Potassium Iodate: More Than Just a Chemical Compound
Historical Development
Decades ago, chemists got wind of the fact that iodine deficiencies could spell disaster for entire populations. The link between iodine and thyroid health popped up in medical literature by the mid-nineteenth century, but it took until the twentieth for potassium iodate (KIO3) to really make a mark. Back then, potassium iodide was the big player, yet potassium iodate slowly gained traction for its stability and shelf life in salt iodization campaigns. During the Cold War, civil defense planners highlighted this compound as a radiation emergency staple, giving the public a tool against radioactive iodine fallout. Human error and environmental shifts forced agencies to adopt more robust and stable solutions in food fortification and emergency preparedness, raising potassium iodate’s profile from dusty chemical to global health asset.
Product Overview
Walk into a chemical warehouse or search an industrial catalog, and potassium iodate stands out as a versatile powder or granule with a strong chemical punch. Its formula, KIO3, is simple on paper, but the uses range from food additive to laboratory reagent. In my own years working with public health teams, I’ve seen it stocked in clinics and disaster preparedness kits, labeled as safe for iodization projects and radiation emergencies. Shelf packs aimed at food fortification programs reflect the global push for iodine sufficiency, especially in regions where goiter and mental impairment due to iodine lack used to haunt entire generations.
Physical & Chemical Properties
Potassium iodate comes as a white, powdery solid—no snazzy colors or tricky textures. It stays stable in typical storage, thanks to its crystalline structure and its lack of reactivity under usual temperatures. Water dissolves it with a bit of stirring, making it ready for dosing in food or tablet production. Handling it in the lab, I’ve noticed the high melting point—over 500 degrees Celsius—and its solid, gritty feel between the fingers (yes, always with gloves, because even safe chemicals deserve respect). The odorless, taste-neutral profile explains why food producers like it: it does its job without altering the eaters’ experience.
Technical Specifications & Labeling
Bottles and bulk sacks of potassium iodate leave little room for confusion. Technical grades bear clear marks: at least 99% purity, a statement of origin, batch tracking, moisture content under 0.5%. Tablet products for emergency use sit in child-resistant packaging, stamped with dosages, hazard pictograms, and expiry info. Labels must obey the demands of regulators—chemical content, potential hazards, warnings about contact with reducing agents, and instructions for food or pharmaceutical use. Across several labs I’ve visited, chemical compatibility and emergency handling instructions sit within arm’s reach of every workbench.
Preparation Method
It’s hard to miss the practical mindset in lab syntheses. Potassium iodate production boils down to an oxidation-reaction between potassium iodide (KI) and a strong oxidizer, like chlorine or nitric acid. In one common route, a potassium iodide solution receives a slow trickle of chlorine gas, letting the iodine oxidize up to the iodate state, after which the solution gets filtered and crystallized. Commercial installations run the reactions under tightly-controlled conditions, minimizing impurities and environmental waste. Time spent in a production facility reveals how batch logs, careful stoichiometry, and temperature controls make the difference between pharmaceutical-grade and industrial byproduct.
Chemical Reactions & Modifications
Potassium iodate acts as an oxidizer with a fairly standard set of reactions. Add it to a reducing agent, and you’ll get either elemental iodine or iodide, depending on conditions. Tossing it into acid or mixing with organic material triggers redox exchanges—sometimes useful, sometimes hazardous. In salt production, food chemists tweak processing temperatures and humidity to keep the iodate from clumping or degrading. The reactivity isn’t just textbook theory: in hands-on work, the right mix means the difference between successful iodization and ineffectual product.
Synonyms & Product Names
Potassium iodate travels the chemical supply chain with many aliases. On invoices, you’ll see KIO3, “Potassium iodate,” “Iodic acid, potassium salt,” and in global commerce, other language variants appear. In public health manuals, the emergency tablets get marketed as radioiodine blockers, though they are never to be confused with potassium iodide (KI) tablets. I’ve seen packaging from five continents with everything from manufacturer codes to plain “Food grade potassium iodate.”
Safety & Operational Standards
No matter the setting, potassium iodate comes with safety rules laid down by regulators and industry veterans alike. The solid doesn’t fly up into the air but can cause trouble if inhaled, ingested in large quantities, or splashed in the eye. Direct contact should always prompt washing and a check for irritation. Safety Data Sheets spell out compatibilities—strong reducing agents mean trouble, and storage containers can’t be reactive metals or acids. Over a decade of lab work, I’ve never witnessed an accident with proper storage and protective equipment. Close supervision in tablet production ensures pharmaceutical safety, while bulk storage follows strict segregation guidelines.
