Sodium Ascorbate: Evolution, Qualities, and the Future of a Key Nutrient
Historical Development
Long before sodium ascorbate found its place on supplement shelves, the story of vitamin C shaped modern nutrition science. In the early twentieth century, sailors dealing with scurvy pushed the world to hunt for the missing link in their diet. Albert Szent-Györgyi isolated ascorbic acid from paprika in the 1930s, setting the stage for derivatives like sodium ascorbate. Moving past straight ascorbic acid, researchers started to tweak the molecule with minerals, aiming for compounds that wouldn’t upset stomachs or dent teeth. Sodium ascorbate, landing in the limelight, gave both chemists and food technologists a stable, water-soluble form of vitamin C that buffered the acidity most folks wanted to avoid. Its rise tracked with expanding food preservation, evolving fortification, and a growing focus on non-acidic vitamin C for infants, adults with digestive issues, and large-scale food production.
Product Overview
You will spot sodium ascorbate in more places than you expect—powder for food processing, tablets for multivitamins, premixes for health drinks. In lab settings, it comes as a white, crystalline powder, usually in heavy drums or bags. In the home, it may sit in a supplement bottle labeled for gentle vitamin C support. The food industry values sodium ascorbate for its ability to boost shelf life and maintain color in cured meats, juices, and dairy blends. The supplement market leans on its less acidic nature for folks prone to heartburn, sensitive teeth, or gastrointestinal sensitivity.
Physical & Chemical Properties
The physical tale is simple—sodium ascorbate flows as a dry, white to pale yellow crystalline powder with a slight salty flavor and a mild tang. Water loves it; you can dissolve grams in just a little liquid without much effort. Chemically, it’s known as the sodium salt of ascorbic acid, C6H7NaO6, with a molecular weight just over 198 g/mol. On the pH scale in solution, it stays around neutral, sometimes landing between 7 and 8—far gentler than straight ascorbic acid. It melts at a relatively low temperature, not above 220°C, and does not take humidity well. In air, it can oxidize, losing potency if left exposed, a key issue for storage.
Technical Specifications & Labeling
Regulators like the FDA and EFSA keep a close eye on how sodium ascorbate gets labeled. The powder must show purity, usually higher than 99%. Moisture levels top out at 0.25%, with nitrate, heavy metals, and oxalate limits set low to meet safety standards. Labels list sodium ascorbate as an antioxidant (E301 in Europe), sometimes calling out the origin of the ascorbic acid or the grade—USP, food, or pharmaceutical. Companies add lot numbers, expiration dates, and directions on storage conditions to slow oxidation. Detailed supplement labels mention milligrams per dose and, for multivitamins, daily value percentages.
Preparation Method
Making sodium ascorbate looks a lot like high school chemistry—just bigger and more refined. Manufacturers start by dissolving ascorbic acid in water, then introduce food-grade sodium bicarbonate (baking soda). The mix bubbles as carbon dioxide escapes, leaving sodium ascorbate behind. Once the reaction settles, operators filter, concentrate, and dry the mixture, yielding the familiar powder. The process demands clean handling, precise measurements, and careful control to avoid leftover acids or sodium ions that could throw off safety or flavor. Large producers use closed reactors to avoid airborne contamination, followed by vacuum drying to keep moisture at bay.
Chemical Reactions & Modifications
On a molecular level, sodium ascorbate serves up plenty of reactivity—it’s a strong reducing agent, generous with electrons. It cheers up antioxidant reactions in the human body, helping mop up free radicals. The chemical community relies on its reducing power for non-biological uses too: maintaining color in processed meat and fixing the color of fruit juices. Under acidic conditions, it can revert to ascorbic acid, but under neutral to alkaline conditions, it holds steady. Chemical modification has produced forms blended with other minerals or chelated to improve absorption or taste—a trend that pops up when companies look to differentiate products in crowded markets.
