Sodium Carbonate: A Practical Look at an Unsung Hero of Industry
Historical Development
Sodium carbonate, often called soda ash or washing soda, traces its roots deep into the story of civilization. The Ancient Egyptians used natron, a natural mix containing sodium carbonate, in mummification and soap-making long before scientists described its chemistry. By the late 18th century, the Leblanc process gave European industry a reliable way to produce sodium carbonate from common salt, filling a growing demand for glass, soap, and textiles. The process spit out nasty byproducts like hydrogen chloride, which fouled air and water. As environmental worries grew, the Solvay process took the lead, driven by a Belgian industrialist in the 1860s. This method, still in use, relies on saltwater and limestone—much cleaner basics. The shift from burning seaweed and natural deposits to deliberate, large-scale manufacturing turned sodium carbonate into a global staple.
Product Overview
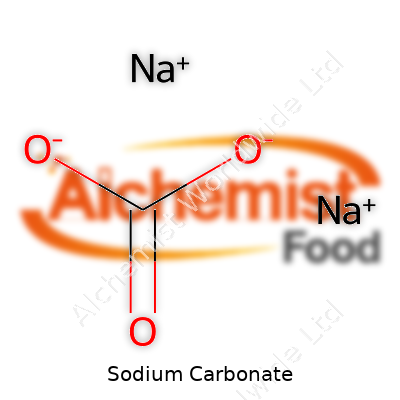
Sodium carbonate has become a warehouse workhorse, sitting on pallets in chemical supply rooms and household laundry aisles alike. It pushes pH levels up in pool waters, comes as a key ingredient in making glass and pulping wood, and scrubs away stains in detergents. The chemical formula, Na₂CO₃, may look simple, yet its uses run the spectrum. In water softeners, it’s an unsung problem-solver. In bakeries, it increases alkalinity so baked pretzels keep their chewy texture and signature brown crust. Its economic importance, from manufacturing to small businesses, can hardly be overestimated.
Physical & Chemical Properties
Sodium carbonate looks like a white, slightly grainy powder or tiny granules. It dissolves fast in water, turning the solution basic with a reassuringly soapy feel on the skin. The melting point hovers around 851°C, making it stable under most working conditions. When heated with other materials, sodium carbonate offers up carbonate ions that help melt glass, smelt ores, and neutralize acidic waste. Unlike caustic soda, it won’t burn the hand, but it can still dry skin due to its alkalinity. Chemists prize its ability to form hydrates, meaning it picks up water molecules—helpful for storage and dosing in industry.
Technical Specifications & Labeling
Manufacturers spell out content, purity level, water content, and any impurities right on the pack. Technical grade sodium carbonate comes with specs revealing losses on ignition, iron or heavy metal counts, and soluble silicates. Food or pharmaceutical grades have tighter restrictions, flagged by purity over 99%, and trace metal levels in parts per million. Packing in waterproof bags or drums, producers stamp hazard codes and Safe Handling advice so that users recognize the basic nature and avoid accidental mixing with acids. The UN number, 1823, signals sodium carbonate’s international shipping identity for safe transport.
Preparation Method
Producers today most often use the Solvay process. They combine brine, meaning saltwater, with ammonia and carbon dioxide. This cocktail forms ammonium bicarbonate and sodium bicarbonate through a string of reactions. Filtering, washing, then heating the sodium bicarbonate produces pure sodium carbonate plus water and CO₂. The leftover ammonia gets recycled, slashing costs and cutting down on waste. This method offers an efficient, environmentally gentler replacement for past processes. Natural soda deposits, like those at Green River, Wyoming, get mined and refined in huge quantities. Both methods continue winning users, depending on local resources and energy prices.
