Potassium Permanganate: A Deep Dive
Historical Development
Potassium permanganate has been turning heads since the mid-1800s, ever since chemist Johann Rudolf Glauber first noticed the remarkable color changes during an experiment. In those early days, it was a real marvel—just watching deep violet crystals mix into water was enough to spark curiosity. Not long after, chemists figured out that this compound stood out for its power to tackle tough stains and odors. Markets for potassium permanganate took root in medicine and water treatment before expanding to everything from textile processing to specialty chemistry labs. For more than a century, it has played a key role across many industries because of its knack for powerful oxidation and disinfectant properties. Some older writings even mention its role as a universal antidote for some poisonings and as a staple for cleaning wounds and disinfecting tools, especially before antibiotics became common.
Product Overview
Potassium permanganate typically appears as bright purple or deep magenta crystals. Each batch tells the story of its origin, usually landing in industrial facilities, municipal waterworks, or emergency medical kits. The vivid crystal color comes from a unique electronic structure that easily accepts and donates electrons. In many countries, large quantities of potassium permanganate help keep water safe to drink, clean up factory effluents, and support specialized chemical processes. Even in its pure crystalline form, it's dependable because purity levels rarely dip below 99 percent. Commercial units supply it in airtight bottles, drums, or bags, giving every shipment a long shelf life—as long as moisture stays out of the container.
Physical & Chemical Properties
Potassium permanganate’s look isn’t easy to forget. It shows off a bold violet color, sometimes shading toward black, with long needle-shaped or prismatic crystals. With a melting point near 240°C, it keeps its shape at moderate temperatures. It stands out for being highly soluble in water, turning solutions a vibrant pink to purple depending on concentration. Chemically, potassium permanganate packs a strong oxidizing punch, which means it willingly snatches electrons from many organic and inorganic substances. That property makes it valuable in tough cleaning situations. Exposure to most fuels, glycerol, or concentrated acids can trigger fast and sometimes dangerous reactions, including fire or explosion. It doesn’t give off fumes or vapors in normal use, yet its active oxygen content (31%) makes it a favorite for reactions where precise oxidation is necessary.
Technical Specifications & Labeling
The best manufacturers of potassium permanganate keep their standards high. Each shipment lists potassium permanganate content, total impurities, chloride, sulfate, and iron content. Labels usually specify grade, manufacturer, batch code, and instructions for safe storage. UN number (1490) appears on packaging, with hazard pictograms highlighting risks. Safe handling details urge users to avoid contact with combustible material and keep out of reach from moisture or sunlight. Even with tight tolerances, regulators in many regions require regular sampling to spot contaminants or clumped crystals. Personal experience with these labels hammered home the importance of diligent handling, especially when the substance lands in hands unfamiliar with its risks.
Preparation Method
Manufacturing potassium permanganate starts with natural manganese dioxide, usually sourced from mined ores. Manganese dioxide reacts with hot potassium hydroxide and an oxidizer, most often air or potassium nitrate. The resulting intermediate, potassium manganate, then faces further oxidation—often using chlorine, ozone, or even additional air exposure—until deep purple permanganate emerges. Chemists carefully wash, filter, and crystallize the product, producing those unmistakable purple needles. Growing up near an industrial plant, the production process made it clear that managing waste streams and effluent required more than just chemistry—it took plenty of planning to keep sharp oxidizers out of waterways and off workers’ hands.
Chemical Reactions & Modifications
In chemical terms, potassium permanganate acts as a workhorse. It quickly oxidizes alcohols, aldehydes, phenols, and certain organic pollutants, changing color through a series of intermediate manganese compounds. It breaks down many pesticides, neutralizes hydrogen sulfide in water, and oxidizes iron and manganese for easier filtration. In organic labs, adding it into reaction mixtures lines up perfectly with breaking down tricky double bonds or cleaning up unsaturated fats. Sometimes, to avoid over-oxidation, chemists use buffering agents or add potassium permanganate dropwise to stay in control. That flexibility has made it a go-to in synthesis, disinfection, and troubleshooting stubborn chemistry challenges. Recently, new catalysts and modified supports have widened its reach in specialty oxidation reactions, letting researchers tune selectivity and efficiency without sacrificing safety.
