Propyl Gallate: Insights, Science, and Future Directions
Historical Development
Propyl gallate first caught attention in the late 1930s, emerging during a period defined by the search for effective food preservatives. Scientists recognized that fats and oils posed a major challenge for food storage and transport, as spoilage from oxidation led to waste and potential health hazards. The hunt for a solution brought folks to gallates, and after a series of evaluations, propyl gallate made its way onto the shelf as a synthetic antioxidant. Its popularity grew post-World War II, when the industrial food landscape started demanding shelf stability for mass production and distribution. Today, propyl gallate has become an established player in food technology, with regulatory bodies in the US, EU, and Asia including it in discussions about food safety and consumer trust.
Product Overview
Propyl gallate stands as a white to off-white powder, sometimes showing up in crystalline or granular forms. Almost odorless, it offers only a faint taste, which works to its benefit in food systems aiming to preserve flavor integrity. This compound shows up under the names E310 or n-propyl 3,4,5-trihydroxybenzoate, but most folks in food labs just call it propyl gallate. It slots in as an antioxidant, sometimes paired with other preservatives, to keep oil-rich foods and pharmaceuticals from turning rancid. Major food producers, supplement makers, and even cosmetic companies tap into this additive to stretch expiry dates and keep undesirable reactions at bay.
Physical & Chemical Properties
Propyl gallate delivers a melting point in the 148°C to 153°C range, which makes it stable for most manufacturing conditions. Water does not dissolve much of it, but alcohol, acetone, and ether do the job just fine, pointing to its affinity for oily or fatty substances. Its chemical structure—essentially a propyl ester of gallic acid—gives it three hydroxy groups, which soak up free radicals before they spark bigger oxidative problems. In practice, this chemical backbone makes propyl gallate strong in its role and mostly resistant to breakdown under light and moderate heat, though strong acids or bases can degrade it. In the real world, this means it holds up in food systems that don’t get overly acidic or exposed to extremes.
Technical Specifications & Labeling
Anybody dealing with food or pharmaceutical regulations quickly learns the rules for propyl gallate. In food, FDA limits typically cap levels at 0.02% by weight of fat or oil content, which roughly mirrors European standards for most categories. Labels must spell out the additive, usually by its E number or its full name. Companies measure purity as part of their sourcing, often demanding at least 98% pure product based on dry weight. Particle size also matters in some applications—smaller sizes may blend more smoothly into fats or fine powders. Safety data sheets require prominent placement, and handling standards highlight dust inhalation as a risk, so companies invest in good ventilation and personal protective equipment in their operations.
Preparation Method
Industrial synthesis of propyl gallate usually starts with gallic acid, which itself comes from the pyrolysis of tannins. Reacting gallic acid with propanol in the presence of an acid catalyst triggers esterification. Manufacturers often use sulfuric acid for this step, followed by neutralization and recrystallization to clean up the final product. Filtration, drying, and particle sizing wrap up the process, yielding a consistent batch that meets purity and quality benchmarks. Some research teams experiment with greener or less energy-intensive processes, but major producers often stick with these proven steps for batch reliability and cost control.
Chemical Reactions & Modifications
The molecular structure of propyl gallate opens up several chemical paths, mainly through modification at the phenolic groups. These hydroxy sites respond to oxidative environments, scavenging radicals that drive rancidity. Researchers have dabbled in tweaking the ester group, swapping propyl for other alkyl chains to tailor solubility or antioxidant strength. Such modifications often help in adjusting the compound for non-food sectors, such as polymers or industrial lubricants. In food or pharma, less is more—most standards aim to keep the molecule unaltered to avoid regulatory headaches.
Synonyms & Product Names
Propyl gallate goes by a range of monikers: E310 in food coding, propyl 3,4,5-trihydroxybenzoate in technical texts, and sometimes simply gallate ester among chemists. Buyers might also see labels showing “Gallic acid propyl ester” or “n-propyl gallate.” In certain regions, the trade names PG or Gallaprop pop up among suppliers. The European Food Safety Authority and US FDA guidelines all point to the same molecule, helping global companies ensure consistency and avoid dangerous mix-ups in international trade.