Synonyms & Product Names
On labels, sodium ascorbate doesn’t hide. It goes by names like ascorbic acid sodium salt, E301, and sodium L-ascorbate. Supplement brands describe it as “buffered vitamin C” or “non-acidic vitamin C.” Older pharmaceutical texts sometimes say "sodium erythorbate,” but that’s a separate chemical with a similar structure. Trademarks come and go, especially among supplement firms that blend it with calcium ascorbate or other mineral ascorbates for proprietary formulations. These names drive both regulatory compliance and marketing strategies.
Safety & Operational Standards
As a food additive and supplement, sodium ascorbate falls under strict manufacturing standards. HACCP, cGMP, and ISO 9001 certification all play a role in factories that make this powder. Operators work in dust-controlled rooms, wearing gear to avoid skin or eye contact, since fine powders can irritate mucous membranes. Down the line, regular lot testing screens for heavy metals, microbial contamination, or other impurities flagged by regulators. Workers keep their eyes on batch records and cleaning logs, since slips in process control can result in contaminated or degraded products. The compound’s safety profile reflects decades of use, but sodium content matters for those limiting salt—important for hospitals and dietitians.
Application Area
Sodium ascorbate’s reach stretches from the kitchen to the manufacturing plant. Food processors use it to slow spoilage in luncheon meats, canned fruits, and beverages. At home, health-conscious shoppers buy it as “gentle” vitamin C, often recommended for people who can’t tolerate the acidity of regular ascorbic acid. In clinical medicine, it supports parenteral nutrition and acts as an antioxidant in specialized IV therapies, though high-dose regimens spark debate on safety and benefit. Some wound dressings for burns and chronic ulcers contain sodium ascorbate for its healing properties. Pet food makers rely on its shelf-life extension. Even in water treatment, it neutralizes disinfectants before lab testing.
Research & Development
Universities and private labs keep sodium ascorbate near the front of antioxidant research. Animal and cell studies probe roles in immune function, iron absorption, and skin repair. Some clinical trials evaluate buffered vitamin C for gut health or athletic recovery. The supplement field investigates blends that pair sodium ascorbate with bioflavonoids or probiotics, aiming to improve absorption and tolerability. In manufacturing, new drying and encapsulation methods seek to protect sodium ascorbate from humidity—one of the top threats to shelf life and potency. Ongoing R&D examines how sodium ascorbate stacks up against other forms of vitamin C in terms of absorption, long-term outcomes, and potential drug interactions.
Toxicity Research
Given the staggering amount of vitamin C sold worldwide, toxicity remains rare but well-studied. Sodium ascorbate, like other forms of vitamin C, rarely causes harm unless taken in massive doses—often over 2,000 mg per day for long stretches. High intake can increase risk of kidney stones, particularly in those predisposed to oxalate buildup. Sodium content also matters—someone with high blood pressure or chronic kidney disease might need to track their sodium intake more closely. Lab studies involving rats and cell cultures suggest high concentrations of sodium ascorbate can produce oxidative metabolites that cause cell stress, but human data stays reassuring for typical doses. Ongoing post-market surveillance flags adverse reactions quickly, especially in populations with underlying health issues.
Future Prospects
The trajectory for sodium ascorbate keeps pointing up. More consumers lean toward supplements, and food manufacturers find ever more uses for shelf-stable antioxidants. Interest in intravenous and therapeutic forms has picked up, with researchers sifting through data sets to separate hype from real clinical benefit. Sustainability questions shape new sourcing and production technologies—companies want greener, less energy-intensive ways to produce not just sodium ascorbate, but all ascorbates. In the supplement aisles, formulas are shifting—blends with other forms of vitamin C, or pairings with minerals and botanicals, open up fresh territory. New delivery forms, like chewables, gummies, and dissolvable powders, hit the scene as customers look for more convenient options. Looking to the next decade, sodium ascorbate seems likely to keep expanding, rooted in a strong safety profile, broad utility, and persistent curiosity in both nutrition science and industry innovation.
What is Sodium Ascorbate and how does it differ from regular Vitamin C?
