Sodium Diacetate: A Story of Science, Safety, and Everyday Impact
Historical Development
Chemistry often changes the shape of food and industry, and sodium diacetate’s story stretches decades. Chemists noticed the need for reliable food preservatives as early as the mid-twentieth century; meat and dairy needed longer shelf lives, and bread kept going stale. Scientists blended acetic acid, familiar from vinegar, with sodium acetate to create a salt that married sourness with salt’s preservative touch. The process matched demand as global supply chains stretched and food storage got trickier. Unlike earlier attempts with single-acid compounds, sodium diacetate held stability and handled transport better in humid or hot conditions. By the 1960s, bread makers and cheese processors grabbed onto it, drawing from research in Europe and the US that spotlighted its success curbing mold and bacteria while keeping taste in check.
Product Overview
Sodium diacetate lands on shelves as a white crystalline powder or granule, easy to handle and lightweight. It comes bulk or packed tightly for industrial kitchens. Most folks know it from that tangy aroma in sour cream and salt-and-vinegar chips, though it plays a bigger role behind the scenes as a preservative and seasoning in processed foods. For industry, it works as a buffer, keeping acidity in line without overpowering the flavors or textures. The ingredient shows up in dozens of everyday food items—bakery goods, snacks, and cured meats—while also finding space in non-food areas like animal feed and certain fungicides.
Physical & Chemical Properties
In physical form, sodium diacetate weighs in as a fine, free-flowing crystalline material. It melts at around 95°C. Water pulls it in fast—a trait that speeds up mixing in dough and seasoning. The pH sits in a mildly acidic to neutral range when dissolved, somewhere near 4.5-5.0 in a 10% solution. Chemically, it’s a blend in equal molar ratio between sodium acetate and acetic acid. The compound resists caking better than pure acetic acid, and clumping in storage rarely turns into a headache during actual use. The taste offers a sharp, slightly salty tang, which helps control off-notes in food without masking other flavors.
Technical Specifications & Labeling
Technical grades and food grades separate on purity and trace contaminants. Food-grade sodium diacetate runs upwards of 99% purity, with tight controls on heavy metals and unwanted residues. Some production lots follow specifications like FCC or E262, a code handed down by food regulators in Europe. Labels typically call it sodium diacetate, E262(ii), or sometimes acetic acid sodium salt. Ingredient lists in snacks, bakery mixes, or cheese-flavored powders almost always declare it by name so regulatory agencies and consumers know exactly what goes in. Transporting it as a solid rather than a liquid makes handling safer for workers and shippers.
Preparation Method
Synthesizing sodium diacetate outpaces the older method of simply mixing powders. Industrial plants react glacial acetic acid with sodium carbonate or sodium bicarbonate under strict temperature control, often in stainless steel reactors. Once the bubbling calms, they evaporate water away. What’s left are pure, dense crystals. Screening removes dust and irregular clumps, guaranteeing a consistent product for big-scale baking or snack plants. Companies invest in closed systems to prevent workers from breathing in sharply acidic vapors or coming into contact with wet acetic acid spills.
Chemical Reactions & Modifications
Sodium diacetate reacts gently in most settings—another reason food processors use it. It keeps to itself until it encounters water. In solution, the product breaks down, releasing acetic acid's characteristic sourness and antimicrobial traits. Labs tinker with the sodium-to-acid ratio, sometimes nudging it off the one-to-one line, to change shelf life or acid strength depending on the food or process. Most real-world changes, though, focus not on direct chemical modification but on co-blending with other preservatives, salts, or acidulants. This tweaking can boost effectiveness against mold in high-humidity storage or in recipes with tough microbes.
Synonyms & Product Names
The chemical world loves aliases, and sodium diacetate proves the point. In regulations and textbooks, it pops up as E262(ii), sodium hydrogen diacetate, or just acetic acid, sodium salt. Snack food companies sometimes call it sour salt or dry vinegar for marketing, although legally, those names need clarification on ingredient labels. Internationally, suppliers market it using these terms, but government bodies insist on clear naming, especially where food safety codes demand it.
