Sodium Benzoate: Past, Present, and Future
Historical Development
Walking through the annals of food preservation, you don't miss sodium benzoate. Far back in the 19th century, chemists working on food safety stumbled upon it while searching for better ways to stop spoilage—especially for pickles and jams—before refrigeration lined every kitchen. As the world shifted toward industrialized diets, sodium benzoate emerged in the early 1900s as a practical safeguard. Early debates focused on its effect on health, sometimes fiery among scientists and policymakers alike; it took decades for regulations to catch up, weighing public benefit against warnings. Today, its legal status rides on precise dosing and reliable science.
Product Overview
Nobody grabs a can of soda to study food chemistry, yet sodium benzoate works quietly to keep it safe. The product shows up as a white, crystalline powder—easy to measure, nearly odorless, fitting snugly into the manufacturing process. Companies value it not for glamour, but for dependability: it holds its ground in acidic conditions, remains soluble in water, and gives a strong safety record for short-term use at approved concentrations. Markets range from food and beverage to pharmaceuticals and cosmetics; few additives move so freely across sector boundaries. Names like Benzoate of Soda, E211, or Sobenate appear on packaging, but it’s the same backbone at work.
Physical & Chemical Properties
Start with the basic facts: sodium benzoate carries the formula C7H5NaO2, along with a molecular weight of just over 144. Water welcomes it easily—about 62g will dissolve in a liter at room temperature—placing it on top of many alternatives that clump or cloud. It melts above 410°C, not a figure that matters in food processing but a reflection of its stability. In pure form, sodium benzoate won’t react wildly in dry air. It originates from benzoic acid—an aromatic compound with a long shelf life. Mixing sodium hydroxide with benzoic acid triggers a salt formation—that’s where sodium benzoate enters the picture, stable on its own yet reactive enough to halt mold or yeast inside an acidic soft drink.
Technical Specifications & Labeling
Regulators have kept a close eye on sodium benzoate’s technical details. For food-grade batches, the purity lands at or above 99%; most nations cap it at 0.1% in finished foods. labs test for heavy metals, water content, and pH to meet safety codes. On shelves, labeling laws force manufacturers to list “Sodium Benzoate” or its code “E211” to inform allergy-prone or cautious consumers. Some regions add requirements for storage, limiting extremes of moisture or temperature to avoid caking or breakdown. Labels extend to color and odor checks—freak results get flagged before products leave the plant.
Preparation Method
Sodium benzoate owes its commercial success to a straightforward method. In industrial facilities, manufacturers react benzoic acid with sodium hydroxide—usually under agitation, sometimes with heat for efficiency. The result drops out as sodium benzoate in water, from which it’s filtered, dried, and milled to the desired granulation. Recrystallization can fine-tune purity further, making the product suitable for sensitive pharmaceutical applications. In smaller labs, chemists may use glassware and heat plates, but the principle stays the same. This simple chain of reactions allows bulk production at low cost, helping keep processed foods affordable at the checkout.
Chemical Reactions & Modifications
The story of sodium benzoate doesn't end in its standard form. It participates in several side reactions that shape product safety discussions. In highly acidic environments where ascorbic acid appears (such as fruit drinks), sodium benzoate may convert to benzene—a volatile compound with carcinogenic reputation. Such findings pushed researchers to adjust recipes and storage instructions, ensuring that levels remain far below safety flags. Elsewhere, companies sometimes blend sodium benzoate with other preservatives—like potassium sorbate—to stretch antimicrobial protection across a range of pH levels and spoilage types. Efforts also go toward altered molecular forms, aiming for better solubility or compatibility with alternative packaging.
Synonyms & Product Names
Markets recognize sodium benzoate under a handful of names. E211 stands tall in food. Pharmacy shelves show “Sobenate,” “Benzoate of Soda,” and plain “Sodium Benzoate USP.” In chemical supply catalogs, the structure repeats: sodium salt of benzoic acid. Even industries beyond food—think fireworks, photographic processes—rely on the same chemical, calling it by its IUPAC or CAS-registry handle for clarity. Despite cosmetic differences, the chemistry stays consistent.