Chemical Reactions & Modifications
Sodium carbonate acts as a classic base, picking up protons and neutralizing acids briskly. Dumping sodium carbonate in an acid bath gives carbon dioxide bubbles, water, and a neutral salt. Mixing it with calcium chloride in hard water pulls out calcium ions, softening the water and forming calcium carbonate sludge. In the glass furnace, sodium carbonate drops the melting temperature of silica sand, cutting energy costs and enabling work at manageable temperatures. Chemists often modify sodium carbonate for special needs, attaching it to molecules in catalysts, or mixing it with potassium salts and phosphates for fertilizer applications. Its transformation into sodium percarbonate by reaction with hydrogen peroxide delivers an oxygen-rich, safer alternative to chlorine bleach for cleaning and sanitizing.
Synonyms & Product Names
This compound goes by a handful of aliases, depending on industry and country. Soda ash and washing soda may pop up in laundry aisles and glassmaker vendors. Chemists call it sodium carbonate, with variants like soda nitre or sal soda turning up in historical records. Some labels show “anhydrous sodium carbonate” or “sodium carbonate decahydrate,” marking the water content in each version. Whether it’s used for textiles, swimming pools, baking, or chemistry sets, sodium carbonate wears more than one hat. The diversity of names reflects its wide reach beyond simple chemistry.
Safety & Operational Standards
Practical safety starts with skin and eye protection. Dust from sodium carbonate doesn’t threaten the lungs like silica, but more than a light whiff can irritate the nose and throat. Prolonged handling dries out skin, so gloves and eye shields offer simple, cheap insurance. If mixed carelessly with acids, foaming reactions can lead to overflows and splashes. OSHA in the United States, along with sister agencies worldwide, sets limits for workplace dust and spells out spill control steps. Storage in dry, closed containers keeps lumps and clumps at bay. Ventilated workspaces and good housekeeping remain just as valuable as safety sheets or labels.
Application Area
Industry relies on sodium carbonate for more than a handful of jobs. Glassmakers stir in huge batches to climb past melting point and fuse the silica. Water treatment plants rely on it to soften hard water and keep pipes scale-free. Paper mills cook wood chips with sodium carbonate to release fibers, bleaching pulp along the way. Laundries benefit from its power to break stubborn stains, and municipal pools use it to bump up pH. In food, bakers sprinkle it on pretzels and noodles to create deep color and snap. Photographers use it in traditional developers, rooting chemistry in art. Cleaners and sanitizing tablets often swap out harsher alkalis for sodium carbonate, giving safer yet still powerful results. Even research laboratories keep it close by for pH control and titrations.
Research & Development
Research focuses on making sodium carbonate cleaner and more efficient, both in production and end-use. Teams at chemical plants work to shrink energy needs and capture emissions from Solvay lines, sometimes integrating waste heat into district heating. In cleaning products, some developers replace chlorine-based formulas with sodium carbonate blends, boosting stain removal without harmful byproducts. Materials scientists test its blend with polymers and minerals for 3D-printing feedstock and water filtration supports. Environmental researchers continue probing ways to recycle sodium carbonate from industrial byproducts or municipal wastewater sludge. Innovations arise from basic chemistry paired with a push to use less, reclaim more, and keep processes as clean as possible.
Toxicity Research
Toxicologists point out that, compared to more caustic alkalis, sodium carbonate carries a fairly low risk for acute or chronic poisoning. A little goes a long way in the body’s natural acid-neutralizing tricks, but swallowing concentrated powder or solution burns the throat, stomach, and gut. Animal research and case studies of workplace exposures mostly show temporary irritation, not lasting harm, unless massive doses are taken in. Regulators don’t class it as carcinogenic or mutagenic, but they insist on solid employee education and personal protection. Wastewater from sodium carbonate-heavy operations demands neutralization before release, since high pH harms aquatic life. Lab testing for bioaccumulation weaves together how it behaves in the body and the wider environment.
