Sodium Acetate: Commentary on a Vital Chemical
Historical Development
Sodium acetate traces its roots to centuries-old discoveries that reveal how necessity and curiosity move science ahead. Medieval alchemists recognized its cooling effect and ability to shift certain reactions, but the real breakthrough arrived with advances in organic chemistry through the 19th century. Factories began making sodium acetate on purpose using acetic acid, which at first came from fermenting grains—think vinegar on an industrial scale. Once chemists understood how to harness acetates in textiles and tanning, this once humble salt marched into mass production. Today, factories churn out sodium acetate in massive quantities, drawn by demands from everything from food preservation to heat packs, reminders of a chemical story that grew with technology.
Product Overview
Many folks know sodium acetate from those reusable hand warmers that turn from liquid to solid with a snap, giving off a cozy heat. Beyond warming hands, the compound keeps showing up in industries that need buffering power, a safe food additive, and a critical agent for controlling pH. Food manufacturers trust it as an acidity regulator that helps keep snacks shelf-stable and tangy. Textile plants depend on it to help fix dyes in natural fibers, ensuring colors come out bold. Scientists depend on it for precise chemical reactions, and waste treatment centers use it to support healthy bacterial growth. Sodium acetate’s low cost and familiar safety record mean it lands on the must-have list for labs, factories, and even classrooms.
Physical & Chemical Properties
Sodium acetate usually arrives as a free-flowing white powder or clear crystals, dissolving easily in water and splitting into sodium and acetate ions. The salt keeps well under most storage, resists clumping from humidity, and packs a salty, slightly vinegary taste thanks to its parent compound, acetic acid. Its melting point around 324°C and high boiling point make it stable in cooking and chemical processing. Unlike some more temperamental salts, sodium acetate stays reliable at room temperature, doesn’t break down on the shelf, and won’t go up in smoke unless temperatures get extreme. Those qualities hang together with its reputation for being safe and dependable in diverse uses.
Technical Specifications & Labeling
Producers sort sodium acetate by grade—industrial, food, or laboratory—backed by standards from organizations such as the Food Chemicals Codex and ASTM. Labels call out purity (almost always above 99%), moisture content, and confirm whether the product uses the hydrated or anhydrous form. Regulations insist on full batch information and clear hazard statements. Each label offers up contact details for emergency handling, storage guidance, and advice about spill management. The food grade carries extra paperwork certifying its safety for human consumption. Companies have learned that accurate documentation of technical standards is more than a regulatory requirement—it reassures users that what’s inside matches precise needs.
Preparation Method
Chemists typically make sodium acetate by mixing acetic acid with sodium carbonate or sodium hydroxide. Small-scale preparations often start with plain white vinegar and baking soda, a familiar classroom experiment that fizzes and foams before leaving behind sodium acetate and water. On an industrial level, acetic acid reacts with sodium carbonate under controlled conditions, followed by careful evaporation to leave clean, consistent crystals. Any stray ions or byproducts get filtered out to hit the exacting standards of the chosen grade. The process keeps getting tweaked for efficiency and environmental impact, often reclaiming water and streamlining energy use to limit the carbon footprint.
Chemical Reactions & Modifications
Sodium acetate’s main job in reactions is buffering, steadying pH in mixtures where exact acidity or alkalinity matters. In biology labs, it supports DNA extraction, shaping the environment for strands to settle together. Mixed with strong acids, sodium acetate lets off acetic acid, which can play a key role in condensing reactions in organic synthesis. Heating pushes some sodium acetate to break down into methane and sodium carbonate—knowledge base for high-temperature work in labs. Chemists sometimes transform it via other acetylation steps, taking advantage of the acetate part for multi-step synthesis when building bigger, intricate molecules. Each tweak expands the compound’s usefulness for those solving tough problems in research and manufacturing.
