Sodium Citrate: From Root Discovery to Modern Importance
Historical Development
Sodium citrate’s story starts in the early 19th century when researchers began isolating citric acid from lemon juice. Eventually, chemists found sodium salts helped stabilize citric acid, giving rise to sodium citrate. By the late 1800s, sodium citrate entered the food world, quickly taking on roles in cheese production, beverage preservation, and laboratory work. Hospitals grabbed onto it for blood storage, noticing its ability to stop blood from clotting, which changed how blood was preserved for transfusions during war and peacetime medicine. Over generations, sodium citrate moved through diverse sectors, cementing a reputation for versatility and safety. My own university lab work always included those crisp, white sodium citrate crystals, an ever-present staple for experiments. Its story tracks ongoing curiosity, invention, and adaptation — not some stagnant chemical, but one with a place on dinner plates, in IV bags, and on lab benches around the world.
Product Overview
Sodium citrate comes up a lot whether you’re in the grocery aisle or mixing reagents in a lab. It’s a salt made from citric acid and sodium bicarbonate or sodium carbonate. In foods, it acts as a flavor enhancer and preservative — think processed cheese, sports drinks, and sodas. The pharmaceutical world knows it as an anticoagulant and a buffer for medications where stability and predictable pH matter. Industrial makers rely on sodium citrate for detergents, textile dyeing, and cleaning agents. Every time a company bottles a fizzy lemon drink or rolls out slices of American cheese, sodium citrate has played a behind-the-scenes role — it stands as a quiet workhorse making mass food production and modern medicine possible.
Physical and Chemical Properties
Sodium citrate shows up as a white crystalline powder that's highly soluble in water and gives off a mildly salty, slightly tart taste. In the right light, the crystals scatter, sometimes glimmering if you get close. Chemically, the compound holds three sodium ions paired with one citrate ion, offering stability even as pH shifts. Its pH lands on the basic side, usually between 7 and 9 for a 5% solution. In kitchens it keeps cheese smooth by binding calcium and adjusting acidity, which everyday cooks and manufacturers both count on to get creamy sauces and shelf-stable snacks. A shelf of sodium citrate, even at home, helps pull off spherification tricks for modernist recipes — its practical chemistry translates directly to hands-on results.
Technical Specifications and Labeling
Guidelines dictate tight technical standards for food and pharmaceutical sodium citrate. Production batches get tested for purity, moisture content, pH level, and heavy metals. Most purity specs demand over 99% content, with limits set on things like arsenic and lead, to keep products safe for consumption and therapeutic use. Labels spell out the chemical name, batch number, manufacturing date, and storage recommendations. Knowing what’s in the package gives both food companies and hospitals confidence in processes — traceable, reliable, and trusted for regulatory compliance. My years working with food scientists taught me scrutiny matters: quality control in manufacturing begins and ends with accurate labeling, because small mistakes ripple through supply chains into real-world risks.
Preparation Method
Manufacturers typically form sodium citrate by neutralizing citric acid, usually extracted from citrus fruits or produced from fermenting sugar with specific mold strains, with a sodium base. This reaction, carried out in stainless steel tanks, produces a solution from which the sodium citrate can be crystallized and dried. The process makes use of temperature control and agitation to ensure fine, uniform crystals. Purification steps remove byproducts, leaving a product pure enough for foods, beverages, and medicines. I remember the hum of evaporation tanks and scent of lemon during factory tours — an industrial process that retains a trace of its citrus origins, blending modern engineering with a chemical that began as fruit.
Chemical Reactions and Modifications
Sodium citrate sits at a crossroads of reactions. In the stomach, it converts back to citric acid, which interacts with stomach acid, producing carbon dioxide — sometimes leading to a gentle fizz. This is why sodium citrate pops up as an ingredient in antacid tablets and effervescent medicine powders. It bonds with calcium and magnesium, sequestering them in hard water and keeping soaps effective. Scientists tweak the ratios of sodium ions and citrate ions to get mono-, di-, or tri-sodium forms, each with different pH and solubility profiles. Product developers treat sodium citrate as a toolkit for pH adjustment, ion binding, and flavor control, a substance that bridges food chemistry with biotech innovation. I’ve seen engineering students test sodium citrate’s chelating powers firsthand, amazed at how much one compound can tame unpredictable systems.