Synonyms & Product Names
Potassium permanganate doesn’t go by just one name. Chemists might call it “Condy’s crystals,” a label swiped from pharmacist Henry Bollmann Condy who promoted its use for water purification. In catalogues, you’ll spot references like “permanganic acid, potassium salt” or simply “KMnO4.” Workers often abbreviate it as “KMnO4” in technical or shorthand notations, while older texts sometimes refer to it as “permananganate of potash.” Local vendors and distributors might brand it under trade names, tying its reputation to legacy uses in waterworks or textile houses. The many names reflect an audience that stretches from farmhands to forensic chemists.
Safety & Operational Standards
Potassium permanganate stands out as a strong oxidizer, which brings immediate safety challenges. Direct contact with skin, especially at high concentrations, causes irritation and brown stains that stick around for days. Breathing dust rarely happens under good practice, but it’s just as unpleasant as accidental ingestion, both of which require fast medical attention. Mixing potassium permanganate with fuels, acids, or organics can touch off explosive reactions, so every step in storage and handling means keeping it cool, dry, and away from fire hazards. Regulations require chemical-resistant gloves, safety glasses, and tight protocols for spills and cleanup. Many safety lessons get learned the hard way—one slipup with cleanup tools dampened with vinegar, for instance, can trigger a fire. Consistent staff training sticks as one of the most effective measures for long-term risk management, together with clear signage and controlled dispensing of the crystals.
Application Area
Potassium permanganate serves in fields where oxidation or disinfection get top billing. In water treatment plants, it strips out hydrogen sulfide, iron, manganese, and some organic contaminants, with operators keenly watching for color changes as a marker for complete reaction. Hospitals and field clinics lean on potassium permanganate solutions for wound irrigation and certain skin conditions, especially where more advanced medications don’t reach. Textile plants put it to use “bleaching” denim and fixing dyes. Across mining and metallurgy, it cleans up effluent streams and prepares ores for further refining. Crime labs and forensics officers use it to reveal fingerprints and highlight traces of blood invisible to the naked eye. Some emergency response teams include potassium permanganate for quick water purification in disaster relief kits, reflecting its versatility outside the laboratory setting.
Research & Development
Scientific research continues to look for more sustainable and safer ways to harness potassium permanganate’s oxidizing talent. Environmental scientists test new approaches to degrade emerging contaminants in water, like pharmaceuticals and personal care products. Synthesis teams experiment with different supports or blending agents to target only the most stubborn materials or pollutants, reducing unwanted byproducts. I’ve watched interest surge in nanostructured composites with embedded permanganate for rapid, on-site remediation of chemical spills. Tech companies explore designs for automated dosing systems, letting water plants keep tighter control over exposures and minimizing worker risk. Medical researchers still revisit old case studies to pin down safe, effective topical applications, balancing antimicrobial activity against the risk of tissue staining or irritation.
Toxicity Research
The toxic profile of potassium permanganate has been investigated for decades, and real-world incidents have shaped much of what is known about safe exposure. Swallowing strong solutions burns the mouth, esophagus, and stomach, triggering vomiting and sometimes even shock. Skin contact almost always irritates, especially for workers who handle broken crystals without gloves. Scientists have linked repeated inhalation of dusts to respiratory symptoms, and in rare cases, tissue necrosis. Most human safety data stems from accidental poisonings or misuse in medical settings—prompted by ingestion or inappropriate application. Ecotoxicologists focus on the compound’s risks to aquatic life, since even modest levels kill fish and disrupt water-based ecosystems. Guidelines now limit permissible dosing in drinking water to prevent chronically high exposures to both people and animals.
Future Prospects
Looking beyond today’s uses, potassium permanganate holds promise in environmental remediation and green chemistry initiatives. Engineers plan new oxidation processes for breaking down persistent organics in wastewater, and pilot studies point to its role in controlling pharmaceutical and microplastic pollution. At the same time, researchers push for lower-risk systems that deliver the right dose in the right place, using encapsulation technologies that limit accidental exposure. Prospectors still explore ways to recycle or reuse residual manganese, closing the loop on waste and shrinking potassium permanganate’s environmental footprint. Advances in smart dosing and blending tech set the stage for more targeted disinfection applications in healthcare and industry, without the byproducts that have dogged older oxidation systems. The challenge going forward lands squarely in credible research, public education, and risk management—keys for letting potassium permanganate meet rising demand without shortchanging safety or trust.