Future Prospects
Looking ahead, sodium carbonate faces both old and new demands. Battery factories—especially for lithium-ion batteries—lean on sodium carbonate in refining and recycling steps. As solar panel production ramps up, the need for pure glass and chemical-resistant processing chemicals follows, boosting demand. Water-scarce regions adopt sodium carbonate for reclaiming brackish sources, running through ion exchange and desalination loops. In sustainable packaging and eco-friendly cleaners, sodium carbonate wins points for its low toxicity and ready availability. Continued improvements in manufacturing have cut down costs, making recycled or bio-based routes more practical as technology matures. Technical conferences and patents burst with fine-tuning dosage forms, blends, and new uses—keeping this old staple both fresh and relevant for industry, science, and the public at large.
What is sodium carbonate used for?
Everyday Cleaning and Laundry
If you’ve worked with old-school detergents or tried making homemade cleaners, you’ve already encountered sodium carbonate—washing soda. It makes hard water easier to use for cleaning by raising pH and helping remove stubborn stains or grime. Before today’s high-tech detergents, many people added a scoop to their laundry. My grandmother used it, tossing it in with her whites, convinced it gave sheets and clothes a real clean feel. It turns out, the reason is simple science: sodium carbonate helps break down grease and keeps minerals from redepositing on fabrics. Even today, many natural cleaning recipes call for it, and eco-conscious folks buy it to avoid harsh chemicals.
Essential for Glassmaking and Industry
Factories rely on sodium carbonate more than people think. Glassmakers need it to shape everything from simple bottles to windowpanes. The compound reduces the melting point of sand, making glass production smoother and cheaper. Without it, costs would spike, and everyday glassware would become a luxury. This role isn’t some holdover from the past—global demand for glass packaging, electronics, and solar panels keeps sodium carbonate in steady use.
Paper manufacturers also lean on sodium carbonate. It helps balance pH and removes impurities during pulping. As someone who worked near a paper mill, I remember the subtle chemical scent from the factory on rainy days. Sodium carbonate was one of the unsung heroes that kept production efficient and the final paper clear and strong for everything from grocery bags to books.
Water Treatment That Makes Life Easier
City water systems depend on sodium carbonate to make tap water safer and easier on pipes. It raises the pH of acidic water, stopping old lead pipes and copper plumbing from corroding. In my town, service calls for rusty tap water tripled after storms until the city started adding sodium carbonate more regularly. It’s a quiet fix, but pipes last longer, and the water tastes better thanks to careful treatment.
Food Uses and Baking Science
Food-grade sodium carbonate doesn't sound appetizing, but bakers and cooks have good reasons to use it. Bagel-makers rely on it for the traditional chewy crust by soaking dough in a sodium carbonate solution before baking. It’s also hidden in recipes from Asian noodles to German pretzels and mooncakes. It gives baked goods that distinct flavor and texture, while still being safe when used at food-level concentrations approved by food safety authorities.
Sodium Carbonate in Laboratories and Schools
Anyone who’s studied chemistry remembers it for neutralizing acids or cleaning glassware. School science labs use it safely to teach real chemistry, from acid-base reactions to simple titrations. Sodium carbonate lets students see actual chemical changes without handling more dangerous substances.
Leading Towards Safer, Smarter Choices
Sodium carbonate holds a trusted spot in industries and homes because it’s effective and well-studied. Cleaner production methods have made it less polluting. As we keep looking for ways to make manufacturing and cleaning less harmful to the planet and our health, reliable compounds like sodium carbonate have a place in that future. We should keep demanding data on safety and sustainability for everyday chemicals, keep exploring new uses, and support transparent regulations that prioritize both industry needs and public well-being.
Is sodium carbonate safe for cleaning?
Unpacking an Everyday Ingredient
Sodium carbonate, often called washing soda, turns up a lot in old cleaning recipes and new eco-friendly solutions alike. Plenty of families had a bright blue box of it tucked behind the laundry detergent or under the sink. It cuts through grease, loosens dirt, and keeps whites looking crisp, but questions about its safety pop up from time to time. Knowing what sits in these boxes and how it interacts with homes, clothing, and skin deserves some attention.