Safety & Operational Standards
Regulators keep propyl gallate under careful watch. While studies since the 1950s have generally shown it to be safe in modest amounts, some evidence links high doses to allergic reactions or limited enzyme inhibition in sensitive individuals. Operations need good ventilation, dust control, and training for safe handling. Companies must also run regular checks against batch contamination, with ISO 22000 and similar standards providing guidance. Responses to spills or personnel exposure focus on basic hygiene and prompt medical monitoring.
Application Area
Propyl gallate steps up in several industries, but food and beverages remain its mainstage. Snack foods, chewing gum, fried products, and bakery mixes owe much of their shelf life to its antioxidant punch. Medicines and cosmetic creams count on its ability to keep oily and fat-based formulations from going off. Beyond the grocery aisle, propyl gallate turns up in certain lubricants, adhesives, and even photographic solutions, where oxidation could compromise performance or storage. It often teams up with other stabilizers—BHA and BHT are common partners to cover a wider range of free radicals.
Research & Development
Modern research continues to probe deeper into propyl gallate’s potential. Teams track its performance against new preservative challenges, like omega-3-rich foods that spoil easily or natural health blends with fewer synthetic additives. Analytical chemistry labs employ improved detection techniques—HPLC and mass spectrometry now spot even trace breakdown compounds, helping set stricter standards. Some research tackles the search for plant-based routes to propyl gallate production, which could answer the call for sustainable and eco-friendly sourcing.
Toxicity Research
Scientific literature reports low acute toxicity for propyl gallate based on animal models, with standard dietary use showing wide margins of safety. Chronic exposure still attracts scrutiny, as some animal studies point to possible liver and kidney impacts at very high levels, well beyond those used in industrial or food settings. Regulatory reviews routinely set acceptable daily intake (ADI) based on this data—currently, the figure hovers near 0.2 mg/kg of body weight per day, a threshold few diets approach. Food allergy advocates urge continued testing, since sensitive individuals may react even at low doses.
Future Prospects
Demand for better shelf life, reduced spoilage, and global food access keeps propyl gallate in the innovation mix. New consumer values, including non-synthetic and clean-label demands, challenge manufacturers to revisit uses or tweak production methods. Bio-based variations, alternate synthesis, and techniques to minimize residues reflect the ongoing dialogue between industry, regulators, and health advocates. As the push grows for transparent and sustainable food systems, propyl gallate finds itself at a crossroads—serving a critical function, but also inviting deeper investigation into alternatives and optimized use.
What is Propyl Gallate used for?
What Propyl Gallate Does in Food
Propyl gallate shows up in packaged foods more than most shoppers realize. You’ll find it listed on ingredients panels for products like chewing gum, margarine, processed meats, and snack foods. The main reason manufacturers add it comes down to keeping fats from going bad too soon. Fats and oils spoil because oxygen breaks them down, so food makers bring in antioxidants like propyl gallate to slow this chain reaction. Without preservatives like this, familiar brands of baked goods or nut butters could pick up strange flavors long before their “best by” date.
Why the Food Industry Leans on Propyl Gallate
This additive started popping up more after researchers realized how oxygen speeds up spoilage. Back then, choices for preserving shelf life were pretty limited. BHA and BHT have been around for a while, but propyl gallate stepped in because it can work alongside those other compounds. It isn’t just for slowing spoilage, though—sometimes it also helps color hold steady in foods under fluorescent lights, especially when combined with ascorbic acid (vitamin C).
Safety and Health Considerations
Most studies in Europe and the United States suggest propyl gallate is safe in small amounts. Regulatory agencies like the FDA set daily intake limits based on decades of animal studies and any human data they can collect. Some people worry about synthetic additives, and these worries aren’t out of nowhere. Whenever researchers study antioxidants like propyl gallate, they keep an eye out for allergies or links to other health troubles. There aren’t any warnings for most healthy adults, but those with certain conditions—like people with asthma, particularly when paired with aspirin sensitivity—should pay closer attention to food labels.
Challenges of Avoiding Additives
A lot of folks want fewer artificial ingredients in their food. Eating habits change over time, and people look for honest labeling or try recipes at home to dodge preservatives. I’ve had friends who go out of their way at the grocery store, turning every box and bag around, trying to spot the smallest print. It takes time and effort, and sometimes that means fewer choices or higher prices. The science says these additives keep food safe longer and help prevent waste, and it’s not so easy for smaller companies to skip them because fresh foods spoil quickly. Eliminating all preservatives would mean more trips to the store and probably tossing out unused food.