Safety & Operational Standards
Worker safety means paying attention to dust and fine particles, since inhaling powdered acids can irritate noses and throats. Protective masks and gloves turn standard in plants where the product moves by the ton. Regulatory agencies, from the US FDA to the European Food Safety Authority, put sodium diacetate through rigorous food safety checks. They cap usage rates to keep both flavor and consumer health in balance, with limits set far below thresholds that might cause harm. Cleaning protocols in factories help remove residues from surfaces, since spills left uncleaned attract moisture and lead to hardening or minor corrosion.
Application Area
Global snack makers reach for sodium diacetate to deliver that signature salt-and-vinegar punch, but the chemical’s reach travels well beyond potato chips. Meat plants use it to curb bacteria in hot dogs, sausages, and deli meats—it helps keep color stable and delays spoilage. Bakeries use it to hold down mold and yeast growth, stretching shelf life for buns, breads, and tortillas. Animal feed manufacturers include it for stability and mild anti-microbial benefits, especially where storing large stockpiles in warm climates. Outside the food world, some pesticide and fungicide blends rely on its acidity to drive off molds in crops or storage bins.
Research & Development
Academic and industry labs take a constant interest in sodium diacetate, investigating new ways to boost preservation in challenging product formulations like gluten-free baked goods or all-natural deli meats. Scientists examine how it interacts with other acidulants, probing whether careful pairing might slow bacteria faster or fight off otherwise resilient molds. Trials for biodegradable packaging sometimes explore sodium diacetate coatings to keep food fresh inside compostable wraps, since antimicrobial effects might cut spoilage waste. Some research digs into flavor release, looking for better delivery in foods with lower sodium content, which matters as consumers push for less salt.
Toxicity Research
Most current data paints sodium diacetate as safe at commonly used levels. Toxicologists and food safety experts run animal tests, digesting both high and low doses, watching out for possible side effects. So far, results back up regulatory approvals, listing mild stomach upset or irritation only at quantities far above human dietary exposure. Studies continue in chronic, multi-year exposures, especially tracking any allergic responses or subtle changes in gut flora. Regulators follow up on emerging research, so long-term safety monitoring stays up to date as the ingredient reaches more processed foods worldwide.
Future Prospects
Changing diets and evolving export rules keep sodium diacetate an active field of study. As the market leans toward “clean label” products, researchers experiment with fermentation-driven forms of the salt, which might match up with organic or minimally processed claims. New food startups look for ways to blend traditional preservation power with plant-based or alternative protein products, using sodium diacetate to solve shelf life issues raised by fewer classic preservatives. Green chemistry labs tweak production methods to cut waste and energy use during synthesis, making it more sustainable and perhaps reducing cost for lower-income markets. The ingredient’s steady hand in food safety guarantees attention from government agencies and industry watchdogs, building pressure for more transparent labeling and ever-stricter purity checks.
What is sodium diacetate used for?
Walking Down the Grocery Aisle
You pick up a bag of potato chips and read through the label. Sodium diacetate pops up in the ingredients list. It’s not some mysterious lab compound created just for food factories—it is a combination of sodium acetate and acetic acid. This isn’t just used to sound scientific. People expect their food to taste good and last on the shelf. Sodium diacetate helps with both.
Flavor Punch and Shelf-Life Boost
Nobody wants stale crackers or chips that lose their kick after a week. Sodium diacetate brings a tart, salty note that you recognize in salt and vinegar chips. Its sharp flavor isn’t from magic—it’s from the acetic acid, the same thing that gives vinegar its tang. Bakers and snack makers reach for this ingredient not just for flavor, but to keep unwanted mold in check. By making the environment less inviting for fungi, sodium diacetate helps foods stay edible longer.