Synonyms and Product Names
On packaging and ingredient labels, sodium citrate may show up under several names: trisodium citrate, E331, or sodium salt of citric acid. Drugstores call it anticoagulant sodium citrate. The dairy world leans on “sodium citrate buffer.” International coding systems, like E numbers in Europe, peg it as E331, with variants like E331(i), E331(ii), and E331(iii) showing the exact sodium-to-citrate ratio. In labs, you want to check the form — mono, di, or tri — before using, since properties shift with each. I’ve juggled bottles of E331 in culinary classes and lab benches alike, learning to treat ingredient labels as more than marketing — they reveal hidden chemistry.
Safety and Operational Standards
Safety assessments from authorities like the FDA and EFSA recognize sodium citrate as a low-risk substance at approved levels for food and drugs. Proper storage involves sealing containers tightly, stashing them in dry, cool places to avoid clumping and degradation. Industrial settings stick to dust-prevention rules and manage spill cleanup with plenty of water. Food workers and chemists alike trust sodium citrate because data backs up its safety, provided good manufacturing practices follow legal standards. Safety data sheets outline low toxicity and recommend basic precautions, such as avoiding eye contact and inhalation of powder. In my early jobs prepping reagents, handling sodium citrate felt routine, though I always respected the rules — even the gentlest chemicals deserve steady attention to safety.
Application Area
The list of uses runs long: food, medicine, cleaning, metallurgy, and textiles. Cheese manufacturers use sodium citrate to keep cheese sauces silky, prevent fats from separating, and maintain a pleasing bite in processed cheeses. Beverage makers adjust flavors and extend shelf life with its buffering and preservative powers. Hospitals keep sodium citrate on hand to stabilize blood, making storage and transfusions safer. Cleaning product makers rely on its ability to soften water, preventing residue buildup in soaps and detergents. Research labs experiment with sodium citrate for DNA extraction, protein precipitation, and cell culture. Even the brewing and winemaking industries find room for it, controlling acidity and preventing unwanted reactions. Every one of these settings trusts sodium citrate to deliver on reliability without complicating recipes, medicines, or processes.
Research and Development
Researchers continue to study sodium citrate’s abilities far past simple food science. Pharmaceutical labs work on novel drug delivery systems using sodium citrate’s buffering abilities. Chemists explore greener production methods, from bio-based citric acid extraction to energy-efficient crystallization, answering the call for sustainability. Biomedical scientists delve into sodium citrate’s anticoagulant pathways, unlocking clues for safer, more effective therapies. Culinary technologists push its boundaries for vegan cheese melts, low-sodium diets, or stable beverages in challenging environments. My own work with food technologists showed how sodium citrate innovations ripple outward quickly, affecting everything from packaged foods to hospital care. Developing better forms and smarter applications means healthier, more efficient products for consumers and patients alike.
Toxicity Research
Investigators have poured over sodium citrate’s safety profile through animal studies, clinical trials, and monitoring of consumer products. At proper doses, the compound shows minimal toxicity and no evidence of carcinogenic effects or reproductive risks. In rare cases, overdose may upset electrolyte balance, mostly in patients with kidney or heart conditions. Toxicologists keep an eye on new data as use expands, but so far, evidence points to a high safety margin for humans. Regulatory limits exist for a reason — always a safeguard against overexposure. I’ve watched debates play out at food safety conferences and in journal clubs, with consensus holding steady: risk remains remarkably low when standards hold up.