Alternatives and Future Solutions
Plant-based antioxidants like rosemary extract and green tea catch a lot of buzz these days. Some companies experiment with them, especially those wanting “clean label” appeal. The cost, availability, and possible taste changes hold others back. More research could help sort out which alternatives offer the same protection for foods, especially for keeping oils stable. Until new options become cheaper and more reliable, propyl gallate keeps its spot on ingredient lists, making that can of nuts or frozen pastry taste just like the one before it.
Why It Matters
Preservatives like propyl gallate mean less waste, safer foods, and more choices for busy people. For folks watching labels, it helps to know what’s in your food and to find brands that match your values. If you have questions or concerns about an ingredient, talking to a trusted dietitian or doctor makes sense. Food science isn’t perfect, but it keeps pushing toward better options for everyone’s table.
Is Propyl Gallate safe for consumption?
Looking at the Ingredient List
Scan the back of processed food packaging, and you might spot propyl gallate. This chemical acts as an antioxidant. It helps oils and fats in food keep fresh longer and delays the rancid taste many folks can't stand. Propyl gallate has turned up in everything from chewing gum to ready-to-eat microwave meals. The question catches more attention than ever: Should people feel comfortable eating products that use it?
History Meets Modern Science
Propyl gallate didn’t pop up yesterday. Food companies started using it after the 1940s, claiming it gives margarine, potato chips, and even cosmetics a longer shelf life. Scientists looked into its safety as foods changed and technology advanced. Health authorities in the United States, the UK, and the European Union have all reviewed it. The US Food and Drug Administration permits limited amounts. The European Food Safety Authority (EFSA) did a review in 2014, recommending a limit for how much children and adults consume.
Risks and Reality
Some who study these things wonder if long-term use next to other food additives brings hidden risks. Researchers dug through lab data, finding that at massive doses, propyl gallate triggered mild side effects in animals—things like skin irritation or respiratory problems. But these doses went far above what anyone usually gets from snacks or cakes. I always look for a common thread in daily life: Most people rarely hit these high levels. Food safety experts set the legal limits well under possible harm to keep a wide margin.
Cancer risk comes up in many online debates. Studies so far don’t link typical levels of propyl gallate in food to cancer or severe harm. Still, nobody serves up propyl gallate on its own for dinner. We eat it with dozens of other substances, so the full picture keeps growing. When looking at claims and panic, reading actual science reports clears the air. Groups like the National Institutes of Health list propyl gallate as “generally recognized as safe” in small doses.
Why It Matters
A big lesson shows up from recent diet studies: People rarely face problems from a single ingredient, but from eating too much processed food altogether. Propyl gallate rarely shows up in whole fruits, vegetables, or fresh meats. If someone snacks on highly processed foods at every meal, the preservatives, sodium, and sugar build up in ways the body doesn’t like. My own routine shifted once I started reading more about ingredients. I cut back on pre-packaged cookies and chips, not because I feared one chemical, but because fresh food gave me steady energy and fewer aches.
Better Choices for Everyday Life
People want food that lasts on the shelf and sits safely in their lunchboxes. Science and regulation help manage that balance. Still, personal habits shape long-term health more than any single ingredient. Focusing on real, recognizable foods works for most families. Parents filling grocery carts with more greens, grains, and whole proteins instead of boxed meals cut down on preservatives—without worrying about every compound by name. Propyl gallate stays low on my personal concern list, as long as my plate looks like something my grandparents would recognize.
What are the side effects of Propyl Gallate?
What Propyl Gallate Does in Food
Propyl gallate keeps food from going rancid. You see it listed on snack labels, some processed meats, and even in cosmetics or pharmaceuticals. Manufacturers picked it for shelf-life, not nutrition. The logic: more antioxidants mean longer-lasting products. But it’s not a natural ingredient in your everyday diet. Instead, it comes from gallic acid, which gets extracted from plants and then mixed with something like propanol in labs.