What are the main uses of potassium permanganate?
A Colorful Tool in Water Treatment
Growing up near an old well on my family’s property, I learned early on about weird stains you can't scrub off by hand. My grandfather always kept a small jar of potassium permanganate in his garden shed. Back then, it looked like magic in a bottle—dark crystals that could turn water deep purple in seconds. We used it to manage stubborn iron and sulfur odors from the well, thanks to its strong oxidizing power. Communities all over the world rely on this compound to treat drinking water. It helps clear iron, manganese, and unwanted smell from both private wells and municipal plants. Studies from the World Health Organization point out that using the right dose can break down contaminants and protect pipes from bacterial buildup.
Sanitizing and Disinfecting
Walk into a clinic in rural regions, especially across parts of Africa and Southeast Asia, and you might notice a purple-tinged solution for cleaning wounds. Medical professionals turn to potassium permanganate to wash ulcers, fungal infections, and even snake bites. Low-concentration soaks ease itching and curb the spread of bacteria, often where antibiotics are hard to get. Some sources from the Centers for Disease Control and Prevention (CDC) confirm its value as an accessible disinfectant in low-resource settings—something I’ve witnessed during brief periods volunteering abroad. It isn’t a cure, but it helps keep infections from spreading.
Emergency Survival and Field Uses
Spend a week in the wilderness with a survival manual, and potassium permanganate stands out for its versatility. Mix a pinch with sugar or glycerin, you get instant fire—a crucial skill for hikers or rescue crews needing warmth, food, or a signal fast. Mountaineers sometimes carry it in first-aid kits to purify water or clean wounds. I recall reading field notes from firefighters who used it in controlled burns. They’d drip a small amount mixed in antifreeze to safely create a firebreak.
Industrial and Environmental Roles
Factories lean on potassium permanganate to manufacture chemicals and remove toxic gases. For example, it tackles odors in waste treatment plants and neutralizes hydrogen sulfide. Research by the Environmental Protection Agency (EPA) highlights its ability to break down industrial solvents that seep into the ground. This keeps water safer for neighboring communities. In mining, workers treat ore with it to extract precious minerals, a step that’s sparked debates about balancing industrial progress and environmental health.
Looking Ahead: How to Use it Safely
Potassium permanganate delivers results, but it’s not a fix-all. Overuse in water can create byproducts that harm aquatic life. Exposure without protection irritates skin and eyes, and swallowing the crystals is dangerous. I’ve always kept strong gloves and an eye-wash nearby when I handle it for household cleaning or gardening projects. There’s a push for teams in healthcare, industry, and public works to adopt better training and proper storage. Some countries now limit sales for safety.
Final Thoughts on its Value
Many people overlook potassium permanganate until they face a problem—rusty water, stubborn infections, emergency survival. Its practical power comes from careful use, clear knowledge, and a healthy dose of respect. With responsible application, it continues to support communities in both simple and high-stakes situations.
Is potassium permanganate safe to use and handle?
Potassium Permanganate Often Helps, But It Bites Back If Dismissed as Harmless
Most folks meet potassium permanganate in science class or from an old household guide. Dark purple crystals dissolve in water, turning it a dramatic violet. Some use it to treat water, others reach for it as a disinfectant for wounds or minor infections. Pool owners and campers discuss its power as an oxidizer. The stuff definitely works, but treating it as just another cleaning solution usually leads to trouble.
Potassium permanganate acts aggressively, which is what lets it kill bacteria and neutralize some toxins. This same reactivity inflicts serious injuries when handled without planning. Accidentally spilling concentrated solution on your skin will stain your fingers brown, but the real issue runs deeper. Even moderate doses can cause burning, especially on sensitive skin. Splash it in your eyes and you'll need medical care fast—corrosive injuries don’t heal the way scrapes do. Inhaling dust during cleanup causes lung irritation. Full-strength crystals also react with a range of organics. Rags soaked with permanganate can ignite or smolder. Stories about ruined countertops and burned hands suggest plenty of users learn safety lessons too late.