Future Prospects
Sodium citrate stands poised for greater adoption as trends push toward safer preservatives, cleaner-label foods, better-tasting pharmaceuticals, and more sustainable manufacturing. Research teams are developing smarter, less energy-intensive methods for producing sodium citrate, such as enzymatic processes fed by renewable feedstocks. The food industry aims for products with lower sodium and more natural ingredients, and sodium citrate’s mild flavor lets it fit right in. Medicine looks to it for precise control in blood therapies and slow-release drugs. As chemists and engineers refine how it's made and used, sodium citrate’s promise grows — practical, adaptable, and friendly to both industry and consumers. Throughout my career, I’ve seen how a familiar chemical serves as a springboard for progress, a sign that simple compounds still offer room for big ideas in science and technology.
What is sodium citrate used for?
From Kitchens to Hospitals
Sodium citrate, the white granular powder you might spot in ingredient lists, has a way of popping up in places you wouldn’t expect. In my kitchen adventures, it first showed up while trying to make homemade cheese sauce for mac and cheese. It turns out, sodium citrate makes cheese melt smoother, stopping it from turning stringy or greasy. Professional chefs champion it because it pulls off that creamy texture without the need for extra fats, and it can save a sauce from turning into a clumpy mess.
The Food Scientist’s Secret Tool
Dig a bit deeper, and food scientists have long embraced sodium citrate for its role in acidity control. In sodas and lemonades, it helps balance flavor, keeping things tart without being sharp. It rounds out sour notes and lets other flavors shine through. In cured and processed meats, it works with preservatives to check the growth of bacteria, giving products a longer shelf life. This blend of food safety and taste makes it a staple across the food industry.
Health and Medicine
My memories of hospitals aren’t fun, but sodium citrate plays an unseen role there too. Emergency rooms rely on it to help with blood clot prevention. Blood taken for testing or transfusion gets treated with sodium citrate to stop it from clotting in the tubes, making sure results come back accurate and that transfusions go safely. Doctors prescribe it to patients with kidney stones to help alkalize urine and cut the risk of painful stone formation. Sodium citrate can ease burning in the stomach by neutralizing extra acid without the risks tied to stronger medications.
Support for Athletes
If you have friends into endurance sports, they might have stories about sodium citrate as part of their hydration routines. Runners and cyclists know it as a buffering agent—helping keep their muscles working longer by limiting the natural acid build-up that saps strength. It doesn’t taste like much, so it works well in drinks without overpowering them. Research points to some real benefits, believing it can give athletes just enough of an edge to handle longer distances, though the boost isn’t dramatic for everyone.
Industry and Everyday Products
Beyond food and medicine, sodium citrate lands in laundry detergents and cleaning products. That surprised me—most detergents need a little help to work better in hard water, and sodium citrate binds with minerals to make water ‘softer.’ That means less soap goes further, leading to savings and less environmental impact through fewer chemicals entering waterways.
Looking at Safety and Alternatives
You’ll find sodium citrate on ingredient labels in foods and over-the-counter medicines, and scientists have agreed that it’s safe in normal quantities. Reports link it to very few allergic reactions, and the amounts used in foods are quite small. For people with kidney problems, doctors watch intake closely since anything that shifts the body’s acid-base balance can have an effect.
Science keeps probing cleaner, greener ways to handle food preservation and manufacturing. Sodium citrate has a spot in the story, both for helping food taste better and making daily life a bit safer. For now, its wide range of uses shows how one humble ingredient shapes everything from taste to health to cleaning up the laundry pile.
Is sodium citrate safe to consume?
What Sodium Citrate Is and Why It Ends Up in Food
Take a look at the back of almost any processed food, and you’re likely to see sodium citrate hanging around on that ingredient list. You’ll spot it in sodas, powdered drinks, gelatin desserts, cheese spreads, and sports drinks. This ingredient crops up so often because it keeps product textures and colors looking right, and it brings a tangy, pleasant flavor. I’ve seen it used to keep melty cheese smooth for nachos, and the science is pretty straightforward: sodium citrate works like an acidity regulator.