Common Side Effects People Notice
I remember tasting cookies as a kid that sometimes left my mouth tingling, not the good kind. Years later, I started reading up on additives and realized propyl gallate was one of the finishing touches in those cookies. For most people, side effects like skin itching, rash, or mild hives pop up first. Healthcare journals have reported occasional cases of swelling—lips or face—after eating foods with this additive. Asthma symptoms can flare up for some after exposure, particularly those who already deal with allergies or sensitive respiratory systems.
Propyl gallate might also be linked to some gut issues. Studies run by food safety authorities found that a minority of people get stomach pain or mild cramps after eating packaged foods with this substance. For those already prone to food sensitivities, this ingredient could make life trickier. One thing that caught my attention in several reports is the uncertainty of reactions: some folks notice nothing, others get a sour stomach or an itchy throat.
Concerns About Long-Term Exposure
Long-term effects aren’t as easy to nail down. Lab studies on animals raise eyebrows. High doses led to liver and kidney changes, raising obvious questions about chronic intake. Some research from international food safety groups called out possible hormonal effects, since propyl gallate acts weakly like a disruptor in certain tests. Epidemiologists keep asking for more human data before deciding what counts as “safe enough” for a lifetime of small-dose exposure.
Cancer research gives a mixed message. Older reports from the World Health Organization mentioned no clear link between human cancer and propyl gallate, but they didn’t rule out concerns about high levels in rodent trials. I trust toxicologists who say eating occasional snacks with this additive probably won’t tip the scales by itself, yet they also highlight how real risks come with steady, daily exposure—especially in kids.
Ways to Lower Your Risk
Cutting back sounds simple, but it helps to grow the habit of reading ingredient lists. Many companies add propyl gallate alongside BHA or BHT, which means you’re getting a mix of antioxidants, not just one. Swapping out processed or packaged foods for fresher options limits your intake. If you do have allergies or asthma, or you’re a parent managing these conditions for your kids, talking with your doctor before trying foods with unfamiliar additives pays off. The medical community has flagged propyl gallate as a possible trigger for people with pre-existing conditions.
I’m in favor of more transparent food labels and ongoing reviews of old additives. The science isn’t locked in stone yet, and as food technology rolls forward, safer substitutes keep showing up. Every trip through the grocery store is a choice—knowing what you put in your cart matters, especially with additives like propyl gallate.
Is Propyl Gallate natural or synthetic?
Digging Into the Source of Propyl Gallate
Walking through a grocery store aisle, I often pick up food products and scan the ingredients. Propyl gallate pops up on labels for different packaged snacks, vegetable oils, and even cosmetics. At first glance, the name sounds a bit mysterious, almost like something straight from a chemistry lab. So the question crosses my mind: is propyl gallate something natural, or has it been whipped up completely by scientists?
Where Propyl Gallate Comes From
Most additives with names ending in "-ate" usually make people think of synthetic chemicals. Propyl gallate is made by reacting gallic acid—found in plants like oak bark and green tea leaves—with propanol, which is a type of alcohol. Gallic acid occurs naturally in berries, green tea, and some other foods. But there’s a catch: the process that makes propyl gallate on a commercial scale usually takes place in a lab. Manufacturers combine these ingredients in controlled settings, using equipment and processes not found in the wild.
That means the propyl gallate added to packaged food and cosmetics isn’t harvested directly from nature. It’s synthesized, even though one of its main components starts out natural. The finished product doesn’t show up in nature in the same form. This draws a clear line between "derived from" nature and "found untouched" in nature. The FDA has classified propyl gallate as "generally recognized as safe" in specific quantities, but most health authorities identify it as a synthetic antioxidant.
Why Propyl Gallate Raises Questions
I like to know what I’m eating and putting on my skin. Many people seek out natural ingredients, hoping to sidestep mysterious or synthetic additives. So it makes sense to want that clear distinction. Years ago, shopping at farmers markets and seeing food without a long list of preservatives, I felt more peace of mind. There's something about processed foods with extra chemicals that leads to uncertainty, even when regulators say the chemicals are safe.
Propyl gallate’s purpose is to keep oils and fats in food from going rancid. It keeps potato chips crisp, helps grain bars last for months, and plays a role in slowing spoilage in beauty products. Without an antioxidant like this, manufacturers would deal with more food waste and shorter shelf lives. Food would be tossed sooner, which isn’t ideal for anyone involved in production or distribution.
