Potassium Sorbate: Deep Dive into a Food Preservative
Historical Pathways
Potassium sorbate stepped into the spotlight back in the late 1800s, surfacing much later as a food preservative after chemists learned to extract sorbic acid from the berries of mountain ash trees. By the middle of the 20th century, food and beverage industries turned to potassium sorbate because of its effectiveness against mold, yeast, and fungi—especially as processed foods became a household staple. Regulatory bodies, like the FDA, reviewed its use through decades of studies and saw value in both ensuring food safety and extending shelf life. I’ve seen manufacturers embrace it for practicality and consistency, something that has reflected in the supermarket aisles for generations.
What Is Potassium Sorbate?
Potassium sorbate presents itself as a white, granular, or powder-like material with a faint odor. Chemically, it’s the potassium salt of sorbic acid. Its E number, E202, shows up on labels around the world—from dried fruits and baked goods to cheese and wine. It’s water-soluble, blending quickly into food products, which allows for a flexible application across varying food and industrial processes. The key appeal lies in its ability to lengthen shelf life without contributing any flavor or odor that consumers could pick up.
Physical and Chemical Properties
Potassium sorbate features a molecular formula of C6H7KO2 and a molecular weight of about 150 g/mol. It dissolves readily in water, at around 139 g/L at room temperature. Melting point hovers near 270°C (when decomposing), and it is stable under normal temperature and storage conditions, but starts decomposing in acidic environments below pH 4. It works best in pH ranges below 6.5, making it a go-to for acidic foods and drinks. On the periodic table, it stands out as a synthetic additive that remains stable through packaging, transportation, and storage.
Technical Specifications and Labeling
Manufacturers generate potassium sorbate to meet detailed specifications such as food-grade purity, low moisture content, and limited impurities. Regulatory authorities, including US FDA and European Food Safety Authority (EFSA), require that products carry clear labeling with either the full name or E202. In practice, food packages must reflect usage amounts, and technical datasheets give details on particle size, solubility profiles, and recommended concentrations—often up to 0.1% in foodstuffs. Most countries strictly limit how much can be added, as part of ensuring consumer safety.
Making Potassium Sorbate
The preparation often starts by neutralizing sorbic acid with potassium hydroxide or carbonate. As someone who has worked in industrial settings, I’ve watched this chemistry play out in large reaction tanks, managed under temperature control to prevent unwanted byproducts. Once the reaction finishes, crystallized potassium sorbate is separated, washed, and dried, giving rise to the final powder or granular product. Consistency in manufacturing standards remains crucial here, as off-spec material can raise safety, efficacy, and economic concerns through the value chain.
Chemical Reactions and Modifications
Potassium sorbate acts as a mild, unsaturated carboxylic acid salt. In acidic setups, it can convert back to sorbic acid. As a double bond-rich molecule, it is sensitive to strong oxidizers or heat, causing it to break down and lose its antimicrobial grip. Food technologists sometimes seek derivatives with similar structures to stretch uses in non-food preservation or explore shelf-life extension under harsher conditions. Efforts to tweak its structure, though, mostly run into functional and cost barriers that keep the classic version in use.
Synonyms and Product Names
In ingredient lists, one sees potassium sorbate under the names E202, sorbistat-K, or simply sorbate. European and Asian markets might use their own trade names depending on the chemical supplier. A few packaging materials or safety documents note it simply as the potassium salt of hexadienoic acid. Consumers rarely know these scientific names while noticing only the preservative note in the fine print.
Safety and Operational Standards
Every batch must pass strict quality audits before heading from plant to supermarket. Good Manufacturing Practices (GMP) and Hazard Analysis and Critical Control Points (HACCP) protocols remain non-negotiable. Facilities need solid air handling, closed production systems, and regular environment monitoring to catch particulate leaks or moisture intrusion. Product labels require correct lot marking and expiration dating, with periodic in-house or third-party testing for contaminants such as heavy metals and pesticide residues. Regulatory agencies provide oversight and conduct audits, working to shield public health from lapses.