A Closer Look at What Sodium Carbonate Does
The mineral comes from either mined trona deposits or a chemical process that’s been around for close to two centuries. Sodium carbonate lifts grime in a way that reminds me of helping my grandmother scrub kitchen floors. It softens water, making soap bubbles foam rather than flatten. It zaps tough stains and raises the pH in laundry, improving cleaning power without harsh fragrances. Many people, myself included, have used it for soaking stained tea mugs, wiping countertops, and even as a gentle oven cleaner when mixed with warm water.
Safety: Fact Over Fear
Here’s where experience and evidence come together. Sodium carbonate, in its dry form, feels gritty and dries the skin fast. Touching it once in a while during a big cleaning day never led to any burns or long-term irritation for me, but some folks with sensitive skin end up with redness or chapping. Handling large amounts or letting the powder sit on bare skin for a while may cause more discomfort. Inhaling the dust stings a bit, much like any dry cleaning powder. That’s not unique to sodium carbonate—regular flour or plaster dust can irritate lungs, too.
The U.S. Food and Drug Administration recognizes food-grade sodium carbonate as “Generally Recognized As Safe” (GRAS). At home, a pinch tossed into a dishwasher or laundry machine cleans deep and rinses out. The ingredient appears in many commercial dish soaps and detergents for this very reason. Animal studies point out that swallowing large spoonfuls would upset the stomach and raise some risk for burns, but these conditions do not match practical household use.
Making It Work Safely at Home
I found the biggest bumps in safety come from careless handling, not from the ingredient itself. Leaving the box open invites moisture and makes the powder lumpy. Letting kids or pets get curious with cleaning buckets risks upset stomachs or eye irritation. Simple fixes include labeling containers clearly, storing them out of reach of young ones, and using gloves if skin dries out easily. Most packaging tells you to rinse with water if the powder lands in the eye or on the skin for too long—common sense works well here.
Treat sodium carbonate the same as you would any powdered cleaner. Open windows on breezy days, wear gloves if your hands crack in winter, and steer clear of mixing it with strong household acids like vinegar, which won’t explode but won’t clean much either. In the end, with responsible use, sodium carbonate cleans deeply and rinses away safely, earning its spot beside baking soda and borax as a trusted, low-cost staple in the home. If anyone feels uneasy, there are always milder options, but the science and decades of family experience both back its reputation as safe when used wisely.
What is the difference between sodium carbonate and baking soda?
Everyday Encounters with Two Kitchen Staples
Many people keep both sodium carbonate and baking soda on their shelves, but they don’t always know what actually separates one from the other. Both powders might look nearly identical. Both have ‘soda’ in the name. That’s pretty much where their similarities end. Growing up helping bake holiday cookies, and later figuring out how to get stubborn stains out of old tiles, I learned just how different they are—and why those differences matter.
Chemistry Shows True Colors
Sodium carbonate, often called washing soda, and baking soda, known chemically as sodium bicarbonate, both come from minerals. Baking soda has the formula NaHCO3. Sodium carbonate goes by Na2CO3. The extra sodium atom in washing soda gives it a stronger alkaline punch. That shift in power makes a world of difference at home and in the lab.
Using Baking Soda in the Kitchen and Beyond
Baking soda steps into recipes as a leavening agent. Mix it with an acid, and it fizzes, creating bubbles that lighten dough or batter. Anything from chocolate cake to pancakes can trace their fluffiness to that reaction. This reaction releases carbon dioxide quickly, perfect for recipes that go right into the oven. The FDA lists baking soda as safe for food. It ends up in toothpaste, antacids, pool treatments, and a hundred other places.
Sodium Carbonate: Cleaning Powerhouse
Washing soda steps up where extra cleaning strength or higher alkalinity is needed. It leaps into action stripping grease from cookware, tackling bathtubs, or boosting laundry detergent’s effectiveness. The increased pH breaks down tough stains, even those that laugh at regular washing. Unlike baking soda, it can irritate skin or eyes, so gloves make sense for bigger chores. Eating washing soda can be dangerous, which is why it stays far from the kitchen cupboard.