Application Area
Food fortification still takes priority in many developing world programs: potassium iodate goes into table salt to keep populations healthy, especially where natural iodine levels in soil and water fall short. In the nuclear preparedness circles I’ve joined, it comes up as a backup to potassium iodide for blocking radioactive iodine uptake in the thyroid. Farmers use it sparingly in animal feeds; researchers depend on it as a reliable oxidizer in chemical analysis routines. From the pantry shelf in rural clinics to the gloveboxes of academic chemistry departments, the reach of this compound goes far past the stereotype of “simple salt additive.”
Research & Development
Scientists remain curious about maximizing bioavailability and ensuring every grain in fortified salt reaches the person who needs it most. Trials focus on tweaking humidity resistance and preventing caking, especially in hot, damp climates. Pharmaceutical researchers pay attention to its efficiency as a radioiodine blocker, especially after nuclear accidents in places like Fukushima reignited public concern. I’ve worked alongside teams measuring uptake rates and shelf stability, balancing public health needs with tight budgets. Newer analytical methods trace potassium iodate residues and test breakdown products, pushing quality assurance programs forward.
Toxicity Research
Potassium iodate, for all its benefits, must be administered in controlled doses. Toxicity research outlines safe thresholds, showing how excess intake leads to thyroid suppression, renal issues, or digestive upset. Animal studies and human clinical data form the backbone of international guidelines, which health agencies keep updating based on large-scale fortification projects. Public health departments track rare but real cases of overdose—often due to mislabeling or accidental ingestion from bulk stocks. As a chemist involved in some of these field studies, seeing the direct effect of dosage miscalculation always creates urgency for clearer instructions and better community training.
Future Prospects
Global health trends point to more targeted use of potassium iodate, not less. Environmental scientists worry about iodine cycling in depleted soils, making chemical supplementation critical for years to come. As more regions face the risk of nuclear incidents, demand for reliable radioiodine blockers only goes up. Food manufacturers seek even smarter formulations that resist packaging breakdowns and withstand extreme climates. My hope is that cross-disciplinary collaboration keeps improving both the safety and reach of potassium iodate—balancing chemical innovation with boots-on-the-ground realities in clinics, disaster relief zones, and household kitchens.
What is Potassium Iodate used for?
A Medicine Cabinet Staple You Might Not Know
Potassium iodate doesn’t usually hit the headlines, but it has earned its place in medicine, food security, and even nuclear safety. I remember reading about potassium iodate tablets being distributed in parts of Ukraine during the early days of the Chernobyl disaster — families were told to take them to protect their thyroids from radiation exposure. The science behind this action is straightforward. When radioactive iodine enters the body, the thyroid gland latches onto it. If you saturate your thyroid with stable iodine from potassium iodate beforehand, the radioactive version finds no empty space and passes right through.
This isn’t just a relic of the past. Governments everywhere still keep potassium iodate in stockpiles as a reliable emergency measure. The World Health Organization names iodine tablets as a crucial tool against nuclear emergencies. For many, potassium iodate is a safety net—one that protects against a threat most don’t like to think about but governments plan for all the same.
Guarding Public Health With a Simple Addition
For people who live nowhere near a nuclear plant, potassium iodate comes into play in another way—through salt. In places where soil doesn’t hold much natural iodine, people suffer from thyroid problems and intellectual disabilities. Countries facing iodine deficiency in their populations add potassium iodate to table salt. The effects show up over time at the family dinner table. Eating iodized salt every day fights off goiter and leads to healthier children, sharper minds, and fewer birth defects.
I grew up in a family where salt shakers on the counter simply carried iodized salt. As a kid, I never realized the purpose. Only years later did I learn that this small public health decision—fortifying salt with potassium iodate—helped wipe out a host of preventable illnesses in communities like mine around the world. UNICEF and the Iodine Global Network both celebrate this as one of the simplest and most cost-effective public health achievements worldwide.
Challenges and Practical Solutions
There’s no magic solution to ensuring every household has enough iodine. Logistical hiccups still exist, especially in rural or isolated regions. Sometimes, local producers cut corners and use plain salt to save money. Education plays a big role here. If policy makers push clear labeling and testing, they reduce the odds of fake or under-iodized salt ending up on store shelves.