Main Application Areas
Potassium sorbate turns up mostly in food processing—think cheese, yogurt, wine, soft drinks, dried sausage, baked goods, and fruit snacks. In my experience with food labs, bakers depend on it to keep mold out of cakes, while wineries use it to keep bottles from popping their corks. Pharmaceutical firms look to potassium sorbate for topical creams or liquid medications to slow spoilage without sensitizing skin. Cosmetics get in on the action too, using it to fend off bacteria and mold in lotion bottles, powders, and wipes, especially those with high water content.
Research and Development
Researchers continue to study new delivery techniques and synergistic preservatives. Some studies target biofilm formation by yeast or bacteria, trying to fine-tune how potassium sorbate works alongside natural extracts, vinegars, or low-pH flavoring agents. In the last decade, lab teams have published findings around encapsulation methods—maybe embedding potassium sorbate in edible films or coatings—reducing direct contact and possibly optimizing effectiveness over longer shelf times.
Toxicity and Health Research
Extensive toxicology studies chalk up potassium sorbate as among the safest food preservatives at permitted levels. The WHO and other authorities point to low acute toxicity and a track record of safe consumption in humans. In rare cases, sensitive individuals report slight skin or eye irritation—more an occupational hazard than a consumer one. Studies have dug into its metabolism, showing that the body breaks it down and excretes it through natural pathways. Long-term feeding studies in animals have not uncovered cancer or significant chronic side effects. Regulatory updates continue as new scientific tools sharpen the picture.
Future Outlook
Food safety and health trends may pressure sectors to rethink reliance on chemical preservatives. That said, potassium sorbate’s legacy and safety profile keep it on ingredient lists around the world. New research focuses on precision applications—whether reducing overall use through smarter blends, improving residue detection, or combining it with natural antimicrobial agents. Seasoned product developers know the market wants freshness and safety, and potassium sorbate still sits in that sweet spot, even as "clean label" movements gather steam and drive more innovation.
What is potassium sorbate used for?
Why Potassium Sorbate Shows Up Everywhere
Potassium sorbate pops up on ingredient lists all over grocery shelves. It’s not a name I grew up hearing at the family dinner table, but learning more about what’s behind food labels matters to anyone who cares about what goes into their body. Producers reach for potassium sorbate because it keeps food fresh longer. Nobody picks up a yogurt expecting green fuzz or cracks open a bottle of soda looking for bubbles and sourness that’s way off. Potassium sorbate slows down molds, yeast, and certain bacteria, giving both manufacturers and shoppers a little more security in each product’s shelf life.
Where You’ll Find It
Walk through a supermarket, and you’ll spot potassium sorbate across aisles. Cheese, dried fruit, baked goods, jams, syrups, wine, soft drinks, and even supplements often rely on it. In wine, it stops post-bottling fermentation, so people get a stable flavor instead of surprises. In the bakery section, it helps take the edge off spoilage in muffins and tortillas, keeping them looking and smelling as they should. It may even show up in cosmetics as a preservative for lotions and shampoos since no one asks for moldy moisturizer.
Is It Safe?
People worry about chemical names, and for good reason. Knowing what lands in our bodies is important. Potassium sorbate comes from sorbic acid, a compound originally found in the berries of the rowan tree. Many regulatory bodies, including the FDA and the European Food Safety Authority, have studied it and allowed its use in food under strict limits. These authorities base their decisions on evidence, not just tradition. That kind of oversight matters for building trust over time.
The acceptable daily intake sits at about 25 mg per kilogram of body weight. Research hasn’t turned up links to cancer or toxins at normal amounts used in food, but anyone can react to anything, and people with allergies or sensitivities sometimes report skin irritation—especially if a product sits on the face or scalp.
Why Preservatives Aren’t All Bad
Nobody wants to waste food. Preservatives like potassium sorbate help reduce spoilage and foodborne illness, which hits hardest on families without extra grocery money to spare. In my own kitchen, moldy leftovers mean frustration and a hit on the wallet. When used as intended, a preservative can support both food safety and availability. That makes a difference, especially in places cut off from steady deliveries of fresh food.
What Could Make Preservative Use Better?
Preservatives keep food on shelves, but shoppers want short ingredient lists and clarity about what’s inside. Transparency helps: clear labeling in language people understand, with support for those who want to avoid certain additives for health reasons or allergies. Using potassium sorbate responsibly, in small concentrations proven safe by science, shows respect for consumers.