Some claim potassium permanganate works wonders for cuts on kids or rashes from the garden. There's a reason registered professionals almost always dilute it carefully before using. Pharmacies don’t just hand out crystals. In chemical plants, operators wear gloves, goggles, and aprons. Schools keep storage tight, since this powdery solid can react with sugars, dust, or other cleaning agents. It breaks down toxins and germs, but handling needs care.
Household Use Crosses Into Risky Territory Too Easily
People sometimes reach for potassium permanganate because it’s affordable and effective. Rural health campaigns have promoted it for water purification. The doses used are tiny—too much in drinking water tastes bitter and can turn mild poisoning serious. Swallow a concentrated solution and you’re in for nausea, burns in your mouth, and possible organ damage. Pets that drink from the bucket end up at the vet. Very low concentrations help soak wounds, but pharmacists know to label everything and instruct clearly. Problems happen when folks skip directions or experiment without checking safe limits.
A quick scan through medical reports reveals cases of skin injuries, eye accidents, or poisonings caused by misunderstanding directions. Cuts and scrapes benefit from diluted baths under a nurse’s guidance, but concentrated mishandling causes delays in healing or scarring. Water treatment stories show why training matters: a few extra crystals thrown into a small tank can make water undrinkable. Experienced water engineers use precise tools, not guesswork or spoons from the kitchen drawer.
Practical Guidelines Prevent Mishaps
Small packages often come with instructions, but who reads them fully? Making this compound safer means clear communication on both the label and through those leading training sessions. Schools should focus not just on what it does, but on what can go wrong. Simple fixes such as color-changing test strips, single-use sachets for wound treatment, and mandatory safety goggles in classrooms tackle real risks. Talking openly about emergencies—what to do if it splashes in eyes or mouth—beats silent hope that nothing will go wrong.
Communities and first-time users benefit from reminders: check your source, measure carefully, use gloves if handling crystals, wear goggles if mixing. Keep jars out of reach from curious children. Don’t mix it with cleaning products or acids. Throw out leftover solutions safely. Knowing someone who got stained, burned, or poisoned turns these warnings into daily habits, not just fine print. A small amount does the job; respect and preparation keep potassium permanganate helpful, not harmful.
How should potassium permanganate be stored?
Why Safe Storage Matters
Potassium permanganate stands out in science labs, water treatment plants, and even emergency supply kits for a reason. Its ability to react strongly brings many uses, from disinfecting water to treating certain skin conditions. Strong reactions mean that mistakes in storage can lead to real consequences. There’s nothing complicated about it—one small problem, like a leaky container or a pileup of the wrong chemicals, invites potential danger.
Direct Experience Shows What Works
I remember working on a water purification project, where a few crystals of potassium permanganate got spilled on a damp counter. We acted fast, but the purple stains stuck around for months. That simple incident drove home that the powder reacts even to small amounts of moisture. People who overlook small details often pay the price later.
Clear, Practical Storage Guidelines
Store potassium permanganate in dry, tightly sealed containers—always. Glass, high-density polyethylene, or certain alloys all do the job, but no one wants to risk a leaky lid. Keep the container far from direct sunlight, any heat sources, or open flames. Moisture leads to clumping and sneaky chemical reactions, so only cool, dry rooms make the cut.
The placement of the container matters as much as the material. Put it on a dedicated shelf, away from acids, paper, oils, and other combustibles. A single careless mix with organics or reducing agents transforms an ordinary day into an emergency. In labs, storage cabinets get labeled with oxidizer symbols and warning stickers, so there’s never a doubt about what sits inside. At home, a plastic box with a clear sign—“Oxidizer: Keep Dry, Keep Away from Everything”—works just as well.
Fact-Backed Practices
Potassium permanganate ranks as a ‘strong oxidizer’ under OSHA and NFPA. Its reaction with glycerin, sugar, or even dust can spark a fire, even without a match. The Centers for Disease Control and Prevention urges facilities to keep it at least a meter from anything organic or flammable. Following these rules keeps insurance costs manageable and prevents fines, but, more importantly, it keeps people safe.