Keeping Safety in Mind
Food safety can’t be ignored. Some organisms want nothing more than to spoil stored foods. Sodium diacetate creates challenges for them. The research says acetic acid shifts the pH balance, and many spoilage microbes prefer a higher pH. Low pH keeps bacteria in check. Multiple studies confirm its protective role, and the FDA recognizes sodium diacetate as safe for foods in specific amounts.
Real World Uses—Beyond the Snack Bag
Pick up a packet of tortillas or a loaf of bread off the shelf. Manufacturers add sodium diacetate to protect against mold growth, especially where packaging may trap in moisture. You’ll find it in processed meats like sausages and cured ham. It works alongside other preservatives, helping keep Listeria and Salmonella at bay in those cold cases.
Not just food—some farmers use sodium diacetate in animal feed, where it helps stave off spoilage in high-moisture silage. It pops up in spices, salad dressings, and even in home-canned vegetables. Having cooked for large groups, I know how quickly bread and cheese can go off. Seeing sodium diacetate on a label often assures me that what I buy is less likely to turn moldy a day after I open it.
The Discussion Around Its Use
People rightfully worry about what goes into their food. While sodium diacetate sounds chemical, it’s found naturally in vinegar. Eating too much isn’t wise, just like anything else. Regulators have set limits and companies must follow strict guidelines. The industry also keeps searching for ways to cut artificial preservatives, but sodium diacetate sticks around because it’s reliable and has an established safety record.
Looking Ahead: Innovation and Choice
Consumers look for clear labels and more natural ingredients. Companies now research combinations of sodium diacetate with rosemary extract or cultured sugar, aiming to reduce the total amount used. Some small bakeries rely on shorter shelf life to skip preservatives, selling foods meant to be enjoyed the same day. Meanwhile, large producers depend on sodium diacetate to meet global demand for safe, tasty products.
Sodium diacetate lives at the intersection of food safety, shelf life, and taste. Whether stocking your pantry for emergencies or preparing lunchboxes for a week, this ingredient plays a key role behind the scenes, keeping everyday foods safer and fresher.
Is sodium diacetate safe to consume?
What Is Sodium Diacetate?
Sodium diacetate pops up on labels of foods like chips, bread, and seasonings. I’ve noticed it in snack aisles, especially on salt and vinegar chips, salad dressings, and even some tortillas. Used mostly as a preservative and acidulant, it stops bacteria and mold from ruining food and gives that signature tang you taste in some processed snacks. It’s made by mixing sodium acetate and acetic acid — both related to common household vinegar.
What Science Says About Eating Sodium Diacetate
Plenty of us have questions when we spot chemical-sounding names on ingredient lists. I’m no exception. Diving into research and food safety guidance, I see that sodium diacetate has been studied for decades. The US Food and Drug Administration labeled it as “Generally Recognized as Safe” (GRAS). That means experts have reviewed the evidence and haven’t found problems when it’s used in normal amounts. International health bodies, like the European Food Safety Authority, reach the same conclusion.
Studies haven’t turned up harmful effects at levels used in foods. Rats fed large quantities didn’t develop health risks, either. Humans would never get close to those doses by eating packaged food. Regular vinegar contains acetic acid, which makes up half of sodium diacetate. Most people eat foods with vinegar all the time — pickles, salad dressing, ketchup. No strong evidence links sodium diacetate to cancer, organ damage, or allergic reactions.
Why We Still Worry About Preservatives
Despite reassurances from food safety groups, some people feel uneasy about preservatives in general. Oversized ingredient lists make food feel less natural. I share that concern, especially with kids or people with health issues eating a lot of processed foods. It’s easy to blame preservatives for any stomachache or headache after junk food binges, and sometimes, the real culprit is the salt or fat content, not sodium diacetate.
Chronic disease often ties back to too much sodium, sugar, and unhealthy fats. Many products using sodium diacetate already push the limit on salt. Swapping convenience foods for fruits, vegetables, or homemade meals makes it easy to keep additives in check. That’s a lesson I learned after seeing a rise in my blood pressure before age forty.