Food science never stands still, and companies test new ways to keep food clean and safe, like natural extracts from rosemary or green tea. Until enough evidence stacks up for those alternatives, potassium sorbate remains a useful tool in the food industry’s kit. Keeping a close watch on how much ends up in products, tracking research, and listening to customer concerns will keep this preservative in its proper place: useful, but not invisible.
Is potassium sorbate safe to eat?
What Potassium Sorbate Does in Food
Potassium sorbate pops up in a lot of foods lining supermarket shelves. Think about cheese, yogurt, dried fruits, and even some baked goods. The job it does isn’t glamorous, but it protects foods from molds and yeast, stretching their shelf life. Manufacturers use it in small amounts, keeping products stable without overpowering the taste or texture. For a long time, people thought homemade bread always tasted better than the kind from the store. Growing up, I noticed store bread never seemed to mold, which made me wonder what got put in there. Potassium sorbate played a big part in keeping those loaves fresh.
Experts Weigh In
The Food and Drug Administration on one side of the Atlantic, and the European Food Safety Authority on the other, both called potassium sorbate “generally recognized as safe” or GRAS. Human and animal studies looked at how the body handles this preservative. Most of it leaves the body through urine. Long-term studies show no bump in cancer rates or other serious health issues tied to normal use. That puts it in a different league than some other preservatives, such as nitrates.
Why People Worry
People feel uneasy about additives because they don’t recognize what the label says. Potassium sorbate sounds more like something in a chemistry kit than a kitchen. Social media spreads fear, and personal blogs amplify confusion by claiming links to allergies, gene damage, or even cancer. Studies failed to confirm those alarming claims at the normal doses found in food. Some individuals show mild reactions, often sun-like rashes or itchiness, usually from cosmetics that use higher concentrations than any food would. Folks who have especially sensitive skin, or deal with chronic hives, might want to pay close attention to their own reactions.
Eating Too Much of a Good Thing
Our diets have shifted toward packaged foods, and that’s pushed up how much preservative the average person takes in. The World Health Organization set what is called an “acceptable daily intake” for potassium sorbate. Most people fall far short of that line. The typical diet rarely comes close, unless someone lives on nothing but processed foods. Over decades, I’ve met plenty of people convinced that “processed” equals “bad.” The story isn’t so simple. Preservatives stop food from spoiling, which also keeps many people from eating something dangerous or getting sick.
Looking Ahead: Balance Matters
Our biggest risk doesn’t come from potassium sorbate itself, but from eating heavily processed foods all day, every day. A diet full of fresh fruits, vegetables, and home-cooked meals cuts down the intake of any food additive without much effort. Reading labels and cooking at home more often gives control back to families. Food preservation has a place, especially for folks without easy access to fresh produce or those who can't shop often.
Small Decisions Add Up
Potassium sorbate keeps food safe and fresh, but moderation always wins. People worried about additives should look past scare tactics and ask tough questions. They can trust established scientific bodies more than viral claims. Making a few more meals at home and eating whole foods most days keeps preservatives and their risks in check. It's not about hunting for “chemical-free” shopping carts—just keeping a thoughtful balance.
Does potassium sorbate have any side effects?
What Is Potassium Sorbate?
Potassium sorbate gets used a lot to help food products last longer. It's a preservative made in labs, not something you stumble across at the farmer’s market. It limits mold, yeast, and some bacteria from taking over everything from dried fruit to bottled lemon juice. Some cheeses and wines also rely on it for freshness. Food makers trust it because it’s cheap, tasteless, and does the job without fuss.
Why People Care About Side Effects
Eating less processed food has gotten popular as folks learn more about what’s in their kitchens. Preservatives have taken some heat. Potassium sorbate often pops up in these conversations. Health sites and blogs buzz with claims around it—some accurate, some wild. I’ve run into confusion myself when standing in a grocery aisle, squinting at an ingredients list. Is this stuff safe, or will it cause problems?
What the Science Says
Studies say potassium sorbate does its job with little drama for most people. The U.S. Food and Drug Administration and the European Food Safety Authority both say it’s safe in regulated amounts. It doesn’t hang around in your body for long; the body breaks it down into water and carbon dioxide after you eat it.