Addressing Common Mistakes
Cutting corners rarely ends well. People sometimes pour excess crystals back into the main bottle after use. This introduces small amounts of dirt, wetness, or incompatible residues. Over time, the risk snowballs. Using dedicated scoops and never returning used powder goes a long way. Old bottles should get labeled with the date and any signs of caking or odd smells mean it’s time to discard them with care, following hazardous waste guidelines.
Improving Storage Habits
Basic training makes the biggest difference. Anyone handling potassium permanganate should learn the symptoms of an oxidizer incident: sudden heat, sparks, or strange colors near the material. Protective gloves and safety goggles count as bare minimums. Keeping a spill kit nearby—think sand, not sawdust—prepares people for a fast cleanup. In bigger setups, proper ventilation and humidity control keep the area as safe as possible.
Raising the Standards
Each accident with potassium permanganate becomes a lesson for the next person. Over time, better storage practices reach everyone, from school science students to city water treatment workers. Updated labels, regular safety reminders, and accessible training all work together to push risks lower.
Can potassium permanganate be used for water purification?
Potassium Permanganate: The Purple Crystal with Big Promises
In small towns where water sometimes comes out of the tap looking like weak tea, older neighbors used to drop a few crystals of potassium permanganate in their storage tanks. The deep purple color made it feel like magic, seeing cloudy water clear up. Some folks swear by it, especially in places where proper water treatment plants don’t reach. Yet this chemical is more than a colorant—its ability to fight certain germs and oxidize metals sparked wide interest.
How Does This Chemical Work in Purifying Water?
Potassium permanganate acts as an oxidizer. It attacks iron, manganese, and even some sulfur, turning dissolved metals into solids that filters can catch. After a few minutes, orange and black stains appear in your pipes or tanks because the chemical forms rust or manganese oxides. In theory, this makes it easier to filter these minerals from your water. Some bacteria—like those that produce rotten egg smells—have a hard time surviving after a proper dose of this stuff. In fact, municipal water plants sometimes use potassium permanganate as a pretreatment step. But they manage dosing carefully, using precise instruments.
Risks and Limitations Most People Miss
At home, the kitchen table chemistry gets risky quickly. Too much potassium permanganate can turn your water pink or purple, or worse, make it unsafe to drink. Short-term overexposure irritates your stomach, throat, and sometimes even burns your skin. Swallowing high doses causes serious damage. A tiny amount—usually less than one milligram per liter—removes certain metals and microbes, but hitting the right dose without lab equipment becomes guesswork.
This chemical doesn’t remove every danger, either. Parasites, industrial chemicals, or pesticides will slip right through if they’re present. Potassium permanganate doesn’t kill every virus or bacteria. It works on some but not all. It can’t physically remove dirt, and it doesn’t destroy harmful organic chemicals. Real safe drinking water calls for more than one trick.
My Experience with Rural Water Struggles
After spending a few years working in a developing area, I saw firsthand how desperation pushes people to grab whatever works. Even in city apartments where water tanks catch bird droppings from rooftop pigeons, some residents believe potassium permanganate cleans the mess. I realized that the real problem stays hidden underneath: poor access to municipal treatment, broken pipes, over-reliance on narrow fixes. Local health clinics report cases of chemical poisoning each year, usually after someone tried fixing “smelly water” with too much chemical.
Better Ways and Responsible Use
Affordable water filters—sand, activated carbon, or reverse osmosis units—help people avoid these risks. Boiling water or using chlorine, when properly guided, tends to be safer and more effective than misusing chemicals with potential toxicity. Many public health agencies stress that proper community education stops people from harming themselves with well-intentioned quick fixes.
In emergencies, experts suggest potassium permanganate as a last resort for disinfecting water, but only with measured doses and clear instructions. Generally, anyone without training or proper measuring tools should leave this purple powder to professionals. Equipment that can test water quality and monitor residual chemicals makes all the difference between safe and hazardous.
Relying on a single chemical won’t fix deeper water challenges facing many communities—consistent investment, maintenance, and clear public information do. While potassium permanganate shows that science can offer hope, real trust comes from approaches grounded in experience, accountability, and the facts.
What precautions should be taken when using potassium permanganate?