How to Make Safer Choices at the Store
Learning which ingredients show up in your regular groceries helps with smarter choices. Reading ingredient lists takes extra time, but I see benefits almost immediately. I don’t stop buying everything that lists sodium diacetate, but I ask whether the food stays in my pantry for months or if I’ll eat it quickly. Breads and tortillas are fresher from a local bakery, where the preservative is rarely used. Chips and processed cheese taste just as good in moderation and won’t tip your dietary balance if you’re eating them as a treat instead of a staple.
Curiosity about food labels pays off. If you stick to mostly whole, recognizable foods, your intake of all types of synthetic preservatives stays low. Real safety comes from a balanced diet — something every doctor and nutritionist I’ve talked to stands behind.
What Regulators and Companies Can Do Better
Bigger print on ingredient labels and more public information would go a long way. I’ve seen companies responding to demand by making “no preservative” versions. More clear, honest communication from food producers encourages trust and helps everyone make informed choices. Researchers can keep an eye on preservatives, updating safety guidance when new evidence appears. Our job as consumers is to stay informed without panicking over every mystery ingredient.
What are the benefits of using sodium diacetate in food?
Making Food Last Longer Without Fuss
Growing up in a busy household, the kitchen always stayed full of smells, half-eaten meals, and leftovers waiting for another day. Nobody wants food going bad before everyone gets a taste. Sodium diacetate helps fight off mold and bacteria in products like baked goods, salsa, and snacks, so that half-eaten bag of potato chips still tastes fresh after days in the cabinet. The trick is pretty simple: this compound works to lower pH, making an environment most spoilage bugs can’t stand.
Some people worry whenever they see a chemical-sounding name in the ingredients. Yet, the science backs up sodium diacetate’s safety. The U.S. Food and Drug Administration gives it the green light as a food additive. It’s made from sodium acetate and acetic acid, the same acid found in vinegar, just blended in a way that handles food preservation and flavoring with a much lighter touch. It breaks down in the body just as vinegar does, and top food safety experts around the globe accept its use.
A Cleaner Food Label and Proven Results
Food companies face a lot of pressure to keep things simple on the label. Sodium diacetate can step in for harsher chemical preservatives and manage the job with a smaller amount. That makes it easier for people to recognize what’s in their snack or loaf of bread. Fewer grams of it are needed compared to older alternatives, which avoids overwhelming the recipe or leading to a fake, “chemical” taste.
Pretzel and chip producers, for example, lean on sodium diacetate to help snacks hold up through humid summers and cross-country shipments. No one wants stale or soggy snacks, and shelf life can mean the difference between profit and waste. Studies show sodium diacetate slows yeast and mold growth in baked goods. For chips or tortilla shells, it helps them survive more than a few weeks past their fry date.
Flavor Without Overpowering
Acidic foods call for just the right snap of tang. Sodium diacetate hits that note for salt and vinegar chips, spicy peanuts, even French bread. Too much straight vinegar turns food rubbery and harsh. Blended with salt, sodium diacetate brings out tartness evenly, so products taste the same every time.
Restaurants and home cooks take shortcuts too. I’ve mixed it into popcorn seasoning mixes before long road trips—it stands up to car heat and humidity better than liquid vinegar. Bakers, too, use it to sharpen flavors in artisanal pan loaves or bagels.
What About Health and Allergies?
Plenty of folks live with dietary restrictions or food allergies. Sodium diacetate doesn’t contain gluten, milk, soy, or any major allergens. Food safety groups, including the World Health Organization, recognize it as safe when used as directed. For people with hypertension, it’s worth noting it does add a little sodium, though the amount used is usually too low to matter much for an average serving.