Side effects don’t show up often. Still, they’re not unheard of. Some people have reported mild reactions. If you use it in cosmetics or lotions, there’s a small chance of skin irritation. For food, it’s even less likely. On rare occasions, people have noticed hives, itching, or trouble breathing after eating foods with potassium sorbate. Those cases usually come from people who already have allergies to preservatives or other food-related sensitivities.
Animal studies tested high amounts—far more than you’d ever eat—without causing big health issues. No strong links connect potassium sorbate to cancer, gene damage, or major organ problems in regular use. Rumors about serious illness haven’t held up in quality scientific reviews so far.
Why It Matters
Even if most folks never notice a side effect, awareness is key. The food industry uses a lot of additives, and knowing your own body’s quirks always helps. I have friends who love reading labels and avoid certain preservatives just because it gives them peace of mind. Food allergies have taught people to stay curious. A handful of folks do get rashes or stomach issues and benefit from avoiding certain preservatives, potassium sorbate included.
One thing to watch: potassium sorbate works best in acidic foods. So, foods that use it might contain other acids or preservatives too. Sometimes it’s hard to know which ingredient is actually causing a problem, especially with allergies or food intolerances that already complicate meal choices.
What Can Be Done?
Staying informed stands as one of the best ways to protect yourself. Reading food labels and looking up unknown ingredients before buying something new can help. If someone in your family starts sneezing or itching after a new snack, write down the ingredients and see if patterns show up. When in doubt, doctors and allergy specialists can run tests for specific sensitivities.
Food makers might consider clear labeling and transparency. Sharing not just ingredient lists but also why certain chemicals are used could build trust. Consumers benefit from having enough info to make a call that works for their bodies and their beliefs about food.
Final Thought
Potassium sorbate plays a real role in making our pantries more convenient. Most people experience no trouble, but anyone can take steps to avoid additives that bother them. Take time to know your body and speak up if something doesn’t feel right. That’s often the best way forward, no matter what’s on the label.
Is potassium sorbate natural or synthetic?
Where Does Potassium Sorbate Come From?
Walk into almost any grocery store and read the labels on foods like shredded cheese, dried fruit, or baked goods. Chances are high you’ll spot potassium sorbate somewhere in the list of ingredients. The name might sound like it could belong in a chemistry lab, but the real story sits in the way manufacturers make it and where it begins.
Potassium sorbate starts out with a molecule called sorbic acid. People first got sorbic acid from the unassuming berries of the rowan tree, but picking fruit doesn’t yield enough for food production needs. Companies now create sorbic acid by combining crotonaldehyde and ketene, two compounds that don’t exist in nature but are made in industrial settings. Combine sorbic acid with potassium hydroxide and you’ve made potassium sorbate.
What Does “Natural” Really Mean?
If you ask different folks what “natural” means, you’ll hear a handful of answers. Some say the label belongs on anything that started with a plant or mineral. Others claim the method matters — if it takes a lab and a string of chemical reactions, they leave the “natural” label out. In the United States, the FDA doesn’t have a tight definition for what counts as “natural,” but typically, anything that goes through heavy chemical processing lands in the synthetic group.
Potassium sorbate doesn’t appear in nature in big quantities. No farmer grows it, nobody mines it, and you won’t dig it up in your backyard. Even though it shares roots with a natural acid found in fruit, the process to make enough for the grocery market uses synthetic steps. Food safety authorities and scientists call it synthetic.
How Safe Is Potassium Sorbate?
People often see “synthetic” and start to worry. My own family once cut out foods based on unfamiliar chemical names, thinking we’d dodge some hidden danger. I later learned that potassium sorbate does something pretty important — it stops mold, bacteria, and yeast from taking over packaged foods. That work makes food safer, stretches shelf life, and helps families avoid waste.
Researchers have run dozens of studies on potassium sorbate. The FDA and the European Food Safety Authority both reviewed the evidence and found that, in the amounts used in food, potassium sorbate doesn’t pile up in the body or act toxic. Sensitive folks can experience mild effects if they eat a huge amount, but the quantities in what lands on the average dinner table don’t spark problems for most people.
Is the Synthetic Label a Dealbreaker?
Some shoppers swear by all-natural products, making the “synthetic” label enough reason to put an item back on the shelf. But purity on its own doesn’t define health. Plenty of natural substances, like certain mushrooms or plants, turn out dangerous. On the other hand, preservatives like potassium sorbate help protect millions from foodborne illness, allowing bread, cheese, and even yogurts to travel long distances or store safely at home.