Looking Beyond the Usual Orange Tablet
Most folks know vitamin C as that orange-flavored chewable or something doctors recommend for colds. The labels usually mention ascorbic acid, but sometimes you spot another name: sodium ascorbate. At first glance, it looks like a minor chemical tweak, yet this difference affects how our bodies take in and use vitamin C.
Sodium Ascorbate Versus Ascorbic Acid: What Sets Them Apart
Ascorbic acid comes as a pure acid form of vitamin C. If you’ve ever taken a high dose and felt a little heartburn, that’s because of its acidic nature. In contrast, sodium ascorbate adds sodium into the mix. This small change shifts its pH, making it less acidic and gentler for people with sensitive guts or those prone to stomach problems from supplements.
Some nutritionists recommend sodium ascorbate for those with acid reflux or stomach irritation. I remember switching during a long work project loaded with takeout food and little sleep; the regular vitamin C tablets left me uncomfortable, and sodium ascorbate helped avoid those sharp stomach pains.
Absorption and Bioavailability
Research shows both ascorbic acid and sodium ascorbate help raise vitamin C levels in the blood. A study from the Journal of Nutrition found the body uses both forms about equally well in healthy adults. There’s no need to chase sodium ascorbate for the sake of “better absorption,” but if your stomach acts up, it deserves a look.
There’s a catch, though. Every gram of sodium ascorbate gives you a small dose of sodium. It’s not a huge amount, yet folks keeping close tabs on salt, like those with high blood pressure or kidney issues, might want to keep count. For most people, especially active folks who sweat, this sodium bump doesn’t matter much.
Practical Uses and Day-to-Day Choices
In the supplement aisle, sodium ascorbate pops up in non-acidic vitamin C powders and some “buffered” vitamin C packs. Some people dissolve it in water during a cold or stock it in their emergency cabinet. Parents often prefer sodium ascorbate for kids who balk at tart, sour tablets.
It finds a home in the food industry, too. Sodium ascorbate works as a preservative in processed meats to keep color looking fresh and reduce harmful compounds like nitrosamines.
Quality, Purity, and Choosing Wisely
Quality varies from brand to brand, so checking for third-party testing and clean labeling becomes more important than grabbing the cheapest bottle. Reputable supplements don’t mix in fillers or heavy metals. ConsumerLab and NSF conduct tough testing—those seals on a bottle mean a lot.
Wrapping Up The Real Differences
People who want a gentler option than basic ascorbic acid or those with a sensitive stomach might like sodium ascorbate. For most healthy adults, both work about the same. Pick what feels best—not what sounds most high-tech.
Real value comes from fruits and vegetables on your plate. No powder or pill can match a well-rounded diet. If supplements fill a gap, then knowing these small differences lets folks make a smarter, more comfortable choice.
What are the health benefits of taking Sodium Ascorbate?
Understanding Sodium Ascorbate
Vitamin C always seems to spark debate, especially during cold and flu season. Most folks reach for ascorbic acid, but there’s another form—sodium ascorbate—that deserves a closer look. This type stands out because it blends vitamin C with sodium, shifting its pH to a less acidic level. The body still receives vitamin C, but the ride usually feels gentler, something many people with sensitive stomachs notice right away.
Better Tolerance Means More Consistent Use
Anyone who’s ever experienced heartburn after a glass of orange juice knows the sting that comes with acid. I’m one of those people. Swapping to sodium ascorbate changed my relationship with vitamin C. Instead of skipping doses or fighting through discomfort, I could keep up daily without stomach issues. Several nutritionists confirm this approach helps many clients stick with supplementation, especially elders or those wrestling with reflux.
Supporting Immunity and Cell Health
Vitamin C plays a direct role in cell growth, collagen formation, and the body’s defense systems. Research shows that sodium ascorbate stands on equal footing with ascorbic acid for boosting white blood cells. Hospitals often use sodium ascorbate intravenously for patients who can’t take acidic forms. Scientific reviews confirm that vitamin C shortens the duration of colds in many people and strengthens the immune system by supporting phagocyte function. So, the benefits aren’t just theoretical—they show up in day-to-day resilience against common bugs.