Safety & Operational Standards
Over the years, tough scrutiny followed sodium benzoate into every application. Regulators from the FDA, EFSA, and WHO set strict daily intake allowances—typically around 5 mg per kg body weight—based on comprehensive toxicological reviews. Facilities track every batch from synthesis to shipment, logging storage temperatures, contamination risks, and employee exposure. Workers handling raw powder keep dust at bay using gloves and masks; spills demand immediate cleanup thanks to moderate eye and mucous membrane irritation. Food inspectors test shelf products for purity, and some countries mandate export certifications before sodium benzoate leaves production sites. These steps reflect a hard-learned lesson: strict oversight underpins broad consumer trust.
Application Area
Walk down grocery aisles—sodium benzoate pops up in carbonated beverages, fruit juices, jams, pickles, soy sauce, and even toothpaste. Restaurateurs and food scientists add it for extended shelf life at low pH, lowering the odds that spoilage microbes ruin a batch. In medicine, it stabilizes liquid pharmaceuticals and cough syrups. Cosmetics companies count on it in shampoo and lotions to stave off mold and bacteria, especially in fashionable “clean” formulas with limited ingredient decks. Niche applications span fireworks, where it anchors certain sound effects, and industrial labs, where it acts as a rust inhibitor or corrosion control agent. So, this molecule bridges everyday living with heavy industry, shaping safety and consistency.
Research & Development
Plenty of effort goes into finding new avenues for sodium benzoate. Universities and industry-backed labs publish regularly on antimicrobial mechanisms, looking for finer control over resistance and spoilage in dairy or fruit processing. Current R&D targets combinations with plant-based extracts to lower total preservative content but maintain safety. Scientists also pursue improved detection methods to catch any benzene formation, relying on advances in chromatography and analytical standards. With the shift toward “clean label” foods, innovators keep chasing modified benzoate derivatives that break down faster or reduce salt intake. The pharmaceutical sector aims for longer-acting or better-absorbed versions to tackle metabolic or rare diseases. This research matters, because global food chains and, frankly, growing populations put pressure on every safety lever in processing.
Toxicity Research
Sodium benzoate’s safety record stands higher than many food additives, but it has never escaped skepticism. Robust studies show that, at legal doses, it leaves the body quickly—metabolized in the liver, cleared in urine, rarely causing harm in healthy individuals. Researchers tracked allergic reactions as low-risk, though asthmatics and those with urticaria sometimes report rashes or mild irritation. The benzene issue in drinks keeps regulators alert; public pressure after the 2006 soft drink incident changed how manufacturers test and design recipes. Animal studies digging into long-term effects haven’t shown major organ damage at typical exposure, yet watchdogs demand continued monitoring, not “once and done” conclusions. Real risks rise only at doses many times what appears in the food supply; problems like hyperactivity in children remain ambiguous. Science never promises zero risk, so ongoing research and public access to findings matter a great deal.
Future Prospects
Pressures stack up for every food and chemical preservative as consumers ask sharper questions and tech opens new doors. Sodium benzoate keeps its role for the simple reason that alternative preservatives rarely match its cost or performance. Industry trends lean toward lower doses, smarter mixtures, and periodic reassessment of cumulative exposure from processed foods. “Natural” preservation using fermentation byproducts or essential oils garners headlines, but old standbys like sodium benzoate stick around to fill safety gaps in mass-market products. Environmental questions—such as wastewater breakdown and microbe resistance—start to shape new studies and regulatory reviews. Continued investment in transparent testing and fine-scale monitoring should keep sodium benzoate relevant, even as food systems grow more complex and personalized. The most likely future sees it playing a reduced, but still vital, role in safeguarding food and everyday chemicals.
What is sodium benzoate used for?
