ingredient, like this one, helps keep spoilage and harmful bacteria away from the snacks and drinks we enjoy.
Supporting Proper Hydration
This ingredient helps turn some oral rehydration salts into effective tools for easing dehydration. Ask any nurse who has prepped rehydration solutions after a bad gastro bug, and they’ll say a balanced mix keeps kids and elderly folks safer. By adjusting the acidity and supporting electrolyte balance, sodium dihydrogen citrate does its part behind the scenes.
Safe Handling and Side Effects
No one wants surprises with medical or food additives. Taking sodium dihydrogen citrate in prescribed amounts keeps things safe. Too much can mean dangerous electrolyte shifts or upset stomachs, so medical advice is a must. You can’t just scoop it into a drink and hope for the best—years of research and regulation shape how much shows up in medicines and foods. Doctors know it inside out, which reassures both patients and their families.
Room for Better Access and Information
Not everyone learns about these ingredients in school or from a doctor. Public health programs could step up by sharing easy-to-follow guides. Pharmacies and clinics might update patient leaflets. Technology helps, too, with clear online information supporting families who want to feel empowered but safe.
Summary of its Place
Across kidney care, gout management, food safety, and hydration, sodium dihydrogen citrate proves its worth day after day. By supporting the body’s chemistry, it helps real people live with fewer symptoms and less risk, showing how one compound can make life a little easier for so many.
How should Sodium Dihydrogen Citrate be taken?
What this compound does
Sodium dihydrogen citrate sounds like a mouthful, but it’s often given for one straightforward purpose: to make urine less acidic. Someone dealing with kidney stones or certain urinary tract issues may spot this name on the label. Doctors often use it to help prevent stones from forming. While this isn’t the flashiest topic, it matters a lot for folks prone to kidney pain or uncomfortable symptoms that come with acid buildup.
How to take it safely
From what I’ve seen in pharmacy practice, people usually see this medicine as a powder or liquid. Most folks get it in single-use sachets. The old rule of thumb holds up here — dissolve the powder in a glass of water and drink it right away. It tastes a bit salty, sometimes with a twist of sourness, so don’t expect it to be tasty. Adding it to a flavored drink might help, but one must make sure not to use juice with extra acidity because that misses the point of taking the medicine altogether.
Always follow the dosing your doctor gives you. For adults, prescriptions usually fall between two and six grams daily, split across two or three servings. Kids take less, based on their size and age. A lot of people forget the importance of spacing out doses throughout the day, not piling everything into a single drink. Skipping this advice runs the risk of up-and-down acid levels, lowering the positive effects and sometimes leading to new symptoms. Chugging more thinking it will speed up results can actually create new problems, like high sodium in the blood, and make someone feel pretty crummy.
Timing and combinations matter
Taking sodium dihydrogen citrate right after meals lowers stomach upset. Downing it on an empty stomach can cause light nausea or a mild tummy ache — a fact many find out the hard way. Chugging a glass of water afterward helps avoid dehydration, defeats saltiness, and keeps urine flow up, which is important for flushing out those crystals. People who already have high blood pressure or heart rhythm problems should mention any ongoing symptoms to their doctor, because extra sodium sometimes makes these conditions worse.
Mix-ups and what to avoid
Some folks think all citrates are the same. They aren’t. A cousin, potassium citrate, serves a similar job but isn’t interchangeable. Sodium overload sneaks up when people double doses or combine this medicine with a salty diet or sports drinks. Reading labels closely matters. Patients on a long list of medicines — water pills, heart pills, anti-inflammatory painkillers — should ask a pharmacist to check for clashes before adding sodium dihydrogen citrate to their routine.
A practical word on follow-up
Doctors sometimes order regular urine or blood tests, since this compound slowly changes acid levels. Symptoms don’t always show up right away, so keeping appointments gives people and their care teams time to make careful adjustments. This medicine offers clear benefits for many, but only with respectful use and steady, honest feedback between patient and professional. Support and knowledge here make a world of difference for anyone hoping to kick kidney pain or keep stone trouble from coming back.
What are the possible side effects of Sodium Dihydrogen Citrate?
What Happens In The Body?
Nobody walks into a pharmacy to grab sodium dihydrogen citrate just for fun. The compound helps the body rid itself of excess uric acid, and health professionals prescribe it to manage certain types of urinary tract problems or gout-like symptoms. Still, every medication brings its own list of possible tradeoffs.