Synonyms & Product Names
Sodium acetate hides under several labels, from “sodium ethanoate” when chemistry texts stick to systematic naming, to trade names specific to suppliers. Food packaging may list it as E262. In textile sectors and water treatment literature, the compound simply answers to its sodium acetate identity. Users benefit from a consistent name regardless of the context, though it pays to read the fine print; hydrates and anhydrous forms behave slightly differently, a detail critical for precise recipes or sensitive chemical steps.
Safety & Operational Standards
Labs and workers credit sodium acetate for a sterling safety record. Handling the compound with gloves and goggles in large amounts limits accidental contacts, but it rarely triggers allergic reactions or dangerous fumes under normal use. Agencies like OSHA and the European Food Safety Authority keep the compound on their safe lists. Storage relies on keeping packages dry and away from acids or strong oxidizers, which could trigger unwanted reactions. Fire departments classify sodium acetate as low risk, but still expect users to be alert to spills and to avoid slinging dust in the air. In schools, the salt is often chosen for teaching basic chemistry because of its low hazard profile.
Application Area
Sodium acetate’s applications cut across sectors. The food industry counts on it for flavor enhancement in potato chips, as a pickling agent, and a mild preservative. Textile factories need it for dye baths where pH precision prevents fabric damage and locks colors deep into every thread. Engineers lean on its phase-change properties in heat packs and thermal storage applications, taking advantage of its high heat of fusion. Environmental specialists dose water treatment systems with sodium acetate to promote carbon feeding for denitrifying bacteria. Lab workers use it as a reagent for nucleic acid extraction and protein precipitation, a supporting player in molecular biology breakthroughs. The compound’s cost, efficacy, and safety guarantee its spot in everyday products and advanced manufacturing alike.
Research & Development
Universities and industrial R&D teams treat sodium acetate as a building block for testing greener production methods and new synthetic routes. Scientists explore improved crystallization processes to hit tighter purity targets needed for pharmaceuticals. Researchers look at hybrid materials mixing sodium acetate with other phase-change materials for solar energy and off-grid home heating. Food technologists examine its interactions with new preservatives and flavors. Biochemists continue running studies on using sodium acetate to alter fermentation pathways, aiming for cleaner biofuel production and better protein synthesis in cell culture. Making sodium acetate production more sustainable stands out as a research focus, with many labs seeking lower energy inputs or less dependence on petroleum-derived starting materials.
Toxicity Research
Toxicologists and food scientists have consistently found sodium acetate to be safe at levels found in food, with studies showing it won’t build up in the body or contribute to long-term health risks when consumed in moderation. High concentrations in laboratory animals sometimes bring on digestive discomfort, but human risk stays low in typical exposure scenarios. Researchers watch for sensitive populations and study any allergic or environmental impacts, but evidence keeps supporting its safety profile. Waste management experts keep monitoring for aquatic toxicity, since massive discharges could shift water chemistry, but present-day usage patterns don’t threaten municipal water quality or food supply chains.
Future Prospects
Looking ahead, sodium acetate finds itself woven into plans for energy efficiency, advanced food science, and sustainable chemistry. As companies target lower carbon footprints, sodium acetate’s key role in phase-change storage could support grid stability in renewables-heavy power networks. Food scientists working to reduce artificial preservatives may boost interest in organic acid salts, with sodium acetate standing out as both proven and well-understood. As gene editing and synthetic biology mature, the salt’s support for DNA-related processes will likely scale up, making it even more crucial in biotechnology labs. Clean production processes developed in industries from textiles to chemicals may reward new approaches to making sodium acetate using local, bio-based raw materials, cutting dependence on fossil inputs and creating more resilient supply chains.
What is sodium acetate used for?