Guarding Against Spoilage
A lot of food in the supermarket would spoil twice as fast if it weren’t for sodium benzoate. This simple white powder steps into processed foods, sodas, and salad dressings mainly to keep mold and bacteria from turning a product into waste. Before I cared what went into my pantry, I never paid much attention to food labels. My curiosity grew after seeing food recalls linked to spoilage; I looked into what actually keeps toppings and snacks safe for weeks or months after buying.
Manufacturers add sodium benzoate in small amounts, usually less than 0.1 percent by weight, to acidic foods. Soda, pickles, fruit juices, jams, energy drinks, and sauces often lean on this ingredient for its ability to restrain the growth of unwanted microbes. It works best at low pH, which is why you rarely see it added to bread or other alkaline foods. Instead, it’s all over soft drinks and tangy condiments.
Safety and Everyday Use
People worry about chemicals in food, and they have good reason. Companies must prove that food additives like sodium benzoate are safe, and organizations such as the FDA and EFSA review studies every few years. Most results show that the amounts used in food don’t cause harm, though high doses can irritate some people. Researchers found that mixing sodium benzoate and vitamin C can form benzene, a compound linked to cancer, under certain conditions. The levels found in sodas have dropped as recipes changed. Reading about this made me rethink how many sodas I grabbed from the gas station fridge in my twenties.
Medical researchers have also explored sodium benzoate’s effects on the brain. A few clinical studies have tested it as a possible treatment for conditions such as schizophrenia. Results haven’t pointed to any miraculous effects, but they show how much scientists still find new uses for chemicals once seen as basic food preservatives.
Household and Industrial Uses
Sodium benzoate pops up in plenty of products beyond lunchboxes. If you have a bottle of mouthwash at home or a tube of toothpaste, chances are it contains sodium benzoate. The same goes for some cosmetic items like face creams or moisturizers. It’s there for the same reason as in food—to keep bacteria from spreading. In the garage or garden shed, car coolant and antifreeze sometimes list sodium benzoate. It helps protect parts from corrosion.
Balancing Need and Awareness
Every year, food waste grows. Preservatives let food travel farther and last longer, giving people more options. The trick is using them wisely. Higher intake sometimes comes from diets heavy in processed foods, so people can lower exposure by cooking at home or reading ingredient lists. Laws set upper limits on how much companies can add, and keeping tabs on research keeps standards up to date.
Sometimes I think back to the days before widespread refrigeration or global shipping. Salt, vinegar, and careful canning did the job for years. Now, sodium benzoate offers a consistent and affordable way to prevent spoilage while pushing fewer resources into landfills. The real value lies in balancing safety, sustainability, and transparency, showing that trace ingredients can carry more weight than expected.
Is sodium benzoate safe to consume?
Diving Into the Details
Sodium benzoate is a name most folks rarely hear unless they’re reading tiny text on a can or bag at the grocery store. Food and drink companies use it because it keeps products shelf-stable, stops mold, and helps punch up those brightly colored soft drinks. The FDA has given sodium benzoate a thumbs-up as a food additive, so you’re likely to find it in carbonated sodas, fruit juices, salad dressings, and a whole lot of processed stuff in the pantry. Seeing it show up so often, I started digging deeper to see if it’s really alright to eat, whether you’re a parent packing lunches or someone who just likes a fizzy drink with lunch.
The Science and Real-World Choices
Many food preservatives have a tough job, dealing with microbes and extending shelf life without impacting taste too much. Research has spent decades looking at sodium benzoate’s effects. In small amounts, it leaves your body pretty quickly, filtered out by the liver and kidneys. When studied by groups like the Joint FAO/WHO Expert Committee on Food Additives, low daily intake levels (up to 5 mg per kg of body weight) didn’t show clear risks for most people. For reference, drinking an average soda doesn’t get you anywhere close to this limit.
Stories surface online about sodium benzoate causing hyperactivity in children. These came from a British study linking certain colorants and the preservative to more erratic behavior in kids with ADHD. What researchers found was something, but not a smoking gun—no guarantee that every can of orange soda brings restlessness. Some health experts do call for more caution when it comes to kids though, especially if your family struggles with behavior swings or ADHD.