Less Acidic, Gentle on the Body
Folks on certain medications or with stomach ulcers usually walk a fine line. Acidic supplements can trigger pain or interact with medicines, causing more harm than good. The milder nature of sodium ascorbate comes from its pH. It lands around neutral, giving the gut lining a break. Lining up stories from community clinics and the testimonies of pharmacists points to better tolerability, especially for children and seniors.
Keep an Eye on Sodium
Add sodium to anything and it’s smart to check total intake. People struggling with hypertension or kidney issues may need to watch sodium levels. While the extra sodium in sodium ascorbate isn’t huge, it can build up over weeks if a person is taking large doses regularly or eating a salty diet. Physicians recommend talking things over before settling on a new supplement routine, as balancing vitamins and minerals sometimes takes a little trial and error.
Practical Choices and Trusted Sources
Buying vitamins shouldn’t be a gamble. Always look for brands with third-party testing and clear sourcing. Supplements aren’t all created equal, and quality varies widely. ConsumerLab and USP provide reliable certification if you’re shopping in the US. Talking with a healthcare provider who knows your history is worth it, too—especially if you plan to megadose or tackle more serious health issues with sodium ascorbate as part of your plan.
Diet Still Sits at the Foundation
Sodium ascorbate works best as a complement to a food-first approach. Fresh fruits and veggies bring more than just vitamin C. They add texture, flavor, and all the small helpers like fiber and phytonutrients. Supplements step in when life or a medical situation makes it tough to get enough from food alone. In those cases, sodium ascorbate stands out by offering the protection vitamin C brings, without some of the common downsides tied to other forms.
Are there any side effects or risks associated with Sodium Ascorbate?
Looking Beyond the Promise of Vitamin C
Sodium ascorbate pops up on shelves in supplement stores, often sold as a gentler form of vitamin C. Plenty of folks reach for it, hoping for an immunity boost, quicker recovery from a cold, or just better overall health. The story, though, isn’t only about benefits. Like any supplement, sodium ascorbate brings its own set of concerns that shouldn’t get ignored.
Sensitive Stomachs and Digestive Woes
Some brands push sodium ascorbate as easier on the stomach than plain ascorbic acid. Speaking from some rough experience with heartburn after high-dose vitamin C, this version can indeed feel less harsh for certain people. Swapping the acid for the sodium salt may reduce nausea and help folks with sensitive guts. The flip side? Too much, too fast can still upset digestion. Loose stools, gassiness, or even outright diarrhea pop up if someone takes big doses.
Salt Intake Sneaks In
People often forget that sodium ascorbate contains real sodium. This isn’t much concern if you’re only taking minor amounts. If you’re on a sodium-restricted diet, the math changes. Each gram can pack around 111 milligrams of sodium, and that adds up for folks with hypertension, kidney disease, or heart issues. Too much sodium, no matter the source, can raise blood pressure or make conditions worse over time. Some people juggling several supplements or meal replacements with sodium ascorbate might find this unnecessary sodium creeping into their day.
Kidney Complications Aren’t Just Theoretical
A family member of mine once had kidney stones and the doctor circled back to his high-dose vitamin C habit. Large doses of ascorbate, whether plain or as the sodium salt, bump up oxalate production. Oxalates raise the risk of kidney stone formation, especially in people prone to stones already or who have certain metabolic quirks. The risk varies person to person, but it should stay on the radar, particularly for people with a history of kidney trouble.
Taking More Isn’t Always Better
It’s easy to assume that if a little helps, a lot must help even more. For vitamins, especially vitamin C, the body hits a limit on what it can use. Excess often washes out in urine, but that doesn’t mean megadoses are harmless. Ongoing excess vitamin C, from sodium ascorbate or any form, may interfere with other medications — like blood thinners or certain chemotherapy drugs. A study published in the American Journal of Clinical Nutrition showed that very high vitamin C intake can tinker with blood sugar measurements, which spells trouble for diabetics trying to manage their dose or track their A1C.