Everyday Chemistry in Action
You might not see it by name on grocery shelves, but sodium acetate drifts through daily life in places you might not expect. Most people come across it in those click-to-activate heat packs you grab for sore muscles. This crystal salt stores energy and delivers heat in a way that blends science with real comfort. You click a small metal disk, a cloud whirls through the pack, and warmth spreads almost like magic. This compact burst of heat comes from sodium acetate shifting between its solid and liquid states. I used these packs during winter afternoon soccer games to keep my hands moving when gloves weren’t enough, so I know the value the salt brings during cold snaps.
Kitchen Chemistry and Food Preservation
Food makers turn to sodium acetate for more than just thickening. As a food additive, it brings sharp, tangy notes to chips and snacks under the label E262. Think of that sour, almost vinegary kick in salt and vinegar chips — sodium acetate stands behind much of that punch. It also discourages bacteria from finding a home in packaged bread, so loaves last longer. Groups like the U.S. Food and Drug Administration approve sodium acetate as safe, though moderation stays important. The flavor and shelf-life boost make this chemical a regular part of many modern pantries, whether people realize it or not. Understanding what sneaks into snacks keeps anyone tuned into health choices.
Laundry and Lab Benefits
Textile workers use sodium acetate too. Fabric dyeing stains fabrics evenly, but unwanted minerals in water, like calcium, can cause blotchy colors. Adding sodium acetate softens water, letting colors stay bright and even. During a summer job at a laundry, running those large dyeing machines, we measured out compounds like this to keep our colors from streaking. Testing taught me how some chemistry quietly supports jobs that keep your shirts looking sharp, long after you buy them.
Science classrooms and research labs need buffer solutions to keep their tests accurate. Sodium acetate helps control pH levels, making it a stand-by for biochemistry experiments or DNA work. If pH wobbles, results go sideways. Years of research highlight how even small imbalances derail whole experiments. Colleges use sodium acetate because it’s reliable, predictable, and safe compared to stronger chemicals.
Environmental Cleanup and Road Safety
Clearing icy roads without harming cars or the environment gets tougher as cities hunt for safe alternatives to salt. Sodium acetate melts ice at lower temperatures and doesn’t corrode metal like rock salt, so city crews use it around airports and bridges. In areas with protected water systems, sodium acetate poses less threat to fish or plants, compared to sodium chloride that runs off into rivers. Switching to it cuts down on winter rust repairs and environmental cleanup, though cost and supply keep its use from spreading too fast.
No-Frills Science, Big Impact
Sodium acetate’s reputation stands on its real-world jobs — packing lunches, heating hands, cleaning clothes, and keeping cars on the road. There’s something eye-opening about tracking an ingredient from your dinner table to a runway. By recognizing what this chemical actually does and staying curious about ingredients in everyday life, it’s possible to make smarter choices at home and in the world beyond our front doors.
Is sodium acetate safe to handle?
Everyday Encounters and Common Uses
Sodium acetate pops up in everyday life more than most people realize. Cafeteria kitchens use it for flavoring, it helps turn hand warmers into little pockets of heat, and cleaners rely on it for buffering. Its use stretches from the chemistry lab to the warehouse and even into school science kits. That kind of spread hints at a substance that’s not likely to cause havoc. Still, safety isn’t something to leave to chance just because a product is familiar.
Touching, Inhaling, and Eating: Where Does Risk Appear?
Grabbing a handful of sodium acetate powder or crystals feels a lot like table salt. If any gets on the skin or under the fingernails, the body doesn’t react in any dramatic way. Kids might encounter it during science demonstrations, and I’ve dissolved it a dozen times with nothing more than a soap-and-water rinse afterward. Accidentally inhaling the fine dust can irritate the nose and throat—sometimes, the lungs don’t appreciate strange chemicals floating by. I’ve seen coworkers develop coughing fits in poorly ventilated classrooms, which calmed down quickly after fresh air.
Spilling sodium acetate isn’t headline news. Most spills sweep up easily. Flushing with water does the trick if any lands on clothes or skin. Ingesting small quantities, as happens sometimes when inspecting flavor packets, doesn’t set off alarms for healthy adults. The FDA includes it on the list of food additives that are “generally recognized as safe.” Consuming huge amounts would lead to stomach troubles, though. No food ingredient gives a free pass to ignore dosage.