People looking for alternatives can seek out foods preserved with vinegar, salt, or refrigeration, but those options sometimes change how food tastes, feels, or lasts. If transparency matters, staying aware of ingredient sources gets easier as companies respond to customers asking smarter questions. I often remind friends: reading labels sparks better choices, but it’s the knowledge behind those words that leads to better decisions, not just the length or origin of the ingredient list.
Can potassium sorbate cause allergies?
Potassium Sorbate’s Place in Food and Home Life
Potassium sorbate lands in a lot of pantries and kitchens. It keeps bread fresh, preserves the tartness in yogurt, and even helps dried fruit keep its glossy look. Folks count on it because it slows mold and yeast, stretching the shelf life of everything from cheese to salad dressing. The U.S. Food and Drug Administration puts it on the “Generally Recognized as Safe” list for most people, and for decades, these yellowish granules have kept food losses down across the grocery aisle.
Why Allergies Grab Our Attention
The issue of allergies creeps up with any food additive. Once in a while, people end up with hives, a rash, or an upset stomach after eating something with potassium sorbate. Medical studies show that the chance of a true allergic reaction looks rare. A review in the journal Contact Dermatitis found very few cases of skin rashes in people touching strong solutions, usually in cosmetics and not food. The skin does react to a lot of things, and sorbates sometimes pop up in patch tests, but the numbers stay low.
Eating the preservative seems even less likely to cause trouble. Most of it breaks down as the body digests the food. A person would have to eat a lot more than what a regular meal contains before seeing any symptoms. The FDA allows levels up to 0.1% in food, and most manufacturers aim for less. According to the European Food Safety Authority, daily potassium sorbate intake among the general population rarely even comes close to the acceptable daily intake.
Outliers and Personal Experiences
In real life, allergic reactions run the spectrum. I remember a neighbor who avoided anything with strange names on the ingredient list, especially with her son’s asthma and eczema. They learned to look out for potassium sorbate because one lotion brought out a rash. Yet in my own family, with food allergies and decades in restaurant kitchens, I’ve never seen a true allergy to it. For people already navigating nut or shellfish allergies, preservatives don’t seem to stir up the same worries at the doctor’s office.
Every so often, forums or support groups buzz about additives like sulfur dioxide or sodium benzoate. Potassium sorbate gets mentioned, but never as much as those older preservatives tied to breathing trouble or migraines. Most reactions turn out to stem from natural sources — berries, dairy, or spices in the dressing — and not the potassium sorbate itself.
Facts, Caution, and Straight Talk
Scientists keep studying food additives for a reason. Children, people with eczema, or anyone ultra-sensitive should take care with new lotions, creams, or foods. Reading labels helps, though potassium sorbate provides little reason for fear based on current evidence. Still, some would say that a cleaner ingredient list matters. If folks want to cut their risk, using fresh or minimally processed foods at home helps avoid most additives, potassium sorbate included.
Doctors do encourage parents to trust their instincts if a rash, gut issue, or bad bout of itching follows a meal. Even if science says the odds are tiny, personal comfort means something. In those rare cases, doctors can run allergy patch tests or food challenges under guidance. Local health agencies and Allergy & Asthma Foundations also offer lists of known allergens for reference.
Cutting Down on Preservatives: What Works?
Cooking more from scratch often gives the most peace of mind. Families who bake, ferment, or freeze at home rely less on shelf-stable goods. Grocery shoppers can look for certified organic labels, which restrict many preservatives. Institutions like Mayo Clinic and Harvard Health have published tips for reading food labels and spotting hidden additives. Apps now scan ingredient lists to flag preservatives, dyes, and common allergens.
In the end, most will never worry about potassium sorbate reactions. Still, news stories surfacing every now and then push shoppers to keep checking, asking questions, and looking out for each other. Staying aware gets easier with honest information, strong science, and a touch of commonsense skepticism.