Safe Use Means Personalizing the Approach
It pays to remember that not everyone’s needs match up. Doctors and registered dietitians remain the best voices on the right dose for you. For most healthy adults, a well-rounded diet handles vitamin C needs. Supplements like sodium ascorbate can play a role when health conditions interfere with absorption or boost requirements. Still, reading labels, checking in with healthcare professionals, and paying attention to any new symptoms after starting a supplement all count. The goal is to keep benefits up and risks down, through smart choices and open conversations.
What is the recommended dosage for Sodium Ascorbate?
Getting Straight to the Facts
Sodium ascorbate, a form of vitamin C mixed with sodium, lands on shelves in bottles that look a lot like their better-known cousin, ascorbic acid. Label reading often raises more questions than answers. For adults, the FDA draws the line for vitamin C intake at 2,000 mg per day. That number doesn't only apply to sodium ascorbate, but to all vitamin C taken in by mouth. So, one teaspoon of standard sodium ascorbate powder—around 2,500 mg—already nudges above that guideline. Keep in mind that sodium ascorbate delivers some sodium too, about 110 mg per gram. People watching their sodium intake for blood pressure or kidney reasons might need to pay closer attention.
Why Dosage Matters
Some folks pop vitamin C like candy at the first tickle of a cold. Others want a boost for skin or immune health. I've watched busy parents and gym enthusiasts aim for extra vitamin C, hoping for more protection against illness or faster recovery. What many don't realize is that excessive dosages can backfire. Too much sodium ascorbate risks stomach cramps, diarrhea, nausea, even kidney stones, especially for people prone to them. Even a regular, modest dose can throw off sodium balance for anyone with high blood pressure or who follows a low-salt diet. The lesson here: more isn't always better. Clinicians and registered dietitians regularly stress that going over recommended doses rarely increases benefits—the body flushes out the extra, sometimes along with unwanted side effects.
Recommended Dosage for Different Groups
Doctors generally point to 500 mg to 1,000 mg daily of vitamin C for most adults who want to supplement, counting all sources from food and supplements. For sodium ascorbate, that’s about 560 mg to 1,100 mg of powder daily. Kids need much less. The National Institutes of Health lists 400 mg a day as the upper safe limit for toddlers, 650 mg for elementary-aged children, and 1,800 mg for teens. Pregnant or breastfeeding women fall near the upper edge of the adult range, yet should check with healthcare providers about the right amount. People with kidney problems, certain genetic risks (like G6PD deficiency), or those on strict low-sodium diets often hear strict advice to avoid self-dosing.
Can Diet Fill the Gap?
Plenty of people ask about supplements before they look at their diet. Oranges, broccoli, kiwi, peppers—those foods bring vitamin C minus the sodium load. In practice, unless someone faces absorption issues or dietary restrictions, real food covers most needs. The push for supplementation should come after looking at eating habits and advice from a trained provider, not just buzz from the latest wellness post online.
Practical Tips and Safer Choices
Whenever people add sodium ascorbate, it pays to measure. Scoops are handy, but powder clumps and density shifts between brands. A kitchen scale outperforms a random spoon for avoiding accidental double-dosing. Always check supplement labels: some powders deliver all sodium ascorbate, others mix in binders or flavorings. Trustworthy products share full details, including batch testing and certifications from third-party testers.
People responding to social media fads sometimes think large doses mean quick fixes. If a label or influencer promotes high daily intake, check the science, not just the claims. The safest bet is to talk with a provider about diet, family history, lifestyle, and current medicines. The internet might hype megadoses, but practical health sticks to basics: steady, manageable amounts keep risk low and benefit clear.
Is Sodium Ascorbate safe for pregnant or breastfeeding women?
Vitamin C plays a key role in staying healthy, especially when you have a baby on the way or a newborn at your side. Worth remembering, though, is that vitamin C doesn’t just come in the form of oranges or lemons. Sodium ascorbate is popular as a supplement, sometimes favored for its gentler effect on the stomach compared to ascorbic acid. The question comes up a lot: does sodium ascorbate make sense for pregnant or breastfeeding women?