Mistakes Can Have Real Consequences
A lot of folks hear “soda” and substitute one for the other—especially online, where incorrect home remedy advice travels fast. Swapping washing soda for baking soda in cookies means serving something truly unsafe to eat. Dropping baking soda into a dirty pot instead of washing soda might leave that pot greasy. The wrong choice doesn’t just mean ruined food; it can create a hazard.
Health, Safety, and Smarts
Baking soda stands out for its safety—sprinkle it, taste-test it, and even use it to soothe the odd itchy insect bite. Safety data shows that small, reasonable amounts don’t pose a risk. Sodium carbonate means business: use it to degrease or descale, but keep it labeled and out of reach. Safety organizations agree. Household cleaners with sodium carbonate require different storage and even disposal.
Clear Choices, Real Solutions
Every year, I see questions from friends and family about using the right “soda” for various tasks. Making the best choice starts with knowing how they act, not just relying on the label. Reading instructions and understanding labels goes a long way. Maybe it doesn’t sound exciting, but it prevents ruined dinners and dangerous accidents. Reliable sources—including the CDC, FDA, and health agencies—outline the importance of using chemicals safely according to their actual purpose.
Wrapping Up
Choosing between sodium carbonate and baking soda comes down to chemistry, safety, and intended use. From baking fluffy muffins to cleaning grime that just won’t budge, each compound proves its worth in different corners of home life. That difference matters a lot more than meets the eye.
Can sodium carbonate be used in pools?
What Pool Owners Face
If you’ve owned a pool, you know the challenge of keeping the water just right. Test strips in hand, you juggle different chemicals to keep the pool safe and comfortable. Many of us have looked at bags of sodium carbonate—commonly sold as soda ash—lined up next to chlorine and wondered whether it actually does the job claimed by pool stores.
How Soda Ash Impacts Pool Chemistry
Sodium carbonate serves a specific purpose for pools. Its primary job is raising the pH level. Low pH water doesn’t just irritate swimmers’ eyes and skin—it can also damage liners, corrode metal parts, and make chlorine less effective. Soda ash is strong stuff. Only a little bump in the bucket will shift pH upward without raising total alkalinity too much. Pool pros choose soda ash over baking soda when pool water tests below the recommended pH range (usually 7.2 to 7.6).
People sometimes confuse sodium carbonate and sodium bicarbonate, but these are not the same. Baking soda (sodium bicarbonate) raises total alkalinity more but doesn’t pack as much punch for pH. Soda ash fixes pH without throwing off other chemical balances, assuming the right amount gets used.
My Experience as a Pool Owner
Owning a pool in the Midwest taught me fast: skip on balancing pH, and you will pay for it. One spring, rainwater sent my pool’s pH plummeting. The chlorine didn’t work, and metal fixtures started to rust. A local store owner steered me to soda ash, warning me to start with a small dose. I witnessed clear water return within hours. My swimmers happily stopped complaining about itchy eyes. It worked so well because soda ash changes pH quickly, and one bag lasted the whole season.
Possible Pitfalls of Sodium Carbonate
Raising pH isn’t the only factor to watch. Many people dump soda ash into the skimmer all at once. I learned the hard way that clouds of undissolved soda ash sometimes land on the pool floor, crusting up if circulation isn’t strong. Always dissolve soda ash in a bucket of pool water before slowly adding it around the edge. That keeps the water from turning cloudy and spares you extra scrubbing. Overdoing soda ash can spike pH too high, creating chalky scale on tiles and reducing chlorine efficiency.
Better Results Through Good Practice
Soda ash helps pools when it’s used properly. Reliable commercial brands stick to clear labeling with specific dosing instructions. The best results come from testing the water before and after adding soda ash. Digital testers work, but even old-fashioned test strips—used regularly—catch small problems before they grow.