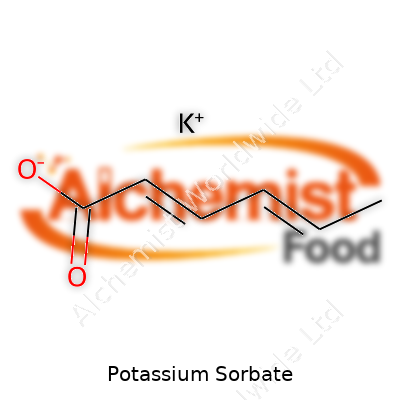
| Names | |
| Preferred IUPAC name | potassium (E,E)-hexa-2,4-dienoate |
| Other names |
Sorbistat-K E202 Potassium (E)-hexa-2,4-dienoate Potassium salt of sorbic acid Sorbic acid potassium salt |
| Pronunciation | /poʊˈtæsiəm ˈsɔːrbeɪt/ |
| Preferred IUPAC name | potassium (2E,4E)-hexa-2,4-dienoate |
| Other names |
Sorbic acid potassium salt E202 Potassium (E,E)-hexa-2,4-dienoate Sorbistat K Sorbistat |
| Pronunciation | /pəˈtæsiəm ˈsɔːrbeɪt/ |
| Identifiers | |
| CAS Number | 24634-61-5 |
| Beilstein Reference | 3592934 |
| ChEBI | CHEBI:53444 |
| ChEMBL | CHEMBL1409 |
| ChemSpider | 11753 |
| DrugBank | DB11097 |
| ECHA InfoCard | 100.022.329 |
| EC Number | 202-768-7 |
| Gmelin Reference | 135635 |
| KEGG | C07398 |
| MeSH | D011104 |
| PubChem CID | 23672387 |
| RTECS number | WSQ1886X0V |
| UNII | 1VPU26JZZ4 |
| UN number | “UN2811” |
| CAS Number | 24634-61-5 |
| Beilstein Reference | 3594971 |
| ChEBI | CHEBI:61047 |
| ChEMBL | CHEMBL1350 |
| ChemSpider | 10106 |
| DrugBank | DB11197 |
| ECHA InfoCard | 100.029.218 |
| EC Number | 202-768-7 |
| Gmelin Reference | 77038 |
| KEGG | C00200 |
| MeSH | D011110 |
| PubChem CID | 23668386 |
| RTECS number | WN6500000 |
| UNII | 1VPU26JZZ4 |
| UN number | UN1390 |
| Properties | |
| Chemical formula | C6H7KO2 |
| Molar mass | 150.22 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.363 g/cm3 |
| Solubility in water | 58.2 g/100 mL (20 °C) |
| log P | -1.27 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.76 |
| Basicity (pKb) | pKb = 10.23 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.536 |
| Dipole moment | 1.97 D |
| Chemical formula | C6H7KO2 |
| Molar mass | 150.22 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.363 g/cm³ |
| Solubility in water | 139 g/L (20 °C) |
| log P | -1.47 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.76 |
| Basicity (pKb) | pKb: 12.5 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.336 |
| Dipole moment | 1.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 375.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -971.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3665 kJ/mol |
| Std molar entropy (S⦵298) | 253.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −971.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4188.7 kJ/mol |
| Pharmacology | |
| ATC code | A07AA10 |
| ATC code | A07AX02 |
| Hazards | |
| Main hazards | May cause mild skin irritation. Dust may cause eye and respiratory irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "May cause respiratory irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 1, Instability: 0, Special: - |
| Autoignition temperature | 270°C (518°F) |
| Lethal dose or concentration | LD50 (oral, rat): 4920 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2,600 mg/kg (rat, oral) |
| NIOSH | RN3830 |
| PEL (Permissible) | PEL for Potassium Sorbate: Not established |
| REL (Recommended) | “Use GMP” |
| Main hazards | May cause eye, skin, and respiratory irritation |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 1, Instability: 0, Special: - |
| Autoignition temperature | > 450 °C |
| Lethal dose or concentration | LD50 (Rat, oral): 4920 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2,630 mg/kg (oral, rat) |
| NIOSH | WN2156000 |
| PEL (Permissible) | 300 mg/kg |
| REL (Recommended) | 300 mg/kg |
| Related compounds | |
| Related compounds |
Sorbic acid Sodium sorbate Calcium sorbate |
| Related compounds |
Sorbic acid Sodium sorbate Calcium sorbate |