Researchers check these additives for side effects, but no serious health concerns turn up in the literature. It’s not known to trigger digestive issues or headaches in healthy people either. So for schools, hospitals, or families with young kids, it does its job quietly and safely.
How to Use It Better
Food manufacturers have room to use sodium diacetate to cut down on waste, especially for products shipped long distances or stored in hot climates. They can pair it with other natural preservatives—like rosemary extract or ascorbic acid—to keep foods fresher even longer without pushing people to worry about ingredient lists. Snack brands can also look at lower sodium recipes, balancing taste with health when using any additive. Industry leaders can support more transparency and easy-to-read packaging, letting shoppers decide for themselves how much is too much.
Keeping food safe for longer, improving flavor, and doing it without complicated ingredients—that’s why sodium diacetate sticks around. Food waste gets cut, flavors stay bold, and families get a break from tossing groceries too early.
Does sodium diacetate have any side effects?
What People Are Eating
Sodium diacetate shows up in plenty of foods people eat every day. Breads, chips, processed meats—once you start reading labels, its name is easy to spot. It keeps products from spoiling and offers a sharp, tangy flavor. For the food industry, it’s a convenient way to boost shelf life and taste, two things shoppers expect. Most folks probably never realize it’s in their snack bag or sandwich bread.
Health and Safety
Questions about safety pop up for any chemical found in everyday foods. Research points out that sodium diacetate breaks down into sodium and acetic acid, things already in many diets. Regulatory agencies like the FDA and European Food Safety Authority have studied its safety at levels used in foods. Both agencies allow it based on current evidence, and the Acceptable Daily Intake (ADI) covers hundreds of times more than most people would get in a day.
Still, being “generally recognized as safe” does not mean zero concern. The big thing that stands out is the sodium. Sodium diacetate adds to total daily sodium intake, which is often higher than recommended for most adults and kids. High sodium intake links closely with high blood pressure and higher risk of heart disease and stroke. It sneaks into the diet through packaged foods, making it tough to keep sodium in check.
Digestive Issues and Sensitivities
I have friends who try to avoid processed foods after running into digestive trouble. Complaints like a sour stomach, mild nausea, or feeling bloated—while rare—come up after eating foods high in acid or preservatives. Scientific papers do not blame sodium diacetate directly for these symptoms, but some people appear more sensitive to components like acetic acid. Anyone with a sensitive stomach or on a restricted sodium diet should pay extra attention to ingredient lists. For others, downsides tend to show up only after eating far more than a healthy serving.
What About Allergies?
Sodium diacetate is not a common allergen. No major reports tie it to allergies in the same way milk, nuts, or wheat can cause problems. That said, some individuals react badly to chemical additives, so it always makes sense to try new foods in moderation—especially if someone suspects sensitivity to food acids or preservatives. Trusting your body’s signals helps more than taking anyone’s word for it, mine included. Symptoms like itching, rashes, or trouble breathing need a doctor’s opinion right away, even if this chemical isn’t usually the cause.
Taking Small Steps
Shelf-stable food has its place—nobody wants moldy bread in days. But sodium diacetate raises important reminders about balance. Fresh food, home cooking, and keeping processed foods as treats all help bring down daily sodium and exposure to preservatives. Food companies respond to folks demanding less sodium, offering options with lower levels or removing certain additives. Reading packages and making swaps—whole fruit over a sealed snack; a bagel from the bakery over packaged bread—gives control back to shoppers.
The Long View
No chemical in the pantry sits in isolation. All those small choices add up. With sodium diacetate, the everyday risks stay low when diets favor fresh foods, and the body works best when overloaded sodium stays off the table. Being picky about labels and reducing processed foods works better for health than stressing over a single ingredient.
How should sodium diacetate be stored?
Why Storage Matters More Than Expected
A lot of people overlook how easy it is for small missteps in storing food-grade additives to snowball into bigger headaches. Take sodium diacetate, for example. It’s a widely used food preservative. Bakers, snack producers, and even home cooks rely on it to keep products tasting fresh and safe. The thing is, all that trust can crumble if sodium diacetate gets stored in the wrong conditions.