We can’t forget food security either. In baking, potassium iodate acts as a bread improver in some places, helping dough rise and bread keep its shape. Although some countries turn to other additives, the basic need stays: healthy bread, consistent quality, and plenty of micronutrients.
Trust and Quality Control
Potassium iodate does its job only if it’s actually present in what we eat and use. Trust matters. Food safety agencies enforce tough standards and regular checks — an essential safeguard against shortcuts. Over the years, I’ve seen the impact of enforcement. When agencies keep a close eye, fewer kids end up with thyroid disorders down the line. Community health workers, teachers, and parents all play a piece in this puzzle. Their voices matter in demanding safer, more reliable food fortification.
Is Potassium Iodate safe for consumption?
What Potassium Iodate Does and Why It Shows Up in Your Food
Potassium iodate turns up in food, salt, and even emergency supplies. Health authorities decided many years ago that folks not getting enough iodine in their diet land themselves in trouble. Iodine supports the thyroid and keeps hormone levels on track. Without it, goiter and mental impairment grow into big problems, especially for kids. I remember learning about this in health class—salt got “iodized” so these problems wouldn’t come back.
In plenty of places, potassium iodate does the job that potassium iodide does in others. Both add iodine, but potassium iodate lasts longer on the shelf and resists spoilage. That’s why big salt brands and some public health programs reach for it. Aid groups hand out potassium iodate tablets during nuclear emergencies, too. This chemical floods the body with safe iodine and blocks harmful radioactive iodine.
What Science Says About Safety
Trust in food doesn’t grow from fancy packaging; it’s science that builds that trust. Potassium iodate sits on the “generally recognized as safe” list with the U.S. Food and Drug Administration—up to a certain amount. The World Health Organization and the Food and Agriculture Organization agree with that stance for fortifying salt. Millions of people worldwide eat it daily in tiny doses and show no harm.
Problems creeps up only if someone eats too much. The body only needs about 150 micrograms of iodine each day, though that goes up for pregnant women. A single teaspoon of most iodized salt delivers a safe, useful dose. Massive doses—over years—might mess with your thyroid, making it too active or shutting it down. People with thyroid disease or on special diets know they need to check their iodine intake. Downing bottles of potassium iodate tablets or eating large industrial amounts crosses a line, but that’s a rare situation.
Supply Issues and Quality Control
Anytime a chemical ends up in food, questions about safety and quality follow. Countries need to set and enforce standards. Some cut corners: suppliers push untested stuff onto the market, or stores sell expired stock. This problem isn’t unique to potassium iodate. Regular testing and rules help build trust. I’ve seen stories about salt without the right iodine levels because of weak regulation. That leaves whole populations at risk of both deficiency and possible overdose.
What Helps People Stay Safe
Clear labeling helps more than most people think. If a salt label says “iodized,” check for ingredients and iodine level. Doctors and nutritionists should talk about iodine at checkups, not only with expectant mothers but with everyone. Public health programs reach more people when they work with local shops. In places where food variety shrinks, health officials should make sure everyone gets the right amount—no more, no less.
Practical Advice on Potassium Iodate
If someone eats a balanced diet, including fish, dairy, eggs, and some iodized salt, they shouldn’t worry at all about potassium iodate in their diet. For folks with thyroid problems, a quick check with the doctor helps. For people stocking nuclear emergency kits, following the dosage on potassium iodate tablets matters a lot. This isn’t something to fear or ban, but like most things in nutrition, the right amount means everything.
How should Potassium Iodate be stored?
Why Proper Storage Isn’t Just a Technicality
Potassium iodate shows up in more places than people realize. Some know it from science labs, others from emergency supplies during nuclear incidents, and bread-makers use it as a dough conditioner. The tricky part isn’t getting it, but keeping it safe and stable for when it’s really needed. Nobody wants to open a container and find useless clumps or, even worse, a risk to their health.
Understanding What the Substance Responds To
Potassium iodate keeps better in some conditions than others. Exposure to moisture in the air creates lumps and lowers its effectiveness. Direct sunlight or heating triggers its breakdown, causing a loss in strength. Even a little contamination from other chemicals or hands can taint it, especially if stored close to acids or reducing agents. Looking back at my years handling chemicals in shared laboratory spaces, I learned quickly: what you store it near matters almost just as much as the temperature or container.