Common Problems After Taking It
Stomach seems to notice change right away. People sometimes report mild pain or discomfort, gas, or a feeling that food does not sit well. These signs usually pop up soon after the dose and fade in most cases, once the body gets used to the medicine. One study published in Therapeutic Advances in Drug Safety mentioned loose stools or an urge to visit the restroom a bit sooner than planned. These effects seldom last long, but anyone feeling them should still mention it to a doctor, especially if nausea or cramps linger.
Sodium And Water Balance
Chemistry in the body can roll off track if sodium loads pile up. People on a salt-restricted diet or with heart or kidney conditions need to stay cautious. The body balances its fluids with strict rules—too much sodium pulls in water, swelling ankles or causing shortness of breath for the sensitive group. Fluid overload is rare but serious, and checking with a healthcare provider before starting any sodium-based product makes a difference.
Metabolic Shifts Worth Watching
Sodium dihydrogen citrate alters urine’s acidity, which solves one problem but sometimes creates others. Lowering urine acidity can let certain kidney stones sneak in, especially in folks who have struggled with stone formation in the past. Lab tests tracking urine composition and kidney function help spot trouble early if the medicine needs to stay in the routine for a while. Hydration plays a key part—water keeps the chemistry in check and provides a simple way to reduce risk.
Uncommon But Serious Reactions
Rarely, allergies make an appearance—rash, swelling, or trouble breathing always demand emergency help. People should not try to “wait out” these symptoms, as quick action might prevent a bigger crisis.
Not Everyone Reacts The Same Way
Older adults, people with impaired kidney function, or those battling high blood pressure live with a different risk profile. Their bodies do not flush sodium and water with the same efficiency as a healthy twenty-year-old. Reports in clinical bulletins from sources like the Journal of Clinical Pharmacy and Therapeutics remind practitioners to stay alert with these cases, urging careful monitoring and routine check-ins.
What To Do If Trouble Surfaces
Anyone feeling outside their normal after a dose should talk to a healthcare provider—no need to tough it out or guess what is “normal.” Clear communication about new medicines helps spot side effects early. Pharmacists and doctors have worked with these problems before and can offer real advice on adjusting the dose or considering another therapy.
Choosing Safety With Each Dose
Trust builds up with honest conversations. Reading the medicine leaflet, asking questions, and reporting new reactions keeps care safer for everyone. Changing one’s diet, routines, or other prescriptions might change how the body handles sodium dihydrogen citrate. Knowing that these risks exist helps people weigh the true benefit of starting or continuing therapy and prevents side effects from turning into bigger health issues.
Can Sodium Dihydrogen Citrate be used during pregnancy or breastfeeding?
Understanding the Purpose of Sodium Dihydrogen Citrate
Sodium dihydrogen citrate pops up in some medications as a way to help with urinary tract infections, kidney stones, or as an ingredient to make urine less acidic. It’s been around for decades and doctors still write prescriptions for it today, but if you’re pregnant or breastfeeding, the thought of putting anything extra into your body can feel overwhelming.
Safety Concerns During Pregnancy and Breastfeeding
Having kids myself, I remember staring at every label and googling every unfamiliar word. Most pregnant women share this concern, and the stakes feel bigger when nursing a baby. Fact is, research about sodium dihydrogen citrate specifically during pregnancy or breastfeeding is surprisingly thin. Most medical textbooks or databases don’t cover it in great depth.
Pregnancy comes with so many changes—hormones, blood flow, even the way kidneys work. The body naturally works harder to flush out minerals and drugs. Anything taken by a pregnant person needs to make it past the placenta safely, without causing trouble for the growing baby. During breastfeeding, the question turns to whether the substance slips into milk and what effect it might have on a newborn’s kidneys or digestion.
Most drug manufacturers recommend skipping sodium dihydrogen citrate unless a health care professional says it’s absolutely necessary. That caution isn’t just about being overly safe; it’s the result of not knowing if the chemical could quietly cause harm. Without solid studies in humans, safe use relies on experience and common sense rather than strict data.
What Do Health Authorities and Experts Say?
Groups like the FDA or NHS tend to keep hands off sodium dihydrogen citrate specifically for pregnancy and breastfeeding, instead pushing for substances with a long track record of safety. In 2024, a quick check for any official green light on this compound for pregnant or nursing folks brings up a lot of “no clear information available.”