Mixing with Other Ingredients
Safety can shift under certain conditions. Sodium benzoate mixes with ascorbic acid (vitamin C) in drinks can create benzene, a compound tied to cancer. The FDA and soft drink companies adjusted processes in the late 2000s after independent reports picked up on this issue. If drinks sit on a warehouse shelf in sunlight, benzene levels might climb a bit, but routine testing in recent years shows most products don't reach levels considered dangerous for daily human intake. Still, shoppers can look for alternatives—choosing beverages without artificial colors or preservatives gives extra peace of mind.
Making Informed Choices and Looking Forward
Walking the aisles of the supermarket, it’s tough to dodge every synthetic ingredient. Taking the time to read labels brings a measure of control. Keeping processed foods to a minimum, focusing on whole meals, gives families more say over what’s in their diets. Sodium benzoate doesn’t raise alarms for healthy adults in modest amounts, but staying mindful—especially if someone at home has allergies, chronic health issues, or sensitivities—helps to steer choices. Long-term studies still matter. Families, food advocates, and scientists all help keep companies honest, pushing for clear labeling and up-to-date research. We’ve come a long way from the days of secret recipes and guesswork. That’s real progress.
Small Steps, Better Habits
The path to healthier eating often feels complicated, but it can start pretty simple. Swapping out one or two processed snacks for fresh options each week, or picking seltzer instead of a third can of soda, will start making a difference. Living with fewer preservatives in your food isn’t about doom and gloom, it’s about making new long-term patterns stick. In my own kitchen, shaking up habits and learning about food chemistry built more curiosity than fear—and plenty more home-cooked meals on the table.
What foods contain sodium benzoate?
What Is Sodium Benzoate?
Sodium benzoate shows up as a synthetic preservative, added to stop mold and bacteria from growing in food and drinks. Chemically, it binds benzoic acid with sodium, making it easy to dissolve in water and spread through products. Food scientists have leaned on it for over a century, since it blends right in and keeps food on shelves far longer than fresh ingredients alone. I’ve stared at enough ingredient labels to know sodium benzoate crops up far more often than most realize.
Common Foods Featuring Sodium Benzoate
If you sift through your pantry, you might spot sodium benzoate in sugary soft drinks. Cola, energy drinks, fruit punches, and most flavored sodas regularly list it. Companies add it to acidic drinks because it works best under acidic conditions. Lemon-lime sodas almost always carry sodium benzoate for this reason.
Jams, jellies, and preserves use sodium benzoate to stave off mold and yeast, especially the cheaper brands lining the lower shelves. Pickled vegetables and relishes, riddled with vinegar, depend on it too. Brands pushing shelf life, like those squeezable ketchup and mustard bottles, pick sodium benzoate for its reliability.
Some condiments and dressings—think sweet and tangy barbecue sauce, bottled salad dressings, certain hot sauces—use sodium benzoate. Its flavorless nature lets it safeguard taste and texture. Even packaged fruit juices, especially those in cartons and plastic bottles, sometimes rely on it to prevent spoilage between bottling and drinking.
Certain dairy and dairy-alternative products, including flavored yogurts and non-dairy creamers, keep sodium benzoate on the roster, mostly in markets where refrigeration isn’t a given at every store. Sometimes, low-sugar snack foods and baked goods opt for sodium benzoate over other preservatives, to keep texture stable longer.
Why Sodium Benzoate Matters
People debate preservatives all the time. Some claim they’re a public health win, stopping food poisoning; others point to concerns about allergies, asthma, and possible links between sodium benzoate, artificial colorants, and hyperactivity in some children. Researchers from Southampton University flagged this combo back in 2007, sparking calls for more studies and better labeling. The U.S. Food and Drug Administration still recognizes sodium benzoate as safe at up to 0.1% of a product, yet concerns linger about combining it with ascorbic acid (vitamin C), since this can spark the release of small amounts of benzene, a known carcinogen.