Common Mistakes and What Can Go Wrong
Humidity acts like kryptonite for sodium diacetate. If someone leaves a bag open or keeps it in a damp place, clumping and caking show up almost overnight. Pretty soon, what looked like a free-flowing powder turns into stubborn lumps that don’t blend properly in a dough or spice mix. I’ve seen facilities lose a whole shipment just because a pallet got too close to a leaky loading dock window. Moisture sneaks into even the tiniest cracks in packaging.
Air is another silent culprit. It doesn’t just encourage moisture to sneak in — it also carries unwelcome odors. Sodium diacetate likes to pick up smells, whether it’s from spices, cleaning products, or other flavorings stored nearby. A batch with a faint whiff of bleach or garlic ruins more than just a day’s production. There are cases where food recalls traced right back to shared shelving that muddied the product’s aroma.
Practical Steps Everyone Can Follow
Keep sodium diacetate in tightly sealed containers. Plain and simple, a quality seal is the first and strongest defense against both moisture and stray smells. Companies with robust food safety certifications usually use airtight drums or thick plastic bags, and they double-check for rips before signing off on storage. At home, a screw-top jar or even a sturdy food-grade plastic tub will do the trick.
Look for a cool, dry spot out of direct sunlight. Too much heat shortens shelf life and even encourages clumping. Nobody likes to waste money replacing half a container because it sat near a heater or sunlit window. Storage places like clean pantries, shelving away from kitchen sinks, and lockers with strong climate control hold up for the long haul.
There’s also the issue of cross-contamination. Many folks in smaller kitchens stash everything on one shelf. That just leads to trouble down the line. Sodium diacetate should have its own space—far from strong spices, oils, detergents, or anything with a strong fragrance. If powders get jumbled or containers bump against each other, flavors cross over fast. A little organizational effort goes a long way.
Food Safety, Employee Training, and Labeling
Staff training supports safe storage. Giving employees clear instructions about opening, resealing, and handling packaging matters just as much as facility upgrades. Teams that understand why certain rules exist react faster when spills or accidents happen. Some of the best-run kitchens I’ve seen post laminated reminders at storage spots, reinforcing simple rules like “keep lids tight” and “wipe down after every use.”
Labeling every container with the date received and batch information makes all the difference. It keeps inventory rotation simple. The “first in, first out” approach reduces waste, and it helps trace any quality issues back to their source. Plus, it instills a culture of accountability from top to bottom.
Solutions That Make a Difference
Food companies can invest in better packaging — vacuum-sealed bags, desiccant packets, and thicker walls for bulk containers lower the risk of product loss or contamination. At home, folks can take cues from industry best practices by repacking sodium diacetate into smaller air-tight jars after opening bulk bags, protecting what remains for future use.
Anyone that works with sodium diacetate stands to benefit from a little care and planning. By paying attention to environment, packaging, and process, waste drops and safety climbs. That keeps food tasting just the way it should — fresh and safe — every single day.