Eye Contact and Allergic Reactions
Eyes don’t like salts of any kind. Sodium acetate can sting or burn if it gets in there, and using an eyewash station always helps. Allergic reactions look rare with this substance. Some folks with sensitive skin react to almost anything, but I haven’t seen swollen hands or rashes tied directly to sodium acetate at work or at home. That matches data in medical reports and lab safety sheets.
Safe Handling Habits: Wisdom, Not Worry
Lab habits carry over into the kitchen and garage. I always recommend gloves when handling any powder in large amounts—nobody wants grit under their rings. Dust masks make sense for large-scale tasks, even if the chemical isn’t a famous irritant. Keeping chemicals away from food storage bins keeps kids and pets out of trouble. That same set of rules works for sodium acetate. Clean up spills right away and store the container with the label facing out.
Sodium acetate has earned a low-hazard label from the European Chemicals Agency, and SDS sheets from chemical suppliers point out little more than gap-filling advice. It won’t start fires and doesn’t explode under pressure, unlike some household cleaners. Waste disposal doesn’t turn into a nightmare, as environmental agencies classify it as a low-risk pollutant. With truly hazardous chemicals, protocols run much stricter.
Addressing the Role of Knowledge and Training
Chemistry should invite curiosity, not fear. Simple habits keep sodium acetate in the harmless camp: wash hands after use, avoid clouding the air with dust, and keep it far from the eyes. Safety training matters more now that home labs and DIY kits have grown so popular. If kids want to trigger a hot ice reaction, a little grown-up supervision helps everyone stay safe.
Sodium acetate doesn’t deserve a starring role in any safety horror story. Handling it safely comes down to common sense and basic respect for what any chemical can do. With informed habits, folks can keep using it for all those clever hacks, classroom lessons, and wintertime hand warmers—minus the drama.
How should sodium acetate be stored?
The Role of Good Storage in Daily Chemistry
Nobody likes ruined chemicals, wasted resources, or cleaning up mysterious leaks. Whenever I’ve worked in a lab, one lesson always sticks: storage makes all the difference. Sodium acetate looks harmless. It shows up as a white powder, pretty easy to recognize, but even simple chemicals deserve respect. Some chemicals seem to get by with little attention, but sodium acetate lasts longer and acts more reliably if given a decent home.
Keeping Moisture Out of the Picture
Anyone who’s left salt open on a humid day sees the clumping, the crusty edges. Sodium acetate attracts water the same way. That’s why open jars soak up moisture from the air and end up sticky or caked, especially in summer. Once I saw a whole bottle become almost impossible to measure properly. A tightly sealed container fixes most of these headaches. Use a glass or plastic jar with a screw cap that shuts firmly. Make sure every scoop is done with a dry spoon—chemistry teachers harp on this, but it saves materials and cuts back on lab clutter.
Temperature: Don’t Overthink It
People sometimes worry about the cold or the heat. Sodium acetate doesn’t mind regular room temperature. Sticking it in a freezer or baking it in a hot car won’t usually make it dangerous, but heat can shorten shelf life or change the texture. A cool, dark cupboard feels just right. The less direct sunlight, the fewer surprises. I’ve kept jars for a year at school, and the powder stays easy to pour when it doesn’t sit on a sunny bench.
The Right Label Matters
Labels might seem basic, but mistakes happen fast in busy spaces. Once I grabbed what I thought was baking soda for a demo and realized later it was sodium acetate—close, but not the same. Clear labels save time and prevent trouble. Every jar deserves its name, concentration if needed, and the date opened. The simple stuff lowers the chance of mix-ups or accidental spills.