I’ve found that most people don’t realize how often they eat sodium benzoate unless they start reading ingredient labels closely. Many processed foods—the ones easy to toss in a lunch bag for kids or grab from a gas station—contain it, especially if they’re liquid or come with a long best-by date.
Finding Healthier Alternatives
Some companies use potassium sorbate, calcium propionate, or natural preservatives such as vinegar or lemon juice instead. Brands that leave out sodium benzoate usually stamp “no preservatives” on packaging and turn to refrigeration, smaller containers, or pasteurization for food safety. Choosing products with fewer artificial additives sometimes means shorter shelf life or a higher price, and sometimes it means learning to enjoy the taste of things like naturally tart jam or pickles that skip chemical stabilizers.
Anyone aiming to lower sodium benzoate intake can check ingredient lists, especially on sodas, processed condiments, shelf-stable baked goods, and snack foods. Shopping the perimeter of a grocery store—where fresh produce, dairy, and meats sit—cuts down on exposure too. The conversation about preservatives like sodium benzoate isn’t going away. Anyone with health or allergy concerns benefits from learning which foods use them, and, if needed, shopping more carefully by reading every label.
Can sodium benzoate cause allergies or side effects?
What People Don’t Always Realize About Preservatives
Sodium benzoate pops up often in ingredient lists for soft drinks, pickles, and even some personal care items. Think of it as a convenient way companies keep products fresh and safe, blocking the growth of certain bacteria and fungi. But many people feel uneasy when they spot chemical names they can’t pronounce in their snacks. That hesitation makes sense. After all, what goes into our bodies—and our kids’—really matters.
Can It Actually Cause Allergies or Side Effects?
Some folks say they’ve had hives, swelling, or even asthmatic symptoms after eating foods with sodium benzoate, especially if they also have a history of allergies. The U.S. Food and Drug Administration and European Food Safety Authority both recognize it as safe in small amounts, up to 0.1% in most foods and drinks. Still, not everyone reacts the same way. I have a friend who avoids processed foods across the board because she once broke out in a rash after a soda binge. She can’t say with certainty it was sodium benzoate, but it’s the additive she blames.
Science backs up some of these concerns. Studies show sodium benzoate may rarely trigger mild allergic reactions like itching or swelling, especially in those already sensitive to food dyes or aspirin. People with asthma might notice more throat tightness or trouble breathing when exposed to the preservative. Cases of full-blown allergic reactions seem scarce, but the stories are real.
Long-term effects get people talking too. In the lab, large amounts of sodium benzoate have shown up as an irritant or raised questions about hyperactivity in children, especially when combined with certain artificial colors. That study from The Lancet in 2007 linking additives—including sodium benzoate—to more hyperactive behavior in some kids made plenty of parents look twice at juice box labels.
Better Safe Than Sorry
I always read the fine print, not because I expect disaster, but because I know how sensitive my nephew is to additives. Like many, my family learned the hard way: trial, error, and a run to the allergy clinic. Eliminating sodas and brightly colored snack foods from the house made a difference for him. For most people, sodium benzoate in typical portions does not cause side effects. But for the segment with allergies, asthma, or a family history of sensitivities, caution makes sense.
Transparency really helps here. Food companies should give easy-to-find info on what’s in their products and warn about any possible side effects, not just in tiny print buried on the back. Health professionals can talk to patients about food sensitivities, especially for families managing asthma and allergies. Researchers also need to keep digging—not just in animals, but tracking long-term health in people exposed to these ingredients daily.
Making Food Choices That Work for You
Everyone has a different level of tolerance. Some chow down on anything in the pantry and feel fine. Others pay dearly for just a sip of soda. If you’ve ever suspected a reaction, jot it down and take it to your doctor or an allergist. Keeping a food diary worked for my friend—and meant fewer ER visits. Parents swapping advice online or at PTA meetings probably know more firsthand than a label ever could.