Safety in Pregnancy and Breastfeeding
Plenty of folks reach for extra vitamin C when immune systems feel shaky. During pregnancy, the instinct to stay healthy grows stronger. The Food and Nutrition Board recommends about 85 mg of vitamin C per day for pregnant women and 120 mg for those breastfeeding. Anything above 2,000 mg a day for adults gets labeled as the tolerable upper intake level for vitamin C. Going above that, especially for long stretches, brings risks.
Sodium ascorbate offers vitamin C with sodium attached. So, yes—each dose adds a small amount of sodium to your diet. Most healthy adults handle this fine, but folks with high blood pressure or certain heart issues may want to keep an eye on it. During pregnancy, already a time when swollen ankles and extra fluid retention can surprise you, that pinch of sodium matters.
Direct research on pregnant or nursing women taking sodium ascorbate sits in short supply. That makes knowing the risks and benefits even more important. Research on vitamin C overall shows clear benefits. It helps babies grow, supports the placenta, and keeps mom’s immune system ticking. Still, megadoses—the kind way over daily recommendations—bring a real risk. They can lead to kidney stones, digestive problems, and, rarely, affect your baby’s health.
What Health Experts Say
Supplements, whether labeled “natural” or not, don’t replace a healthy meal. Obstetricians and dietitians keep saying the same thing: get as many vitamins and minerals as you can from whole foods. Colorful fruit, leafy greens, and real meals carry the nutrients your body recognizes best. Vitamin C in these foods doesn’t pile on sodium. If your doctor recommends a supplement, double-check the label and stick to the dose. Too much of a good thing can snowball problems you don’t need.
Organizations like the American College of Obstetricians and Gynecologists note that prenatal vitamins supply enough vitamin C for most. They don’t usually suggest extra vitamin C unless there’s a clear deficiency or special need. Adding another supplement without talking to your care provider can throw off your diet’s balance.
Common-Sense Choices and Alternatives
Pregnancy and breastfeeding shape eating habits. Sometimes nausea, food aversions, or busy schedules mean supplements sound easier than preparing a meal. In those cases, sodium ascorbate may offer a gentler alternative to ascorbic acid, but always check with a healthcare professional. Track the total amount of vitamin C and sodium from all supplements and foods. Too much vitamin C won’t turn you into “Super Mom”—instead, it can backfire.
If diet alone falls short, discuss options with your doctor. They might suggest a prenatal with balanced vitamin C or recommend food sources you haven’t considered. Fresh strawberries, kiwi, peppers, and broccoli pack plenty of vitamin C—no pill needed. If your healthcare provider believes sodium ascorbate makes sense for you, follow their advice on dose and duration.
Getting Trusted Advice
In my own family, new parents often ask whether new supplements are needed. The doctors we trust always emphasize keeping things simple: stick to evidence, value moderation, and choose foods that nourish both mom and baby. When in doubt, call your provider or registered dietitian. Good advice beats guessing every time.
Smart supplement choices keep parents and babies healthy, and turning to trusted sources for guidance always pays off. If you have questions, don’t rely just on labels or online chatrooms—your healthcare team knows you best.