What Makes Potassium Permanganate Effective — And Risky
Potassium permanganate always stood out in my high school chemistry set. The purple crystals cleaned fish tanks, cleared smelly drains, and sometimes played a part in science experiments that fizzed and sparked. Out in the world, this same chemical finds its place in water treatment plants and hospitals. Despite those everyday uses, this is still a strong oxidizer — a chemical capable of starting fires or eating right through skin if handled carelessly.
The Risk to Skin and Eyes
I remember a classmate whose hand got stained deep purple for a week after a spill. That’s minor. Direct contact with skin, especially if the solution is concentrated, may burn. Eyes take it much worse. Splashes cause pain and can damage corneas fast. Always keep hands covered with non-reactive gloves. Cheap latex tends to break down, so go with nitrile if you can. Splash goggles keep those purple drops away from your eyes. If you get even a tiny amount in your eye, flush for fifteen minutes and head for medical attention. No shortcuts.
Mixing with Other Chemicals
At a wastewater plant where I once interned, potassium permanganate stood on its own shelf, away from acids and organic chemicals. Pairing it with anything that burns — like glycerin or sugar — creates a risk for fire or explosion. Even mixing it with common things like alcohol, sulfur, or paper spells danger. Store it in a dry spot, away from all fuels and heat sources. Make it a habit to never scoop it out with paddles or spoons that have touched anything else. In a lab or garage, stick to clean, dry tools; cross-contamination turns accidents into emergencies.
Preventing Inhalation or Accidental Consumption
Potassium permanganate creates dust. The fine powder; it floats off the crystals and sits on surfaces. Breathing it burns noses and throats, and frequent exposure risks lung irritation. Always work in well-ventilated spaces, or use a mask if there’s any risk of airborne dust. As with most chemicals, never eat, drink, or smoke while you’re around it. Wash your hands afterwards. Good hygiene isn’t just advice; it keeps accidents from travelling to lunch or a drink after work.
Disposal: Protecting Waterways and Soil
People sometimes throw unused potassium permanganate down the sink. That purple flush doesn’t just disappear — it reacts with organic matter, affects water quality, and even kills aquatic animals. I learned early to collect waste and label it. Municipalities often have hazardous waste drop-off points. Small spills on surfaces get neutralized using a reducing agent, like sodium thiosulfate, before rinsing with lots of water. Never toss it in regular trash or drains without neutralizing.
Emergency Preparedness
Chemicals don’t wait for you to be ready. At every workbench, keep water, a sink, and some kind of eyewash station handy. Don’t just rely on memory; refresh yourself on local workplace policy about chemical accidents and always know the fastest route to the nearest source of fresh water. Every now and then, stop and check that gloves aren’t torn, lids are tight, and tools are dry. The best safety habits become routine.
Respect for Its Power
Potassium permanganate cleans water, helps heal wounds, and clears away rotten smells — all good. But it discovers weaknesses, and it punishes shortcuts. Anyone using it owes themselves — and the people nearby — the discipline to work safely, store properly, and dispose responsibly. The purple stain left on careless hands usually fades; the injuries from neglecting safety stick around longer.