Swimming pools should stay fun, not become chemistry experiments gone wrong. I’ve seen community pools using soda ash to correct stubborn pH issues, and it works out as long as the crew stays on top of regular testing and slow mixing. I always recommend checking with a pool professional if you’re unsure about dosing for your pool’s volume. Simple steps, like pre-dissolving the product and keeping a journal of readings, can save a ton of hassle—and plenty of money on repairs.
Looking Ahead
Sodium carbonate isn’t some magic fix, but it’s a trusted tool for pool owners who care about water quality. Used wisely, it keeps swimming safe, comfortable, and affordable. Education goes a long way—especially for new pool owners facing a barrage of different pool products marketed for pH control. Take the time to learn what soda ash does and how much you need, and you’ll spend the summer enjoying the water, not fighting with it.
Where can I buy sodium carbonate?
Straight Talk About This Everyday Chemical
Sodium carbonate, known to some as washing soda or soda ash, isn’t the sort of thing most people keep in the kitchen cupboard these days. Back in the day, though, my grandmother used it to soften laundry and keep her whites bright. Times change, but this compound hasn’t lost its usefulness. If you’re reading this, you probably have a good reason for needing it — maybe for cleaning, boosting your laundry, pool care, glassmaking, or even a school chemistry project.
Where Regular Folks Can Find It
Step into most big supermarkets, and you’ll notice aisle after aisle of cleaners and detergents. Often, you’ll find sodium carbonate quietly sitting in the laundry section, marketed as washing soda. Arm & Hammer makes a familiar yellow box, and most household brands keep their formulas simple. I’ve bought it at big box stores like Walmart and Target, but I’ve also seen it in local hardware shops and some dollar stores. You won’t usually find a dedicated bag labeled “sodium carbonate” unless you’re going for a specialty store, but the ingredients list doesn’t lie.
Online Shopping: Fast and Direct
Sometimes, your local stores disappoint. That’s where the internet steps up. A quick search on websites like Amazon, eBay, or Walmart.com yields plenty of options. Specialty science supply stores online also list sodium carbonate in larger quantities for classroom or professional use. Always check the product description for purity levels. For home and general cleaning, basic washing soda is fine. If you’re prepping a solution for homemade soap or pool maintenance, you’ll want to make sure you’re getting genuine sodium carbonate, not baking soda, which is sodium bicarbonate — not quite the same thing.
What About Chemical Supply Stores?
If you live near a city, dedicated chemical supply shops are an option. These stores usually cater to labs, artists, and tinkerers. I’ve shopped at these places for school projects and found the staff helpful when it comes to explaining the quality and concentration of what’s on the shelf. Call ahead to check if they deal with walk-in customers. Some stores have strict rules due to misuse concerns.
Be Smart, Stay Safe
Sodium carbonate isn’t hazardous in the way stronger chemicals are, but it’s smart to treat it with respect. Wear gloves if you’ll be handling large quantities or making solutions, and keep it out of reach of kids and pets. Always read safety directions on the box or bag you buy. It’s also good to remember, some applications—especially food-related or lab work—demand higher purity levels. Ask questions if you’re unsure. Many manufacturers provide a safety data sheet, which explains exactly what you’re getting.
Supporting Facts and Solutions
Sodium carbonate’s wide use comes from its ability to remove grease, soften water, and act as a pH regulator. Those facts mean high global demand. According to the U.S. Geological Survey, more than 40 million metric tons get produced every year, mainly for glass and detergent. Environmental regulations influence where you buy it, since sales of bulk chemicals now come under more scrutiny. If you can’t find it locally, check regional pool supply stores — they often sell sodium carbonate under the name “pH Up.”
One tip from experience: always double-check product labels. Online sellers sometimes mix up sodium bicarbonate and sodium carbonate. For most home uses, a trustworthy laundry or pool brand works just fine. For science experiments requiring analytical-grade chemicals, stick with suppliers who’ve built a reputation with schools and labs.