Think About Safety, Not Just Cleanliness
Sodium acetate won’t leap out of the jar at you. It’s not known for explosions or wild reactions, but dust in the eyes or mouth still stings. A good lid isn’t just about keeping things tidy. It helps stop leaks or spills if someone bumps a shelf. The material isn’t flammable, so special fireproof cabinets aren’t required, but keeping strong acids or oxidizers far away avoids weird chemical reactions. I remember hearing stories about bottles left side by side in old storerooms—rare problems, but easy to avoid.
Disposal and Freshness
Older powders sometimes form strange lumps, or the color changes. I learned early that tossing anything suspicious brings longer peace of mind. Most school or home labs have a waste plan for chemicals that no longer seem right. Pouring down the drain isn’t smart unless local guidelines say so. When in doubt, check with local waste services—nobody likes finding out the hard way during a plumbing repair.
Small Steps, Big Results
Sodium acetate rewards a little attention. Store it in dry, sealed containers, mark each jar clearly, and keep it in a cool spot out of direct sun. That little bit of care saves money and keeps experiments running smoothly, year after year. These habits help anyone from a high school student to a professional chemist keep things running safely and smoothly. Small steps lead to real peace of mind with every scoop and label.
What is the chemical formula of sodium acetate?
The Basics Behind Sodium Acetate
Kids in chemistry class usually get their hands dirty making sodium acetate with vinegar and baking soda. After the bubbling calms down, there’s a white, powdery residue left behind. That’s sodium acetate, a compound often overshadowed by flashier chemical cousins in labs and kitchens. Its formula is NaC2H3O2. In plainer terms, the makeup includes a sodium ion (Na+) and an acetate ion (C2H3O2-).
People usually meet sodium acetate in science class, some only see it in heat packs that work magic by snapping a metal disc. What they rarely ask is why this simple combination matters beyond school experiments or soothing sore backs.
Daily Relevance of NaC2H3O2
Sodium acetate doesn’t just stay on the shelf. My restaurant life taught me food-grade sodium acetate helps seasonings and snacks punch through with preserved freshness. In industrial kitchens, it extends the shelf life of chips and keeps salad dressings tart and crisp. Food safety isn’t a distant concern—sodium acetate works behind the scenes so products stay right longer on grocery shelves, reducing food waste on a massive scale.
The heating packs rely on a clever chemical trick. They use supersaturated sodium acetate. Flicking the disc triggers crystallization, causing the pack to release heat in seconds. This practical application reaches campers and athletes every day, especially on cold winter hikes or after a rough soccer match. It’s chemistry that feels like a bit of everyday magic.
Science and Industry
Labs put NaC2H3O2 to work in buffer solutions. These solutions stabilize the acidity during delicate processes such as DNA extraction or protein research. Buffering agents like sodium acetate enable reliable results and consistent data, helping research progress without wild swings in pH. Reliable data gets projects funded and makes breakthroughs possible, so the role of this clear, powdery substance ripples far beyond the test tube.
Textile workers use sodium acetate during dyeing to improve color adherence. Adding sodium acetate to the dye bath lets fabric makers lock in shades more consistently. This means consumers see jeans and T-shirts in lasting colors, rather than faded remnants after a couple of washes. My own closet owes some vibrancy to those unseen chemical allies.
Challenges and Better Choices
Production of sodium acetate doesn’t raise the same red flags as some other chemicals, though the disposal practices matter. Tossing unused sodium acetate down the drain has minimal impact, but in bulk, every compound can add up in municipal wastewater streams. The answer lies in proper disposal and recycling plans at the industrial level.
Many food processors already look for alternatives or ways to minimize synthetic additives in response to consumer demand for fewer “unpronounceables” in ingredients. Continued progress in food science could provide natural sources or fermentative processes to match sodium acetate’s benefits without synthetic touch. Manufacturers stay vigilant for greener processes and cleaner ingredient lists, and as customers, voting with the wallet steers those choices.