| Names | |
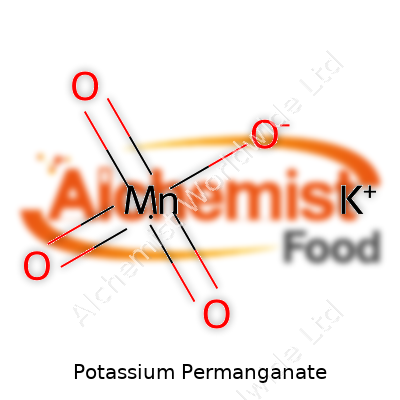
| Preferred IUPAC name | Potassium manganate(VII) |
| Other names |
Condy’s crystals Permanganate of potash Chameleon mineral Permanganic acid potassium salt KMnO4 |
| Pronunciation | /pəˈtæsiəm pərˈmæŋɡəˌneɪt/ |
| Preferred IUPAC name | Potassium manganate(VII) |
| Other names |
Condy’s crystals Permanganate of potash KMnO4 |
| Pronunciation | /poʊˌtæsiəm pərˈmæŋɡəˌneɪt/ |
| Identifiers | |
| CAS Number | 7722-64-7 |
| Beilstein Reference | 0110787 |
| ChEBI | CHEBI:48626 |
| ChEMBL | CHEMBL1200821 |
| ChemSpider | 5667 |
| DrugBank | DB14015 |
| ECHA InfoCard | ECHA InfoCard: 100.029.730 |
| EC Number | 231-760-3 |
| Gmelin Reference | Gmelin Reference: 1730 |
| KEGG | C14334 |
| MeSH | D011103 |
| PubChem CID | 516875 |
| RTECS number | SD6475000 |
| UNII | XB04YZV4M9 |
| UN number | UN 1490 |
| CAS Number | 7722-64-7 |
| Beilstein Reference | 3589883 |
| ChEBI | CHEBI:7754 |
| ChEMBL | CHEMBL1085 |
| ChemSpider | 14110 |
| DrugBank | DB14015 |
| ECHA InfoCard | ECHA InfoCard: 100.028.760 |
| EC Number | 231-760-3 |
| Gmelin Reference | Gmelin Reference: "K 40 |
| KEGG | C14326 |
| MeSH | D011109 |
| PubChem CID | 516875 |
| RTECS number | SD5250000 |
| UNII | 7KM6PO3H2O |
| UN number | UN1490 |
| Properties | |
| Chemical formula | KMnO4 |
| Molar mass | 158.034 g/mol |
| Appearance | Purple or dark purple crystalline solid |
| Odor | Odorless |
| Density | 2.7 g/cm³ |
| Solubility in water | 6.38 g/100 mL (20 °C) |
| log P | -2.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.2 |
| Magnetic susceptibility (χ) | +915.0·10⁻⁶ cm³/mol |
| Dipole moment | 0 D |
| Chemical formula | KMnO4 |
| Molar mass | 158.034 g/mol |
| Appearance | Dark purple or bronze-colored crystalline solid |
| Odor | Odorless |
| Density | 2.7 g/cm³ |
| Solubility in water | 76 g/L (20 °C) |
| log P | -2.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.18 |
| Basicity (pKb) | 9.4 |
| Magnetic susceptibility (χ) | Paramagnetic |
| Refractive index (nD) | 2.41 |
| Viscosity | 0.5–0.7 cP (20 °C, 6% solution) |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 174.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -813 kJ/mol |
| Std molar entropy (S⦵298) | 143.1 J/(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | -813.4 kJ/mol |
| Pharmacology | |
| ATC code | D08AX01 |
| ATC code | V03AB01 |
| Hazards | |
| Main hazards | Oxidizer, harmful if swallowed, causes severe skin burns and eye damage, may intensify fire. |
| GHS labelling | GHS02, GHS05, GHS07, GHS09 |
| Pictograms | GHS03,GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H272, H302, H314, H410 |
| Precautionary statements | P210, P220, P221, P280, P370+P378, P501 |
| NFPA 704 (fire diamond) | 3-0-1-OX |
| Lethal dose or concentration | LD50 oral (rat) 750 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Potassium Permanganate: 1090 mg/kg (oral, rat) |
| NIOSH | MN9600000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Potassium Permanganate: 5 mg/m³ (as Mn, ceiling) |
| REL (Recommended) | 30 mg/day |
| IDLH (Immediate danger) | 500 mg/m³ |
| GHS labelling | GHS05, GHS07, GHS09 |
| Pictograms | GHS03, GHS07, GHS09 |
| Signal word | Danger |
| Hazard statements | H272, H302, H314, H410 |
| Precautionary statements | P210, P220, P221, P280, P370+P378, P501 |
| NFPA 704 (fire diamond) | 3-0-1-OX |
| Autoignition temperature | Decomposes before ignition |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 oral rat: 750 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1090 mg/kg (oral, rat) |
| NIOSH | SN1225000 |
| PEL (Permissible) | PEL: 5 mg/m³ |
| REL (Recommended) | 30 mg/l |
| IDLH (Immediate danger) | 500 mg/m³ |
| Related compounds | |
| Related compounds |
Potassium manganate Sodium permanganate Potassium perruthenate Manganese dioxide |
| Related compounds |
Potassium manganate Manganese dioxide Permanganic acid Sodium permanganate |