A Container Isn’t Just a Box
Plastic bags or thin paper packets might sound convenient, but humidity creeps in faster than most expect. Durable, airtight containers—glass jars with rubber seals or tightly sealed HDPE plastic bottles—protect the powder from air and moisture. Sometimes folks overlook the cap: if the seal dries out or warps, the container turns nearly useless. Each time you open it and re-close, you give a chance for new moisture to sneak in. To solve this, get into the habit of only opening the jar for as long as you absolutely have to. Even in busy workspaces, this stops small mistakes from becoming big waste.
Room Conditions Matter More Than Most Think
Shelf life doesn’t just rely on the jar. A cool, dry, and shaded area slows down reactions that break down potassium iodate. Temperatures above room temp—especially above 25°C—speed up its chemical changes. Place it in kitchens, basements with leaks, or garages with wild swings between hot and cold, and you surrender its reliability. At home, I once lost a jar to dampness during summer storms because I stowed it under a sink instead of a dry, high closet. After that mess, every chemical goes in a locked, temperature-steady cabinet away from water lines and windows.
Label Everything, Even in Small Batches
Handwritten labels tend to fade or fall off, leaving mysterious jars for someone else to clean up a year later. Print or engrave the contents, concentration (for solutions), batch, and date of storage. My old chemistry teacher drilled this habit into me, and it saves trouble every time you rotate stock, check on expiry, or need to trace back a bad batch. It sounds simple, but clear labeling protects families and teams from dangerous mix-ups.
Handling Small Quantities
Even tiny scoops deserve respect. Avoid touching the powder with bare hands—wear gloves and use a dedicated scoop or spatula. This cuts the risk of accidental exposure and stops natural oils or contaminants from breaking down the chemical. After each use, seal the lid tightly and wipe spilled powder from the jar’s mouth. These steps sound obvious, but busy days lead to mistakes, and once cross-contamination starts, there’s no easy fix except throwing everything out.
Final Thoughts on Responsibility
Potassium iodate does its job best if it’s stored with patience and care. The best techniques don’t cost a fortune or take up too much space. A good jar, a steady shelf, a dry environment, and a clear label go further than any fancy gadget. Every time I set up storage at home or in a teaching lab, I remind myself: a few minutes of tidiness keep people healthy, save budgets, and avoid emergencies down the line.
What is the recommended dosage of Potassium Iodate?
Why Everyone Talks About Potassium Iodate
Natural disasters like nuclear accidents often push people to stock up on supplies they wouldn’t normally consider. Potassium iodate falls in that category. I remember the panic-shopping waves during the Fukushima incident in 2011. Shelves emptied out, and people scrambled for any kind of guidance on how much to take, and when. It always bothered me to watch people guessing with something so important. Mistakes with dosing do not just waste money—they can have serious health consequences.
The Numbers: What the Science Says
Potassium iodate acts as a blocker, keeping radioactive iodine out of the thyroid gland. Based on guidance from groups like the World Health Organization and the FDA, most adults should take 170 milligrams once daily at the time of exposure to radioactive iodine. For kids, the dose drops: teens 12 to 18 years old and over 150 pounds also use 170 mg per day; lighter teens and children aged 3 to 12 years take 85 mg; toddlers 1 month through 3 years old need 42.5 mg. Infants from birth to 1 month get 21 mg. Crucially, this isn’t an ongoing supplement—it’s meant for short-term use during nuclear emergencies where radioactive iodine actually enters the air. Taking too much, too soon, or for too long, can do more harm than good.
Not All Potassium Compounds Are the Same
Some people confuse potassium iodate with potassium iodide. Both protect the thyroid, but dosages vary. Potassium iodate gets used where potassium iodide isn’t stable. Misunderstanding the difference creates confusion during emergencies, especially for those who try to cut tablets or use improper substitutes. I’ve seen families keep years-old tablets from overseas trips, only to find different compound names and foreign instructions when it’s too late to ask for help.
Don’t Forget About the Risks
Potassium iodate should never become a daily routine for healthy people. Thyroid problems can pop up for those who take it without real need. Adults over forty without thyroid conditions face a far smaller risk from radioactive iodine, so they usually skip it unless a doctor says otherwise. Side effects—nausea, belly pain, or allergic reactions—can surprise those who take more than needed, especially children, pregnant women, or anyone with existing thyroid conditions. Families with thyroid disease history should check with a doctor before emergencies crop up, rather than play guessing games under stress.
Better Prepared Than Sorry
Clear labeling and instructions save lives. People who bother to read labels ahead of time can act fast during real emergencies. I always recommend keeping the box—don’t write dosages on a sticky note and toss the original. Local emergency services or pharmacies make good sources of information, especially for families with young kids, elderly relatives, or anyone with health uncertainties. Misinformation spreads quickly online, so turn to organizations like the CDC or WHO for guidance and stick to credible websites.