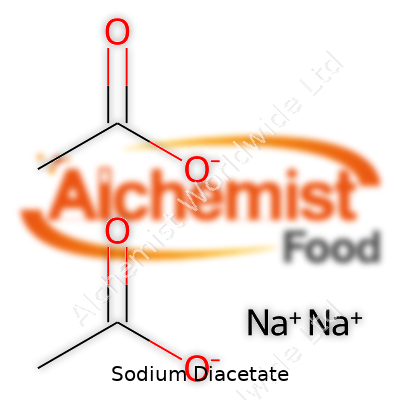
| Names | |
| Preferred IUPAC name | Sodium acetate ethanolate |
| Other names |
Sodium hydrogen diacetate Sodium acid acetate E262(ii) |
| Pronunciation | /ˌsəʊdiəm daɪˈæsɪteɪt/ |
| Preferred IUPAC name | Sodium acetate ethanolate |
| Other names |
Sodium hydrogen diacetate Sodium acid acetate Diacetic acid, sodium salt |
| Pronunciation | /ˌsoʊdiəm daɪˈæsəteɪt/ |
| Identifiers | |
| CAS Number | 126-96-5 |
| 3D model (JSmol) | `[Na+].CC(=O)[O-]` |
| Beilstein Reference | 1731777 |
| ChEBI | CHEBI:62957 |
| ChEMBL | CHEMBL1351 |
| ChemSpider | 56396 |
| DrugBank | DB11124 |
| ECHA InfoCard | 100.028.426 |
| EC Number | 208-807-6 |
| Gmelin Reference | 6767 |
| KEGG | C14448 |
| MeSH | D013482 |
| PubChem CID | 23665763 |
| RTECS number | AJ4300010 |
| UNII | 56Z9Y97BNA |
| UN number | UN2341 |
| CAS Number | 126-96-5 |
| 3D model (JSmol) | `[Na+].CC(=O)[O-]` |
| Beilstein Reference | 1908730 |
| ChEBI | CHEBI:62957 |
| ChEMBL | CHEMBL1201731 |
| ChemSpider | 157359 |
| DrugBank | DB11110 |
| ECHA InfoCard | 100.029.727 |
| EC Number | 262-013-2 |
| Gmelin Reference | 15356 |
| KEGG | C18565 |
| MeSH | D017372 |
| PubChem CID | 23665763 |
| RTECS number | AJ4300010 |
| UNII | UE28H7R94E |
| UN number | UNK1759 |
| Properties | |
| Chemical formula | NaC₂H₃O₂·CH₃COOH |
| Molar mass | 142.09 g/mol |
| Appearance | White crystalline powder |
| Odor | Acetic acid odor |
| Density | 1.528 g/cm³ |
| Solubility in water | Soluble |
| log P | “-4.3” |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa 4.75 |
| Basicity (pKb) | pKb: 9.15 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.420 |
| Viscosity | Powder |
| Dipole moment | 0 D |
| Chemical formula | NaH(C2H3O2)2 |
| Molar mass | 142.09 g/mol |
| Appearance | White crystalline powder |
| Odor | acetic acid odor |
| Density | 1.528 g/cm³ |
| Solubility in water | Soluble |
| log P | -4.3 |
| Acidity (pKa) | 4.75 |
| Basicity (pKb) | 4.76 |
| Dipole moment | 2.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 216 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -706.12 kJ/mol |
| Std molar entropy (S⦵298) | 198.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -722.8 kJ/mol |
| Pharmacology | |
| ATC code | A01AB58 |
| ATC code | A01AB58 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Autoignition temperature | 220 °C |
| Lethal dose or concentration | LD50 Oral Rat 4,930 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral Rat 4,398 mg/kg |
| NIOSH | RN 126-96-5 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium Diacetate: 15 mg/m³ (total dust), 5 mg/m³ (respirable fraction) |
| REL (Recommended) | 200 mg/kg |
| Main hazards | May irritate eyes, skin, and respiratory tract. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry, and well-ventilated place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Use with adequate ventilation. Do not ingest or inhale. |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 0, Special: - |
| Autoignition temperature | 570°C |
| Lethal dose or concentration | LD50 Oral Rat 4,390 mg/kg |
| LD50 (median dose) | LD50 (median dose): 4,390 mg/kg (rat, oral) |
| NIOSH | Not Listed |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium Diacetate: 15 mg/m³ (total dust), 5 mg/m³ (respirable fraction) |
| REL (Recommended) | 10000.0 mg/kg |
| Related compounds | |
| Related compounds |
Acetic acid Sodium acetate Potassium acetate Calcium acetate |
| Related compounds |
Sodium acetate Acetic acid Potassium acetate Calcium acetate Magnesium acetate |