Rumors and real stories about food additives won’t disappear. Even widely used preservatives like sodium benzoate turn out fine for most, but not all—and that’s enough reason for open discussion. People should get the facts and make choices that keep themselves and their families healthy.
What is the acceptable daily intake of sodium benzoate?
What You’re Putting in Your Body
Pick up a bottle of soda or a jar of pickles, scan the label, and there’s a good chance you’ll spot sodium benzoate. This compound shows up all over the place — soft drinks, salad dressings, jams, even medicine. The reason is simple: it stops mold and bacteria, so food lasts longer. But living in a world fueled by convenience, with packaged food at every turn, means we’re chewing through more food additives than our grandparents ever did. And that includes sodium benzoate.
The Science Behind the Number
The Joint FAO/WHO Expert Committee on Food Additives—folks who really dig into food safety—say you should aim for less than 5 milligrams per kilogram of body weight per day. That means a grown person weighing about 70 kilos (154 pounds) ought to stay under 350 milligrams a day. Most people don’t come close to this number just by eating. But toss in a few sodas, snack bars, maybe some breakfast cereal, and that number starts creeping up for heavy processed food fans.
Why Limits Matter
Sodium benzoate on its own doesn’t dig out too many health issues at usual levels. The trouble starts when it partners up with vitamin C (ascorbic acid), where it helps produce benzene, a substance linked to cancer. Even though drink makers keep an eye out and tweak recipes to prevent this, benzene popped up in some soft drinks in the mid-2000s, enough to spark recalls and some tough questions. The U.S. Food and Drug Administration started checking levels more seriously. Since then, most products keep benzene so low that it’s not much different from what’s found in tap water. Still, overusing sodium benzoate walks close to a line that relies on trust, safe manufacturing, and honest labeling.
Bursting the Bubble of “Safe” Processed Food
It doesn’t take a chemistry degree to see how easy it can be to overlook these little numbers. Between the ingredients you can pronounce and the ones you can’t, people reach for what tastes good, what stores well, what’s cheap. After a stretch of reading labels and cooking more at home, I realized how easily the sum of small doses from different foods can add up. Even if every company keeps levels below the legal limit, the slow build across all meals counts. Kids, with their love for bright drinks and sweet treats, risk running higher totals than adults—kids just weigh less, so each milligram counts more.
Better Choices for Everyone
So what can you actually do? Shifting toward fresh food lowers your daily additive intake. It helps to limit the amount of sodas, packaged snacks, and preserved dressings. Read ingredient lists regularly, not out of paranoia, but just to know what’s actually in your food. Pro-choice labeling helps, and pushing for stronger public education on food additives puts the power in people’s hands. Some groceries and cafes now highlight additive-free options, making those choices less of a hassle. With the knowledge about sodium benzoate’s acceptable daily intake, it feels more manageable to balance enjoyment and health. Watching numbers is no substitute for a whole diet shift, but understanding the basics keeps blame out of the supermarket aisles and brings responsibility back to the kitchen.