Building Trust With The Right Information
Everyone wants to protect their loved ones. Relying on hearsay or internet rumors leads to mistakes. Investing a few minutes in checking the correct dosage and guidelines ensures that if disaster strikes, you’re ready. Safe use of potassium iodate starts with knowledge, clear planning, and a bit of humility—there’s no shame in double-checking or asking qualified professionals for advice. People who prepare thoughtfully tend to come through emergencies better, with fewer regrets and more peace of mind. That lesson, more than any number or formula, stays with me each time the headlines start ‘radiation alert’ chatter anew.
What are the side effects of Potassium Iodate?
Unpacking Potassium Iodate’s Role and Risk
Most people don’t think of potassium iodate until a government hands out pills, warning of a nuclear accident. It’s a chemical that helps shield the thyroid from radioactive iodine. Folks swallow it in small white pills, expecting it to protect against radiation’s dangers. But plenty don’t realize too much potassium iodate can bring its own set of headaches. I learned this lesson from years volunteering in disaster response areas. Well-meaning people sometimes hand out potassium iodate without explaining the basics. The pill’s not always forgiving if taken the wrong way or by the wrong person.
Common Side Effects That Don't Get Enough Attention
Too much potassium can throw the body’s balance off. People can feel nauseous, get stomach cramps, or have diarrhea. These hit quickly for some, dragging them down even as they hope for health benefits. Alongside stomach trouble, some report rashes or itchy skin. As a Red Cross volunteer, I once saw lines of evacuees rubbing their arms after taking these pills, looking surprised the helper-pills were the culprit.
Doctors are also wary of the impact on the thyroid. Potassium iodate forces the thyroid to absorb good iodine, which helps for short-term protection. At the same time, a hefty dose floods the body, sometimes sending the thyroid into overdrive or freezing it up. Both options mean real health trouble. Hyperthyroidism shows up as anxiety, sweating, weight loss, and pounding heartbeats. The flip side, hypothyroidism, can sap energy, mess with memory, or bring sudden weight gain. Thyroid issues don’t always show up immediately either, so some folks struggle to connect these symptoms back to the little pill they took during an emergency.
High-Risk Groups Need Special Consideration
Certain people are more likely to land in the danger zone with potassium iodate. Anyone with thyroid conditions like Graves’ disease or Hashimoto’s needs extra caution. The same goes for babies and the elderly. Their bodies handle shocks and new chemicals differently—sometimes badly. Pregnant and breastfeeding women also risk giving too much or too little iodine to their babies. My neighbor, a nurse, warns against giving out these pills blindly, especially to kids and older relatives. All it takes is one dose in the wrong hands to create a thyroid crisis.
Potential Solutions: Information and Access
Clear instructions and medical screening matter a lot. Handing out potassium iodate should always come with a talk about who should take it, how much, and for how long. Not every emergency is nuclear—it’s easy to panic and reach for a pill, but the side effects demand a more careful approach. Public health authorities in Europe and the US now underline that potassium iodate should only be used under clear guidelines and strict supervision.
Doctors recommend watching for any signs of allergy, breathing trouble, swelling, or severe stomach pain after taking the pill. Anyone dealing with these should get medical help right away. Pharmaceutical companies can help by including warnings in plain language, not tiny print.
Real Stories Drive Home the Risks
During the Fukushima nuclear event, local clinics recorded a spike in people coming in with thyroid complaints months later. Some had used potassium iodate without knowing their thyroid was already in bad shape. Detailed health histories disappeared in the panic, stress magnified side effects, and follow-up was harder after evacuation. These stories aren’t uncommon and highlight why education—not just pills—should be at the heart of disaster preparation.