Looking for Better Approaches
People value transparency, and this is where clearer labeling helps. If a component is synthetic, there shouldn’t be vague wording. Truthful information, especially about production and sources, builds trust. Small brands have already started leading the charge by either dropping synthetic antioxidants or providing more detail about ingredient origins. Some have switched to mixing in rosemary extract or vitamin E, both natural antioxidants, instead of synthetic ones. While these may not keep foods fresh quite as long, many shoppers decide they prefer the trade-off.
Research keeps moving, too. Food scientists experiment with natural extracts and combinations, trying to match the protection offered by synthetic preservatives. There’s progress, and with demand on the rise for food made with straightforward, easy-to-understand ingredients, the push for safer, more natural alternatives will only gain ground. As someone who makes choices at the checkout, I want food companies to step up and let us know where our additives really come from.
What products commonly contain Propyl Gallate?
Getting Familiar with Propyl Gallate
Seeing unfamiliar words pop up on ingredient lists can be confusing. Propyl gallate shows up in items at the grocery store and in places you might not expect. Used as an antioxidant, it keeps oils and fats from breaking down and turning rancid, helping foods last longer and stay safe to eat. But foods are not the only things touched by this additive—propyl gallate slips into personal care products, cosmetics, and even pharmaceuticals.
Popular Processed Foods
Anyone eating store-bought baked goods, chewing gum, or chowing down on snack mixes is likely ingesting propyl gallate. It usually hides in items that count on shelf life—think potato chips, breakfast cereals, cake mixes, and instant soups. Margarine, lard, and certain cooking oils often rely on propyl gallate to prevent bad flavors and smells. Even nut butters and some meat products turn to this compound to stay fresh on the shelf.
Food manufacturers sometimes pair propyl gallate with BHA and BHT. The trio teams up inside packaging to keep foods stable. Reading food labels makes it clear: propyl gallate is usually listed toward the end of the ingredients, often next to these antioxidant partners.
Cosmetics and Skincare
Beyond your kitchen, propyl gallate protects products that face exposure to air and heat. Lipsticks, moisturizers, sunscreens, deodorants, and shampoos may contain it, especially if they use plant oils or animal fats. These oils break down over time, so companies add propyl gallate to keep customer favorites smelling and looking like new until used up.
Personal care products don’t last forever, and many only reveal this additive on the package’s tiny ingredient list. If you deal with sensitive skin or allergies, checking those lists becomes more important. Dermatologists often recommend this for those who get rashes or irritation from cosmetics or lotions.
Medicines and Dietary Supplements
Vitamins and drugs stocked behind the pharmacy counter may contain trace amounts of propyl gallate. It helps keep fat-based medications from spoiling. Gelatin capsules, softgels, and multivitamins sometimes depend on propyl gallate to hold their potency. Even over-the-counter ointments and creams may turn to this additive for stability, especially in products that combine several ingredients or rely on oils.
Questions About Long-Term Effects
Questions about food additives swirl around safety and health—propyl gallate is no exception. Animal studies have led scientists to investigate links to allergies and possible hormone effects. The FDA and other regulators set strict limits, but long lists of additives on packages cause shoppers to take a second look.
People can reduce their intake by focusing on fresh ingredients and home-cooked meals. Small steps like reading ingredient lists or choosing products labeled “additive free” help control what ends up in the body. In personal care, seeking out natural, unscented, or preservative-free alternatives gives more options to folks worried about skin or health reactions.
A Bigger Conversation
Propyl gallate has played a role in modern packaging and food safety, stretching expiration dates for busy households. But that convenience comes with more questions than answers for some shoppers. Manufacturers see the value in shelf life, and many consumers value it too. People who want to cut down on propyl gallate will find it takes effort—it’s woven into much of what fills supermarket aisles and bathroom cabinets. Still, with more attention on labels and transparent sourcing, the conversation about what goes into our favorite products grows stronger every year.