So next time someone asks, the answer isn’t complicated. Stores stock it, websites sell it, pool and science shops carry it, and the country keeps hundreds of thousands of tons moving every year. Just know what you need, and read the box before you buy.
| Names | |
| Preferred IUPAC name | Sodium carbonate |
| Other names |
Soda Ash Washing Soda Soda Crystals |
| Pronunciation | /ˈsəʊdiəm ˈkɑːbəneɪt/ |
| Preferred IUPAC name | Sodium carbonate |
| Other names |
Soda Ash Washing Soda Sodium Salt of Carbonic Acid |
| Pronunciation | /ˈsəʊ.di.əm ˈkɑː.bə.neɪt/ |
| Identifiers | |
| CAS Number | 497-19-8 |
| Beilstein Reference | 3539616 |
| ChEBI | CHEBI:29377 |
| ChEMBL | CHEMBL1359 |
| ChemSpider | 72848 |
| DrugBank | DB11310 |
| ECHA InfoCard | 01-2119485498-19-XXXX |
| EC Number | 207-838-8 |
| Gmelin Reference | 822 |
| KEGG | C01353 |
| MeSH | D012948 |
| PubChem CID | 10340 |
| RTECS number | VZ4050000 |
| UNII | 45SY345L3G |
| UN number | UN3077 |
| CAS Number | 497-19-8 |
| Beilstein Reference | 358726 |
| ChEBI | CHEBI:32139 |
| ChEMBL | CHEMBL1357 |
| ChemSpider | 21111 |
| DrugBank | DB09162 |
| ECHA InfoCard | 100.011.769 |
| EC Number | 207-838-8 |
| Gmelin Reference | 232 |
| KEGG | C01442 |
| MeSH | D012964 |
| PubChem CID | 10340 |
| RTECS number | VZ4050000 |
| UNII | 45A3QJQ372 |
| UN number | UN3378 |
| Properties | |
| Chemical formula | Na2CO3 |
| Molar mass | 105.99 g/mol |
| Appearance | White crystalline powder or granular solid |
| Odor | Odorless |
| Density | 2.54 g/cm³ |
| Solubility in water | Moderately soluble |
| log P | -6.19 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10.33 |
| Basicity (pKb) | 3.67 |
| Magnetic susceptibility (χ) | +23.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.378 |
| Viscosity | 300 cP |
| Dipole moment | 0 D |
| Chemical formula | Na2CO3 |
| Molar mass | 105.99 g/mol |
| Appearance | White, odorless, crystalline powder or granules |
| Odor | Odorless |
| Density | 2.54 g/cm3 |
| Solubility in water | Moderately soluble |
| log P | -6.19 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10.33 |
| Basicity (pKb) | 3.67 |
| Magnetic susceptibility (χ) | \(+278 \times 10^{-6}\) |
| Refractive index (nD) | 1.377 |
| Viscosity | Low |
| Dipole moment | 0 |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 135.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1130.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1131.0 kJ/mol |
| Std molar entropy (S⦵298) | 197.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1131 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1131 kJ/mol |
| Pharmacology | |
| ATC code | V03AB37 |
| ATC code | V03AR01 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | Hazard statements: Causes serious eye irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | Sodium Carbonate: 2-0-1 |
| Lethal dose or concentration | LD50 (oral, rat): 2800 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 4090 mg/kg |
| NIOSH | SW8375000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium Carbonate: Not established |
| REL (Recommended) | 1 g/L |
| Main hazards | May cause eye irritation. May cause mild skin irritation. Dust may cause respiratory tract irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | Causes serious eye irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-0-1 |
| Lethal dose or concentration | LD50 Oral Rat 4090 mg/kg |
| LD50 (median dose) | 4090 mg/kg (rat, oral) |
| NIOSH | WJ3675000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.7 mg/m3 |
| Related compounds | |
| Related compounds |
Potassium carbonate Calcium carbonate Sodium bicarbonate Sodium hydroxide Sodium sulfate |
| Related compounds |
Potassium carbonate Ammonium carbonate Sodium bicarbonate Calcium carbonate Magnesium carbonate |