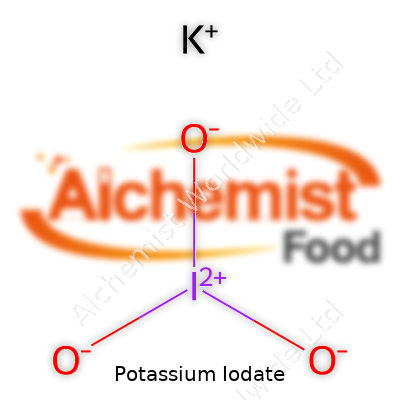
| Names | |
| Preferred IUPAC name | Potassium trioxidoiodate(1-) |
| Other names |
Iodic acid, potassium salt Iodate de potassium Potassium salt of iodic acid KIO3 |
| Pronunciation | /poʊˌtæsiəm aɪˈəʊdeɪt/ |
| Preferred IUPAC name | potassium trioxidoiodate(1-) |
| Other names |
Iodic acid, potassium salt KIO3 Potassium salt of iodic acid |
| Pronunciation | /pəˌtæsiəm aɪˈəʊdeɪt/ |
| Identifiers | |
| CAS Number | 7758-05-6 |
| Beilstein Reference | 358924 |
| ChEBI | CHEBI:83410 |
| ChEMBL | CHEMBL1200827 |
| ChemSpider | 14243 |
| DrugBank | DB14542 |
| ECHA InfoCard | 100.004.504 |
| EC Number | 231-831-9 |
| Gmelin Reference | 16220 |
| KEGG | C05925 |
| MeSH | D017681 |
| PubChem CID | 517165 |
| RTECS number | TT2975000 |
| UNII | 9W94V45T2Q |
| UN number | UN1479 |
| CAS Number | 7758-05-6 |
| Beilstein Reference | 3590482 |
| ChEBI | CHEBI:83414 |
| ChEMBL | CHEMBL1201619 |
| ChemSpider | 60787 |
| DrugBank | DB01345 |
| ECHA InfoCard | 03b0a7e5-7e98-4c62-8f96-24987f9a2c28 |
| EC Number | 231-831-9 |
| Gmelin Reference | 56996 |
| KEGG | C03886 |
| MeSH | D011148 |
| PubChem CID | 24744 |
| RTECS number | TT2975000 |
| UNII | 45O3T9681N |
| UN number | UN1479 |
| CompTox Dashboard (EPA) | UAK6Z6MZ0V |
| Properties | |
| Chemical formula | KIO3 |
| Molar mass | 214.00 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 3.89 g/cm³ |
| Solubility in water | 7.48 g/100 mL (25 °C) |
| log P | -0.701 |
| Vapor pressure | 0.1 mmHg (20°C) |
| Acidity (pKa) | 13.2 |
| Basicity (pKb) | pKb: 6.55 |
| Magnetic susceptibility (χ) | `-84.0 × 10⁻⁶ cm³/mol` |
| Refractive index (nD) | 1.734 |
| Dipole moment | 0 D |
| Chemical formula | KIO3 |
| Molar mass | 214.00 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 3.89 g/cm³ |
| Solubility in water | 7.5 g/100 mL (20 °C) |
| log P | -0.723 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 11.35 |
| Basicity (pKb) | pKb ≈ 6.33 |
| Magnetic susceptibility (χ) | -48.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.734 |
| Dipole moment | 1.65 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 230.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -328.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -295.0 kJ/mol |
| Std molar entropy (S⦵298) | 240.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -328.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -295.2 kJ/mol |
| Pharmacology | |
| ATC code | A09CA01 |
| ATC code | A12CA02 |
| Hazards | |
| Main hazards | Oxidizing solid, may cause fire or explosion; harmful if swallowed; may cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Danger |
| Hazard statements | H272: May intensify fire; oxidizer. |
| Precautionary statements | P264, P270, P273, P301+P312, P305+P351+P338, P330, P501 |
| NFPA 704 (fire diamond) | Health 2, Flammability 0, Instability 1, Special - |
| Autoignition temperature | 540 °C (1004 °F; 813 K) |
| Lethal dose or concentration | LD50 oral rat 2,734 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2,780 mg/kg (oral, rat) |
| NIOSH | SAF120 |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 0.25 mg/kg |
| IDLH (Immediate danger) | Not established |
| Main hazards | Oxidizer, may intensify fire; harmful if swallowed; causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS03,GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H272: May intensify fire; oxidizer. H319: Causes serious eye irritation. |
| Precautionary statements | P261, P264, P270, P271, P301+P312, P304+P340, P305+P351+P338, P312, P330, P337+P313, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-0-1-OX |
| Autoignition temperature | 400 °C (752 °F; 673 K) |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 oral rat 2,730 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2,000 mg/kg (oral, rat) |
| NIOSH | SE3850000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Potassium Iodate: "Not established |
| REL (Recommended) | 0.25 mg/kg |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Potassium bromate Sodium iodate Potassium chloride Potassium iodide |
| Related compounds |
Potassium iodide Sodium iodate Sodium iodide Iodic acid |