Sodium Citrate and Health: Real-World Experience
I’ve learned to be a label reader, picking my way through ingredients because of a family history of heart disease. Looking into sodium citrate, I wondered if it should be a red flag. According to the Food and Drug Administration (FDA), sodium citrate counts as “Generally Recognized as Safe” for use in food. This doesn’t surprise me, since most people eat tiny amounts compared to the heaps of sodium that come from salt. The Centers for Disease Control and Prevention (CDC) says the bigger problem comes from eating too much sodium in general, not this specific ingredient.
Still, for people watching their sodium for blood pressure or kidney reasons, every source counts. Sodium citrate brings sodium to the table, just in a form that doesn’t taste as salty. If you, like me, want to keep your ticker strong, paying attention to your daily sodium from both salt and additives can make sense.
Are There Dangers Lurking With Sodium Citrate?
Everywhere you look online, people stress over food additives. There’s no shortage of headlines warning about things you can’t pronounce. It’s easy to get spooked. But research keeps pointing to sodium citrate as safe for most healthy adults when eaten in typical amounts found in food. Problems show up in some medical situations. For instance, doctors use high doses during kidney treatments to lower acid in the blood, and those doses don’t reflect what we eat. If your doctor has told you to be careful about sodium, it’s smart to check food labels, since every little bit stacks up.
Those with rare genetic disorders affecting mineral balance or kidney issues should check in with a medical professional. In my own home, sticking to whole foods keeps things simple, and it helps me keep track of what goes in every meal. Avoiding over-processed snacks or instant meals means there’s less chance to stack up on the kind of sodium that sneaks in from ingredients like sodium citrate.
Possible Solutions and Smarter Choices
A push for clearer labeling could go a long way. If sodium content stood out more, folks could grab a can of soup or box of mac-and-cheese and quickly see how the numbers add up from salt plus additives. I shop with my eyes open, picking lower-sodium options and prepping meals with fresh produce. Getting in the habit of comparing labels and being a little stubborn in the grocery aisle pays off in the long run.
Food science marches forward, and ingredient safety needs careful, ongoing review. Just because an additive is allowed doesn’t mean there’s no need for questions. Experiences from families like mine show that putting your trust in a wide variety of foods, sticking closer to nature, and being mindful of what’s actually on the label makes mealtimes safer and tastier without giving up convenience.
What are the side effects of sodium citrate?
What Is Sodium Citrate?
People see sodium citrate on drink labels, bags of popcorn, even in blood collection tubes at hospitals. Manufacturers add it to foods for flavor and as a preservative. Hospitals stock it for certain kidney and blood treatments. It helps adjust acidity in all sorts of products. On paper, sodium citrate seems harmless. Everyone agrees its reputation stays pretty clean, but it pays to look beyond the chemistry.
Minor Side Effects That Pop Up
Anyone who’s ever taken a glass of sodium citrate-laced oral solution for a stomach upset or kidney stone prevention knows it goes down sour and salty. It can make people feel nauseated, sometimes even bring on vomiting. Stomach cramps, gas, or mild diarrhea aren't uncommon after downing a dose. These effects don’t sound alarming, but anyone who’s dealt with heartburn or upset stomach after medicine knows it’s more than a minor inconvenience.
Individuals with sensitive stomachs or those just not used to higher salt in their system sometimes see a spike in indigestion. Some report burping or a strange metallic taste lingering in their mouth. Folks sometimes ignore these signs, but they signal the body working overtime to adjust to what it just received.
When Things Get More Serious
Most healthy folks won’t see major trouble from a standard amount in food, but those with kidney problems, heart issues, or electrolyte imbalances need to pay closer attention. Sodium citrate’s job in the body revolves around controlling pH and electrolyte levels. For someone with poor kidney function, it may tip potassium levels into dangerous territory. Too much sodium—for anyone—can push blood pressure up. If you have heart failure or chronic kidney disease, this salt isn’t as innocent.
The FDA and health researchers warn about rare but dangerous complications: high blood sodium, irregular heartbeat, muscle twitching, even seizures. Allergic reactions show up in rare cases, including a skin rash or swelling. Anyone with shortness of breath, chest pain, or swelling after taking medicine containing sodium citrate needs care right away.