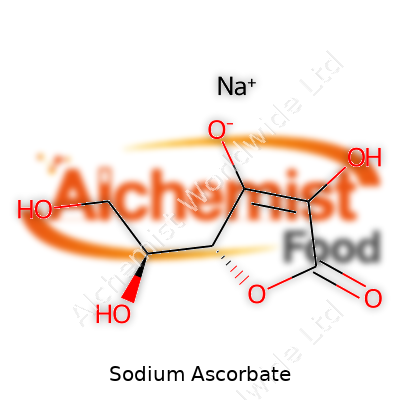
| Names | |
| Preferred IUPAC name | Sodium (2R)-2-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2-olate |
| Other names |
Monosodium ascorbate Sodium salt of ascorbic acid Ascorbic acid sodium salt Vitamin C sodium salt |
| Pronunciation | /ˈsəʊdiəm əˈskɔːrbeɪt/ |
| Preferred IUPAC name | Sodium (2R)-2-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2-olate |
| Other names |
Ascorbic acid sodium salt Sodium L-ascorbate Sodium erythorbate Monosodium ascorbate |
| Pronunciation | /ˌsəʊ.di.əm əˈskɔːr.beɪt/ |
| Identifiers | |
| CAS Number | “134-03-2” |
| Beilstein Reference | 1721942 |
| ChEBI | CHEBI:63258 |
| ChEMBL | CHEMBL1201520 |
| ChemSpider | 59372 |
| DrugBank | DB01213 |
| ECHA InfoCard | 100.031.288 |
| EC Number | 1.10.3.3 |
| Gmelin Reference | 190268 |
| KEGG | C01601 |
| MeSH | D020790 |
| PubChem CID | 23668198 |
| RTECS number | WS7250000 |
| UNII | SQE6VB453K |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID4022650 |
| CAS Number | 134-03-2 |
| Beilstein Reference | 3594400 |
| ChEBI | CHEBI:32979 |
| ChEMBL | CHEMBL1201538 |
| ChemSpider | 11344 |
| DrugBank | DB01373 |
| ECHA InfoCard | 05d63368-777a-4dba-9ff8-7690a504e31b |
| EC Number | 205-126-1 |
| Gmelin Reference | 59382 |
| KEGG | C01366 |
| MeSH | D020789 |
| PubChem CID | 23668193 |
| RTECS number | WS0940009 |
| UNII | 025XOM940Z |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | DTXSID7046138 |
| Properties | |
| Chemical formula | C6H7NaO6 |
| Molar mass | 198.11 g/mol |
| Appearance | White to slightly yellowish crystalline powder |
| Odor | Odorless |
| Density | DENSITY: 1.66 g/cm3 |
| Solubility in water | Freely soluble |
| log P | -7.85 |
| Acidity (pKa) | 11.6 |
| Basicity (pKb) | 8.92 |
| Magnetic susceptibility (χ) | -72.0e-6 cm³/mol |
| Refractive index (nD) | 1.62 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.69 D |
| Chemical formula | C6H7NaO6 |
| Molar mass | 198.11 g/mol |
| Appearance | White or almost white, crystalline powder or granules |
| Odor | Odorless |
| Density | Density: 1.667 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -7.85 |
| Acidity (pKa) | pKa 4.2 |
| Basicity (pKb) | 8.8 |
| Magnetic susceptibility (χ) | -75.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.62 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.53 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 178.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1331.7 kJ/mol |
| Std molar entropy (S⦵298) | 111.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1339.7 kJ/mol |
| Pharmacology | |
| ATC code | A11GA01 |
| ATC code | A11GA01 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | No signal word |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| Flash point | 105 °C |
| Autoignition temperature | > 452 °C |
| Lethal dose or concentration | LD50 (Oral, Rat): 11,900 mg/kg |
| LD50 (median dose) | LD50 (median dose): 11,900 mg/kg (oral, rat) |
| NIOSH | SC7500000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 1000 mg per day |
| Main hazards | Irritant to eyes, skin, and respiratory system |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | No signal word |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| Flash point | > 212 °F (100 °C) |
| Lethal dose or concentration | LD50 (oral, rat): 11,900 mg/kg |
| LD50 (median dose) | LD50 (median dose): 11,900 mg/kg (oral, rat) |
| NIOSH | RN: 134-03-2 |
| PEL (Permissible) | PEL: 15 mg/m³ |
| REL (Recommended) | 750 mg |
| IDLH (Immediate danger) | Not Established |
| Related compounds | |
| Related compounds |
Ascorbic acid Calcium ascorbate Magnesium ascorbate Potassium ascorbate Sodium erythorbate |
| Related compounds |
Ascorbic acid Calcium ascorbate Magnesium ascorbate Potassium ascorbate Sodium erythorbate |