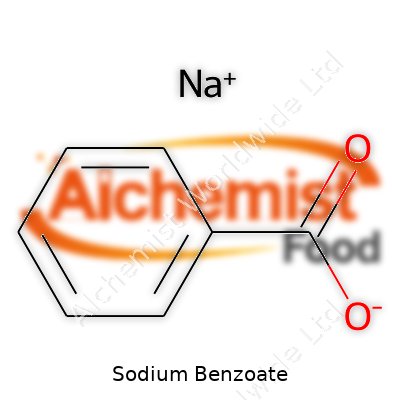
| Names | |
| Preferred IUPAC name | Sodium benzenecarboxylate |
| Other names |
Sodium salt of benzoic acid E211 Benzoate of soda |
| Pronunciation | /ˈsəʊdiəm ˈbɛnzəʊeɪt/ |
| Preferred IUPAC name | Sodium benzoate |
| Other names |
Benzoate of soda E211 Sodium salt of benzoic acid |
| Pronunciation | /ˌsəʊdiəm ˈbɛn.zə.weɪt/ |
| Identifiers | |
| CAS Number | 532-32-1 |
| 3D model (JSmol) | ``` C1=CC=C(C=C1)C(=O)[O-].[Na+] ``` |
| Beilstein Reference | Beilstein Reference: 412629 |
| ChEBI | CHEBI:32971 |
| ChEMBL | CHEMBL135 |
| ChemSpider | 7286 |
| DrugBank | DB02251 |
| ECHA InfoCard | 100.965. καλυπ |
| EC Number | 211-532-5 |
| Gmelin Reference | 613 |
| KEGG | C00120 |
| MeSH | D017366 |
| PubChem CID | 517055 |
| RTECS number | DH6650000 |
| UNII | UJ5458Y6BU |
| UN number | UN2819 |
| CompTox Dashboard (EPA) | DTXSID2020794 |
| CAS Number | 532-32-1 |
| Beilstein Reference | 1908734 |
| ChEBI | CHEBI:32971 |
| ChEMBL | CHEMBL1357 |
| ChemSpider | 6026 |
| DrugBank | DB09462 |
| ECHA InfoCard | 100.011.274 |
| EC Number | 211-581-5 |
| Gmelin Reference | 822 |
| KEGG | C00199 |
| MeSH | D017366 |
| PubChem CID | 517055 |
| RTECS number | DH6650000 |
| UNII | OY889U9N0H |
| UN number | UN3076 |
| Properties | |
| Chemical formula | C7H5NaO2 |
| Molar mass | 144.11 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.44 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.1 |
| Vapor pressure | Vapor pressure: Negligible |
| Acidity (pKa) | 11.5 |
| Basicity (pKb) | Sodium Benzoate pKb = 10.7 |
| Magnetic susceptibility (χ) | -23.0e-6 cm³/mol |
| Refractive index (nD) | 1.49 |
| Viscosity | Viscosity: "1.02 mPa·s (20 °C, 10% solution) |
| Dipole moment | 1.82 D |
| Chemical formula | C7H5NaO2 |
| Molar mass | 144.11 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.5 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.1 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa = 4.2 |
| Basicity (pKb) | 11.0 |
| Magnetic susceptibility (χ) | `-22.0 × 10⁻⁶ cm³/mol` |
| Refractive index (nD) | 1.49 |
| Dipole moment | 1.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 139.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -632.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -339.8 kJ/mol |
| Std molar entropy (S⦵298) | 155.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -587.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1620 kJ/mol |
| Pharmacology | |
| ATC code | A16HA04 |
| ATC code | A16HA04 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory irritation. |
| GHS labelling | GHS07: Exclamation mark |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "P264, P270, P273, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0-"- |
| Flash point | > 100 °C (212 °F) |
| Autoignition temperature | > 570°C (1,058°F) |
| Lethal dose or concentration | LD50 (oral, rat): 4,070 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 4,100 mg/kg |
| NIOSH | RN: 532-32-1 |
| PEL (Permissible) | 5 mg/m³ |
| REL (Recommended) | 600 mg/kg |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, Warning, Exclamation mark |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "May cause eye, skin, and respiratory irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Flash point | > 100°C |
| Autoignition temperature | > 570°C (1,058°F) |
| Lethal dose or concentration | LD50 oral rat 4,070 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Sodium Benzoate: "oral, rat: 4,070 mg/kg |
| NIOSH | RN367 Sodium benzoate |
| PEL (Permissible) | PEL: 5 mg/m³ |
| REL (Recommended) | 500 mg/kg |
| IDLH (Immediate danger) | Not listed. |
| Related compounds | |
| Related compounds |
Benzoic acid Potassium benzoate Calcium benzoate Sodium salicylate Sodium phenylacetate |
| Related compounds |
Benzoic acid Potassium benzoate Calcium benzoate |