Everyday Foods and Hidden Salt
Supermarket snacks, sodas, and processed cheese often contain sodium citrate. Food manufacturers like its tangy taste and ability to keep things fresh. People watching their sodium intake need to look for sodium citrate as carefully as plain old salt on food labels. Individuals with hypertension and kids with kidney sensitivity are especially at risk of getting too much sodium without knowing it. There’s still too little public education about this “hidden salt.”
Solutions: Stay Safe and Informed
Doctors and pharmacists should let patients know about both common and serious side effects before giving sodium citrate for medical reasons. People with heart or kidney concerns can ask about alternatives, especially for long-term use. Reading every label might sound obsessive, but tracking sodium intake matters for many.
Regulators and public health campaigns could do more to keep consumers informed. Clear food labeling and education about less obvious sodium sources would protect more vulnerable groups. For those using sodium citrate for a diagnosed issue, sticking to prescribed amounts and getting regular checkups gives the best protection.
Like so many additives and supplements, sodium citrate works well in the right hands. Yet, those drawn-in by the promise of convenience or “alkalizing” health trends should remember every ingredient on the label has its place—and its risks.
Can sodium citrate be used in food preparation?
Understanding Sodium Citrate’s Role in Cooking
Sodium citrate shows up on ingredient lists for a reason. It brings a kind of usefulness to the kitchen that doesn’t get much attention. This white, almost tasteless powder takes on the job of an emulsifier and a buffer. Regular cooks and professional chefs turn to it for creamy sauces or smooth, stable foods without clumping or grittiness.
Transforming Cheese Sauces and Melts
Cheese lovers probably owe a lot to sodium citrate. Making a creamy cheese sauce from scratch—think fondue, queso, or homemade mac and cheese—can turn tricky. Cheese usually breaks, curdles, or turns stringy because the proteins and fats fight to stay separate. Toss in just a small pinch of sodium citrate and things change. Suddenly, hard cheeses like cheddar become silky and pourable. This trick doesn’t only make food look better; it saves money by letting cooks use what they have at home instead of tracking down ultra-processed cheese blends.
Health Perspective: Safety and Application
People sometimes feel cautious about food additives, but sodium citrate does not linger on the “suspicious” list. The Food and Drug Administration considers it safe, and nutritionists point to its long use in foods and even medicine. As an acid regulator, sodium citrate helps keep the pH of products steady, which can mean less spoilage and a lower risk of foodborne germs multiplying. Anyone watching their sodium intake should pay attention, though. Sodium from any source adds up throughout the day. Still, the small amounts used for home cooking don’t tip the scales as much as everyday salt does.
Sodium Citrate Offers Creative Freedom
Restaurants and food manufacturers reach for sodium citrate to solve technical kitchen problems, but home cooks can tap into that same science. Imagine a soup that refuses to separate, or ice cream with an impossibly smooth texture—cooks often owe those wins to sodium citrate. Curious home cooks can try it out: dissolve a bit into water or milk, add grated cheese, and watch a fragrant cheese sauce pull together without lumps. It doesn’t take a food scientist to get the hang of it.
Balancing Tradition and Innovation
Some people raise an eyebrow at the idea of using “lab” ingredients in home kitchens. Cooking and eating come with tradition and memory. But tools like baking powder, yeast, and vinegar started as technological leaps too. Sodium citrate doesn’t demand replacing old recipes. Instead, it offers a way to tweak old favorites and fix problems that might ruin a batch. A chef I know uses it for vegan cheese, pulling out creaminess from ingredients like cashews and carrots, which would otherwise separate. Cooking at home means having more say in what goes into your food, and sodium citrate puts another option on the table.
Accessible Solutions, Simple Tools
Sodium citrate isn’t locked away in factories or commercial kitchens. It’s sold online, in brewing supply shops, sometimes even on regular grocery shelves. You don’t need fancy equipment or advanced training. With a kitchen scale and a sense of curiosity, anyone can take their cheese sauce, soup, or custard to the next level. That’s something that empowers cooks—not just in flavor or texture, but also in reliability. Mistakes shrink, creativity grows.