| Names | |
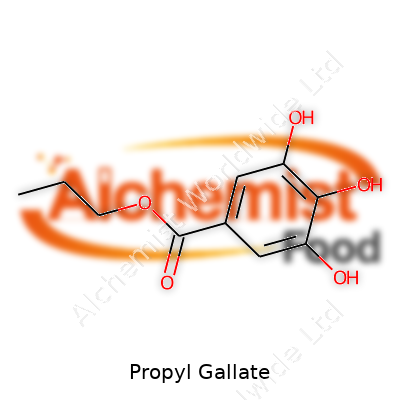
| Preferred IUPAC name | Propyl 3,4,5-trihydroxybenzoate |
| Other names |
Propyl 3,4,5-trihydroxybenzoate E310 n-Propyl gallate n-Propyl 3,4,5-trihydroxybenzoate |
| Pronunciation | /ˈproʊpɪl ˈɡæleɪt/ |
| Preferred IUPAC name | propyl 3,4,5-trihydroxybenzoate |
| Other names |
Propyl 3,4,5-trihydroxybenzoate E310 n-Propyl gallate Propylester kyseliny galove Propyl gallate (food additive) |
| Pronunciation | /ˈproʊ.pɪl ˈɡæl.eɪt/ |
| Identifiers | |
| CAS Number | 121-79-9 |
| Beilstein Reference | 136898 |
| ChEBI | CHEBI:32053 |
| ChEMBL | CHEMBL1409 |
| ChemSpider | 13907 |
| DrugBank | DB03817 |
| ECHA InfoCard | 100.009.165 |
| EC Number | EC 202-307-7 |
| Gmelin Reference | 8832 |
| KEGG | C01227 |
| MeSH | D011376 |
| PubChem CID | 4947 |
| RTECS number | WL5078000 |
| UNII | FD64TTP0ZX |
| UN number | UN3082 |
| CAS Number | 121-79-9 |
| Beilstein Reference | 1361577 |
| ChEBI | CHEBI:32050 |
| ChEMBL | CHEMBL1409 |
| ChemSpider | 5377 |
| DrugBank | DB03817 |
| ECHA InfoCard | 100.007.286 |
| EC Number | 3.4.21.112 |
| Gmelin Reference | 7420 |
| KEGG | C10621 |
| MeSH | D011376 |
| PubChem CID | 4947 |
| RTECS number | WL5075000 |
| UNII | FZ989GH94E |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID3020662 |
| Properties | |
| Chemical formula | C10H12O5 |
| Molar mass | 212.21 g/mol |
| Appearance | White to almost white crystalline powder |
| Odor | Odorless |
| Density | 1.2 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.7 |
| Vapor pressure | <0.1 hPa (20 °C) |
| Acidity (pKa) | 8.35 |
| Basicity (pKb) | 11.89 |
| Magnetic susceptibility (χ) | -7.86e-6 cm³/mol |
| Refractive index (nD) | 1.451 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.64 D |
| Chemical formula | C10H12O5 |
| Molar mass | 212.21 g/mol |
| Appearance | White to practically white crystalline powder |
| Odor | Odorless |
| Density | 1.2 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.7 |
| Vapor pressure | <1 mm Hg (20°C) |
| Acidity (pKa) | 8.2 |
| Basicity (pKb) | 8.08 |
| Magnetic susceptibility (χ) | -65.0e-6 cm³/mol |
| Refractive index (nD) | 1.451 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.69 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 247.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1046.34 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6014 kJ/mol |
| Std molar entropy (S⦵298) | 228.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1078.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6595 kJ/mol |
| Pharmacology | |
| ATC code | A01AD12 |
| ATC code | A01AD18 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause allergic skin reaction. |
| GHS labelling | GHS02, GHS07, Warning, H319, H335 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302, H317 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| Flash point | 170°C |
| Autoignition temperature | 410°C |
| Lethal dose or concentration | LD50 (Rat, oral): 4,800 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 3800 mg/kg |
| NIOSH | WN4725000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 0.0025% |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause allergic skin reaction. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H317 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0- درمیان |
| Flash point | 150°C |
| Autoignition temperature | 410°C (770°F) |
| Lethal dose or concentration | LD50 (oral, rat): 4,800 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Propyl Gallate: 1980 mg/kg (rat, oral) |
| NIOSH | NT8050000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 0.7 mg/kg bw |
| Related compounds | |
| Related compounds |
Gallic acid Methyl gallate Ethyl gallate Octyl gallate |
| Related compounds |
Gallic acid Ethyl gallate Methyl gallate Octyl gallate Lauryl gallate Tannic acid |