Doctors usually weigh the risks and benefits. Chronic urinary tract infections can do real damage if not treated. If there’s no better option, a professional may still use sodium dihydrogen citrate for a short period, but close monitoring is a must. They might check kidney function, adjust dosing for body weight, and keep an eye out for unusual reactions.
Making Health Decisions With Limited Evidence
Pregnant people already get mixed messages about coffee, sushi, or hair dye—sodium dihydrogen citrate is just another on a long list that needs a careful, personal decision. From personal experience, clear, honest conversations with a healthcare provider make a world of difference. Pharmacists can offer advice, too, especially if you worry about mixing this medication with prenatal vitamins or other prescriptions.
Looking for alternatives, doctors might turn to drinking more water, dietary changes, or safer medications that treat the same problems. Cranberry supplements or probiotics are sometimes recommended, though the evidence for those remains limited as well. Prevention can play a central role, with lifestyle shifts helping lower the need for medicines that haven’t been fully tested in pregnancy.
Final Thoughts and Safer Choices
Trust forms the base of good decisions during pregnancy and breastfeeding. That means admitting when we just don’t know enough about a medication’s risks. Until researchers do deeper studies on sodium dihydrogen citrate, the safest move often means avoiding it unless there’s a clear, unavoidable need. Talking through the options with a health professional, and exploring gentler preventive measures, offers a path toward peace of mind.
Are there any drug interactions with Sodium Dihydrogen Citrate?
Understanding Why Sodium Dihydrogen Citrate Needs Caution
Sodium dihydrogen citrate often ends up in the toolkit for treating conditions where urine needs to be more alkaline, like kidney stones or gout. Doctors sometimes recommend it for easing mild discomfort from urinary tract problems. It works by making urine less acidic. This change sounds simple, but the body runs on balance, and even mild changes in acidity can shake up how certain medicines act inside us.
Common Medications That Ask for Extra Attention
Some drugs, especially ones struggling with acid-sensitive absorption, react strongly to shifts in the body’s pH. Antibiotics like tetracyclines or certain medications for fungal infections fall into this category. If you’re on a medicine that asks for acidic urine, like methenamine, sodium dihydrogen citrate gets in the way by making urine less acidic. Suddenly, your medicine isn’t working as well.
Blood pressure medications that use potassium, like some diuretics, pile on another risk. Sodium dihydrogen citrate may increase the body’s potassium, which could tilt the scales toward high potassium—dangerous if you’re already at risk. People using ACE inhibitors, angiotensin receptor blockers, or potassium-sparing diuretics need an honest conversation with a clinician before throwing another variable into the mix.
Health Factors That Shift the Equation
Anyone living with kidney problems, heart failure, or severe dehydration runs into more hazards when their mineral and water balance shifts. Changes from sodium or citrate won’t always show up fast, so a lag between starting the drug and feeling side effects can fool even the watchful. I’ve seen friends try out an over-the-counter remedy, certain it’s mild, only to chase their tails weeks later over something as basic as swollen ankles or fatigue. Monitoring makes the difference.
People taking nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen, especially over the long term or with kidney concerns, need more than a casual scan. The combination with sodium dihydrogen citrate could inch the kidneys closer to injury. Each new medication, even over-the-counter ones, comes with a fresh risk and deserves a medical professional’s quick review.
Facts Behind Citrate and Everyday Medication
Clinicians across primary care and emergency settings agree that sodium dihydrogen citrate doesn’t deliver widespread drug interactions, but the ones that happen can be significant. For example, the U.S. National Library of Medicine warns that certain antibiotics and methenamine get tripped up by altered urinary pH. Reports from Europe show similar caution with potassium-handling drugs.
Plenty of folks use this product when fighting kidney stones or gout. Adding a drug that changes mineral levels, especially in older adults, multiplies chances for a slip-up. Some specialists recommend routine blood checks for people who stay on both potassium-shifting meds and urine-alkalizing agents.
Practical Steps for Safe Use
It starts with clear, straightforward questions before adding anything, supplement or pharmaceutical. A pharmacist or doctor can catch a risk that a quick web search might miss. Checking labels on every medication in use, including herbal teas or powders, makes a real difference—these often hide unexpected ingredients.
Anyone who notices muscle weakness, too much thirst, or an oddly fast or slow heartbeat after starting sodium dihydrogen citrate deserves prompt care. These early warning signs rarely fix themselves and sometimes point to a shift in potassium or sodium that climbs to medical emergencies fast.