Where can I buy sodium citrate?
Sodium Citrate’s Many Roles
In kitchens and classrooms, sodium citrate pops up more often than folks realize. Some know it as that ingredient that keeps cheese sauce smooth, others hear about it in chemistry class, but most households won’t have it sitting next to the salt. This little compound acts like a bridge between science and daily life.
Grocery and Specialty Food Stores
Years ago, if you wanted sodium citrate, you had to hunt around specialty baking sections or hope your local grocer carried it. These days, grocery stores with solid international or gourmet aisles sometimes stock it. Places catering to home cheesemakers or modernist cooks have started to notice the demand. Stores like Whole Foods, Sprouts, or well-stocked food co-ops sometimes carry small tubes or bags of sodium citrate near canning supplies or cheese kits.
Anyone who has spent time chasing ingredients for a new recipe understands the frustration. Employees usually recognize the need for help but might have little info beyond the spice aisle. Asking for help saves time, and a phone call before driving over avoids a wasted trip.
Online Retailers Step Up
Amazon, Walmart, and other giants carry sodium citrate year-round. Packages range from small jars to bulk sizes, great for folks testing new recipes or someone gearing up for a science fair. Kitchen specialty sites like Modernist Pantry or Bulk Apothecary list it with detailed descriptions, purity information, and reviews.
Reading reviews on a product page helps gauge customer trust. People mention batch consistency, delivery speeds, and if recipes worked out as described. If someone shares a story about lumpy mac-and-cheese or quick deliveries, it adds a layer of real-world insight to a purchase choice.
Pharmacies and Health Stores
Sodium citrate occasionally pops up in the pharmacy section. It gets used as a buffering agent in some antacids and medical solutions. Those looking for it for culinary or cleaning reasons won’t want medicated tablets, but it shows sodium citrate is considered safe and has trusted applications. Always double-check packaging for additives that might not work in food.
Local Chemistry Suppliers
For classroom projects or hobby science, local chemical supply stores sell sodium citrate in food and lab grades. High school teachers, science fair coaches, and kitchen tinkerers head here to ask for the type that fits their need. Many of these shops require ID or proof the chemical won’t get misused, which builds trust and protects everyone involved.
Know the Source and Quality
Not all sodium citrate is made the same. Food-grade packs stay pure and unblended. Supplies aimed at science have their own standards, sometimes carrying warnings against ingestion. Detailed labelling matters. Reading labels before clicking "buy" keeps recipes safe and trustworthy.
For anyone nervous about using something new in the kitchen or for their kids’ school projects, look up safety sheets or manufacturer guidelines. Trusted sellers display this info online so customers don’t miss a step. Mistakes in the kitchen can ruin a meal, but a bad batch or poorly labelled product causes more serious trouble.
Supporting Smart Choices
A little research makes a big difference. Choosing an established retailer with clear ingredient listings and a good track record keeps things simple and safe. Chatting with cooks and science teachers online or in local groups often brings up tips about sourcing or using sodium citrate.
Most people find that once they’ve located a reliable source, the hunt for sodium citrate turns into a chance to try new recipes and experiments at home. It’s a small ingredient with plenty of possibilities and getting it shouldn’t mean jumping through hoops.