A Small Formula, Big Impact
Sodium acetate, known to chemists as NaC2H3O2, delivers practical value every day. It shapes comfort in heat packs, keeps food lasting longer, refines textiles, and smooths science behind the scenes. A little knowledge of that formula changes how we see pantry products and even the clothes we wear. Such simple combinations reveal chemistry’s touch in places we rarely stop to notice.
Can sodium acetate be used in food applications?
A Closer Look at Sodium Acetate in the Kitchen
Sodium acetate sounds more like something from a high school chemistry lab than something you'd sprinkle on your dinner, but it’s hardly foreign to food. This compound pops up in flavoring mixes, especially for chips and snacks. Anyone who’s ever tasted salt and vinegar chips has probably crunched on some sodium acetate. Its main job is to bring that tangy, vinegary bite without adding all the extra moisture or hassle of actual vinegar.
Chemists have studied this ingredient for quite a while, and food science backs up its use. The European Food Safety Authority (EFSA) and the U.S. Food and Drug Administration both give the thumbs up on sodium acetate as a safe food additive. You’ll see it labeled as E262 in Europe. It’s not toxic under normal food doses and doesn't hang around in the body. It breaks down to harmless substances: sodium, water, and acetic acid (that’s the main part of vinegar).
Why Chefs and Snack Makers Like It
There’s a practical side, too. Vinegar’s great, but it’s wet and acidic. Sprinkling it straight onto crackers or popcorn turns things soggy fast. Sodium acetate is a dry powder, so it gets the tang across and leaves everything crisp. That’s why you’ll find it in commercial popcorn, chips, and even some reheatable meals. It keeps snack coatings light and helps flavors cling to surfaces better than plain salt.
Preservation matters, too. Sodium acetate raises the acidity a bit, slowing the growth of certain bacteria and molds. Food companies rely on this quality when they want products to last just a little longer on the shelf without using more aggressive preservatives.
Facts Worth Knowing
People sometimes worry about additives with scientific names. Studies on sodium acetate show that it doesn’t cause trouble at common levels. Still, large doses can lead to too much sodium, just like table salt. That means moderation stays important, especially for folks watching their blood pressure. The research is out there—peer-reviewed studies and safety assessments run by government organizations show that sodium acetate breaks down naturally after you eat it.
Its taste isn’t a perfect match for regular vinegar, but it delivers a similar sour punch. Some cooks experiment with sodium acetate for culinary tricks, infusing that sharp bite into ice cream or cocktails without risking a watery mess.
Thoughts on Better Food Choices
The use of additives often raises questions about clean labels and whole food approaches. The food industry keeps looking for ways to give people what they want—bold flavors, long shelf-life, convenience. Food scientists could already try swapping sodium acetate with other acids or salts that come with more recognizable names for consumers.
Reading ingredient labels and understanding what goes into our food always helps. If you enjoy a tangy snack, enjoy it in moderation, and don’t let chemical names scare you off before checking the science. In kitchens both big and small, sodium acetate has carved out a niche because it works and passes the safety tests. But as more shoppers care about ingredient lists, companies can step up with clearer labeling and education so customers know what’s in their food and why. That’s a small but real step toward trust, taste, and health.