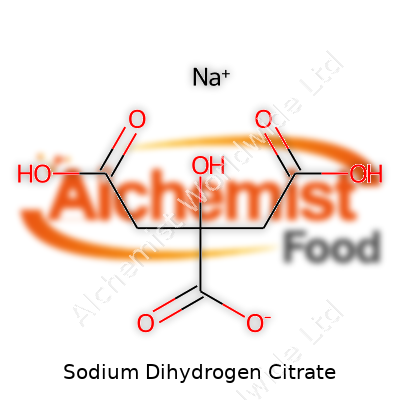
| Names | |
| Preferred IUPAC name | Sodium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Monosodium citrate Sodium acid citrate Sodium dihydrocitrate |
| Pronunciation | /ˌsəʊdiəm daɪˌhaɪdrə.dʒən ˈsɪtreɪt/ |
| Preferred IUPAC name | sodium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Monosodium citrate E331(i) Sodium acid citrate Sodium citrate monobasic |
| Pronunciation | /ˈsəʊdiəm daɪˈhaɪdrədʒən ˈsɪtreɪt/ |
| Identifiers | |
| CAS Number | 18996-35-5 |
| Beilstein Reference | 1902303 |
| ChEBI | CHEBI:62935 |
| ChEMBL | CHEMBL1201570 |
| ChemSpider | 5956 |
| DrugBank | DB14598 |
| ECHA InfoCard | 100.028.335 |
| EC Number | E331(i) |
| Gmelin Reference | 9768 |
| KEGG | C00732 |
| MeSH | D017366 |
| PubChem CID | 23682280 |
| RTECS number | GE8300000 |
| UNII | X5R9R72R10 |
| UN number | UN1760 |
| CompTox Dashboard (EPA) | DTXSID2037468 |
| CAS Number | 18996-35-5 |
| Beilstein Reference | 104281 |
| ChEBI | CHEBI:9120 |
| ChEMBL | CHEMBL1201564 |
| ChemSpider | 22258 |
| DrugBank | DB14547 |
| ECHA InfoCard | 100.031.398 |
| EC Number | E331(i) |
| Gmelin Reference | 131388 |
| KEGG | C00715 |
| MeSH | Diatric Acid |
| PubChem CID | 643398 |
| RTECS number | GE7250000 |
| UNII | RU9T81Y78F |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID5053606 |
| Properties | |
| Chemical formula | NaH₂C₆H₅O₇ |
| Molar mass | 258.06 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.36 g/cm³ |
| Solubility in water | Freely soluble in water |
| log P | -2.6 |
| Acidity (pKa) | 3.1 |
| Basicity (pKb) | pKb 3.14 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.445 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.6 D |
| Chemical formula | NaC6H7O7 |
| Molar mass | 258.06 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.36 g/cm³ |
| Solubility in water | Freely soluble in water |
| log P | -3.5 |
| Vapor pressure | Non-volatile |
| Acidity (pKa) | 3.21 |
| Basicity (pKb) | pKb ≈ 6.40 |
| Magnetic susceptibility (χ) | -56.0×10⁻⁶ cm³/mol |
| Viscosity | Viscous liquid |
| Dipole moment | 6.1 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 206.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1561.9 kJ/mol |
| Std molar entropy (S⦵298) | 248.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –1566.38 kJ/mol |
| Pharmacology | |
| ATC code | G04CA11 |
| ATC code | A09AB16 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Use with adequate ventilation. |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Lethal dose or concentration | LD₅₀ (oral, rat): 17,000 mg/kg |
| LD50 (median dose) | > 8.4 g/kg (oral, rat) |
| NIOSH | WF3150000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1.5–3 g daily in 2–3 divided doses |
| IDLH (Immediate danger) | Not listed |
| Main hazards | May cause irritation to eyes, skin, and respiratory tract. |
| GHS labelling | GHS labelling: "Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008 (CLP/GHS) |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Do not ingest. Use with adequate ventilation. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | > 220°C |
| Lethal dose or concentration | LD50 oral rat 5400 mg/kg |
| LD50 (median dose) | 5400 mg/kg (rat, oral) |
| NIOSH | WM5425000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 6 - 10 gm |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Citric acid Trisodium citrate Disodium citrate Monosodium citrate |
| Related compounds |
Citric acid Trisodium citrate Disodium citrate Monosodium citrate |