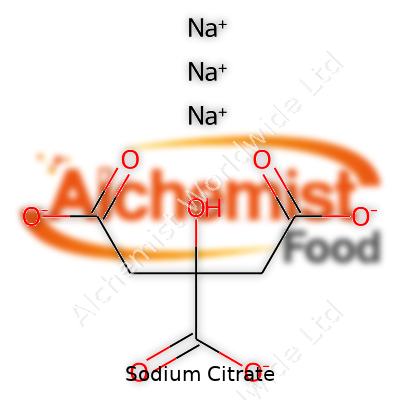
| Names | |
| Preferred IUPAC name | Trisodium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Trisodium citrate Citrosodine Sodium citrate tribasic E331 Citric acid sodium salt |
| Pronunciation | /ˈsəʊdiəm ˈsɪtreɪt/ |
| Preferred IUPAC name | trisodium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Trisodium citrate Citrosodine Sodium salt of citric acid E331 Citric acid trisodium salt |
| Pronunciation | /ˈsəʊ.di.əm ˈsɪ.treɪt/ |
| Identifiers | |
| CAS Number | 68-04-2 |
| Beilstein Reference | 3596802 |
| ChEBI | CHEBI:32139 |
| ChEMBL | CHEMBL1359 |
| ChemSpider | 51107 |
| DrugBank | DB03927 |
| ECHA InfoCard | 100.011.806 |
| EC Number | 200-675-3 |
| Gmelin Reference | 12658 |
| KEGG | C00793 |
| MeSH | D017355 |
| PubChem CID | 6224 |
| RTECS number | GE8300000 |
| UNII | DEB7XGC8BB |
| UN number | UN1759 |
| CAS Number | 68-04-2 |
| Beilstein Reference | 1713881 |
| ChEBI | CHEBI:32139 |
| ChEMBL | CHEMBL1357 |
| ChemSpider | 5366 |
| DrugBank | DB03914 |
| ECHA InfoCard | 100.007.340 |
| EC Number | 200-675-3 |
| Gmelin Reference | 130044 |
| KEGG | C00793 |
| MeSH | D013019 |
| PubChem CID | 67121 |
| RTECS number | GE8300000 |
| UNII | 1Q73Q2JULR |
| UN number | UN1759 |
| Properties | |
| Chemical formula | Na3C6H5O7 |
| Molar mass | 258.06 g/mol |
| Appearance | White or almost white, crystalline powder or granules |
| Odor | Odorless |
| Density | 1.7 g/cm³ |
| Solubility in water | Freely soluble in water |
| log P | -6.2 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa 3.13 (1st), 4.76 (2nd), 6.40 (3rd) |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | -59.0e-6 cm³/mol |
| Refractive index (nD) | 1.49 |
| Viscosity | 30cP (25°C) |
| Dipole moment | 0 D |
| Chemical formula | Na₃C₆H₅O₇ |
| Molar mass | 258.06 g/mol |
| Appearance | White or almost white, crystalline powder or granules |
| Odor | Odorless |
| Density | 1.7 g/cm³ |
| Solubility in water | Freely soluble |
| log P | -3.1 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa1 = 3.13, pKa2 = 4.76, pKa3 = 6.40 |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | -67.0e-6 cm³/mol |
| Refractive index (nD) | 1.48 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 216.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1568 kJ/mol |
| Std molar entropy (S⦵298) | 427.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1567 kJ/mol |
| Pharmacology | |
| ATC code | B05BB02 |
| ATC code | B05BB01 |
| Hazards | |
| Main hazards | May cause mild eye and skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. |
| Precautionary statements | Keep container tightly closed. Store in a dry place. Wash hands thoroughly after handling. Do not eat, drink or smoke when using this product. Wear protective gloves/eye protection/face protection. |
| NFPA 704 (fire diamond) | 0-0-0 |
| Lethal dose or concentration | LD50 (Oral, Rat): 8,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 4,200 mg/kg (oral, rat) |
| NIOSH | WS5600000 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 24 months |
| Main hazards | May cause mild skin and eye irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Hazard statements | Hazard statements: Not classified as hazardous according to GHS. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 (oral, rat): 8,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 8.4 g/kg (oral, rat) |
| NIOSH | WT2690000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium Citrate: Not established |
| REL (Recommended) | 340-1,700 mg |
| IDLH (Immediate danger) | Not listed. |
| Related compounds | |
| Related compounds |
Citric acid Monosodium citrate Disodium citrate Trisodium citrate Potassium citrate Calcium citrate |
| Related compounds |
Citric acid Monosodium citrate Disodium citrate Trisodium citrate Potassium citrate Calcium citrate |