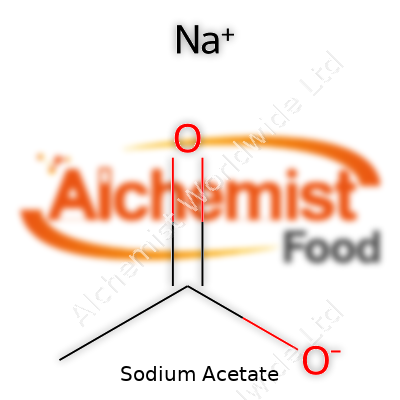
| Names | |
| Preferred IUPAC name | Sodium ethanoate |
| Other names |
Acetic acid, sodium salt Sodium ethanoate Hot ice E262 |
| Pronunciation | /ˈsəʊdiəm əˈsiːteɪt/ |
| Preferred IUPAC name | sodium ethanoate |
| Other names |
Sodium ethanoate Acetate of soda Ethanoic acid sodium salt |
| Pronunciation | /ˌsəʊdiəm əˈsiːteɪt/ |
| Identifiers | |
| CAS Number | 127-09-3 |
| Beilstein Reference | 1718736 |
| ChEBI | CHEBI:32139 |
| ChEMBL | CHEMBL1631320 |
| ChemSpider | 5035 |
| DrugBank | DB03166 |
| ECHA InfoCard | 04dee56a-ccd2-4e7e-94d7-9377f2ae1f3b |
| EC Number | 204-823-8 |
| Gmelin Reference | 6046 |
| KEGG | C00258 |
| MeSH | D019274 |
| PubChem CID | 517055 |
| RTECS number | AJ4300010 |
| UNII | NSU444B46G |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID3023497 |
| CAS Number | 127-09-3 |
| Beilstein Reference | 397445 |
| ChEBI | CHEBI:32139 |
| ChEMBL | CHEMBL1359 |
| ChemSpider | 5059 |
| DrugBank | DB09238 |
| ECHA InfoCard | 100.007.322 |
| EC Number | 204-823-8 |
| Gmelin Reference | 6358 |
| KEGG | C02759 |
| MeSH | D012964 |
| PubChem CID | 517055 |
| RTECS number | AJ4300010 |
| UNII | N9YNS0M02X |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID5021824 |
| Properties | |
| Chemical formula | C2H3NaO2 |
| Molar mass | 82.03 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.528 g/cm³ |
| Solubility in water | freely soluble |
| log P | -4.21 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | 4.76 |
| Basicity (pKb) | 9.25 |
| Magnetic susceptibility (χ) | -42.1·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.464 |
| Viscosity | 1.1 mPa·s (at 20°C, for a 1M solution) |
| Dipole moment | 2.92 D |
| Chemical formula | CH3COONa |
| Molar mass | 82.03 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.528 g/cm³ |
| Solubility in water | Very soluble |
| log P | -4.21 |
| Vapor pressure | <0.01 mm Hg (20°C) |
| Acidity (pKa) | 4.76 |
| Basicity (pKb) | 9.25 |
| Magnetic susceptibility (χ) | -36.8·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.422 (20 °C) |
| Viscosity | 1.0 – 1.1 cP (20°C, 10% solution) |
| Dipole moment | 1.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 86.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −709.32 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -947.7 kJ/mol |
| Std molar entropy (S⦵298) | 86.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -709.33 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -953.7 kJ/mol |
| Pharmacology | |
| ATC code | B05XA03 |
| ATC code | B05XA04 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS07, GHS06 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P370+P378 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 1, Instability: 0, Special: - |
| Flash point | > 250 °C |
| Autoignition temperature | 607 °C (1125 °F; 880 K) |
| Lethal dose or concentration | LD50 (oral, rat): 3530 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3530 mg/kg (oral, rat) |
| NIOSH | SN1225000 |
| REL (Recommended) | 4 mg/kg bw |
| Main hazards | Irritant to eyes, skin, and respiratory system. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 1, Instability: 0, Special: - |
| Autoignition temperature | 604 °C (1120 °F; 877 K) |
| Lethal dose or concentration | LD50 (oral, rat): 3530 mg/kg |
| LD50 (median dose) | 3,530 mg/kg (rat, oral) |
| NIOSH | SN1225000 |
| REL (Recommended) | “3 g” |
| Related compounds | |
| Related compounds |
Calcium acetate Potassium acetate Lithium acetate Ammonium acetate Sodium formate Sodium propionate |
| Related compounds |
Acetic acid Sodium carbonate Sodium bicarbonate Sodium formate Potassium acetate Calcium acetate |

