Potassium Bicarbonate: A Comprehensive Commentary
Historical Development
Potassium bicarbonate has been a reliable player across industrial, agricultural, and household sectors for centuries. Dating back to early chemistry labs in the eighteenth and nineteenth centuries, its utility outshone many contemporaries—serving as a safer alkali salt compared to the harsher potassium carbonate. Before fully refined production methods arrived, folks collected and processed potash from wood ash, eventually landing on more consistent yields with the growth of analytical chemistry. Today, factories harness purified solutions, with the benefits of years of improvements guiding both purity and yield.
Product Overview
Bags or drums of potassium bicarbonate appear deceptively simple, but the content inside solves issues ranging from fire safety to crop protection. Sold in powder or crystalline forms, the product often carries food, technical, or pharmaceutical grades, reflecting how stringently contaminants are managed. Industries stake their confidence on its dependable behavior: in bakeries, greenhouses, fire stations, and pharmaceuticals.
Physical & Chemical Properties
Imagine a white crystalline powder that dissolves easily in water, suggesting versatility—potassium bicarbonate fits that mold. With a molecular weight of 100.12 g/mol and the formula KHCO3, its solubility and gentle, slightly alkaline reaction make it something of a safe bet compared to caustic potash. The salt releases carbon dioxide when heated or exposed to stronger acids, a property that’s found eager adopters in multiple chemical and food processes. Unlike caustic soda or strong alkali, it won’t sear skin or corrode basic containers, which makes storage and handling straightforward for most users.
Technical Specifications & Labeling
Technically inclined buyers focus on aspects like purity, granulometry, and moisture content. Food or pharma snapshots will require potassium bicarbonate to hit a minimum 99% assay, with heavy metal residues and microbial counts kept nearly invisible. Labels capture all the critical details: batch and lot number, fine print on maximum allowed sodium, and company-specific marks for traceability. Regular audits from certifying bodies and customer labs reaffirm these numbers, assuring end users that the bag’s promise aligns with results.
Preparation Method
Potassium bicarbonate is most often produced by bubbling carbon dioxide through an aqueous potassium carbonate solution. As the CO₂ dissolves and reacts, it nudges the chemical balance toward the bicarbonate—a simple, energy-efficient trick chemists have refined over generations. Any remaining carbonate drops out as a byproduct in carefully controlled environments, ensuring the bicarbonate stays pure. Facilities dial in these settings for thousands of tons, processing and packaging the powder in controlled air and humidity to avoid caking.
Chemical Reactions & Modifications
In chemical parlance, potassium bicarbonate showcases itself as a mild base and source of carbon dioxide. Drop it into an acid, and you’ll see fizzing as CO₂ escapes; heat it in a closed vessel, and water vapor and CO₂ gas both emerge, with potassium carbonate left behind. Many commercial processes piggyback on these properties, whether leavening bread or designing safer dry chemical fire extinguishers that knock out Class B and C fires. Chemists keep poking and probing, sometimes blending it with other substances to tailor pH control, solubility, or reaction rates for specialty jobs.
Synonyms & Product Names
Walk into a global chemical warehouse, and you’ll find potassium bicarbonate sold under names like potassium acid carbonate or simply “bicarb potash.” In the fire protection industry, it’s commonly listed as “Purple K.” On a food label, the E number E501(ii) assures bakers of its use as a raising agent. Different trade names still reflect the same, time-tested compound, changing only with the end use and local language flavors.
Safety & Operational Standards
Safety data reveals strengths and some gentle cautions—dust masks are advised for folks regularly handling open bags, since the powder, although considered low in toxicity, can irritate the respiratory system in large quantities. Storage guidelines prioritize dry, sealed containers away from acids or moisture, both to protect the powder and to guard against accidental releases of gas or caking. Fire extinguisher makers pay close attention to particle size and flowability, since these affect the reliability of the chemical in breaking combustion cycles. Most regulatory agencies, such as OSHA and EFSA, list potassium bicarbonate as low-risk, but sensible operational habits—good ventilation, clean storage, respectful handling—underscore every workplace policy.
Application Area
Kitchen pantries, fire station gear lockers, field sprayers, pharmaceutical labs—the reach of potassium bicarbonate feels endless. Bakers use it to make cakes rise without harsh alkaline notes, while greenhouse operators spray it on crops as a gentle fungicide approved for organic use. Firefighters rely on it for a safer, more effective extinguisher against grease or electrical fires. Some folks with kidney disorders use it under medical advice to manage blood acidity, though strict doses matter here. Many folks forget how it softens hard water or neutralizes acids in industrial wastewater, offering a reliable, simple way to fine-tune chemical flows in myriad settings.
Research & Development
R&D teams probe potassium bicarbonate for smarter, more sustainable uses. Trials explore how well it can replace harsher chemicals in organic farming, particularly since it breaks down to natural components that don’t accumulate in soils or waterways. Food scientists refine how it interacts with new gluten-free baking formulations, balancing leavening and taste. Environmental labs look into its use as a buffering agent in greener fire retardants, chasing lower toxicity not just for users but for wildlife, especially as regulations tighten around every part of the chemical value chain.
Toxicity Research
Potassium bicarbonate enjoys a strong track record on safety. Animal studies and chronic exposure reports rarely flag major risks when handled and consumed as intended. The main debate focuses on potassium ion load in people with kidney or heart concerns; for the general population, it passes muster as a safe food and pharma ingredient. Toxicologists continue watching for subtle, long-term trends, but decades of widespread use foster a level of comfort not easily rivaled among chemicals of its class.
Future Prospects
As climate, health, and environmental pressures mount, potassium bicarbonate stands ready for new roles. Its eco-friendly profile fits the mold for sustainable agriculture, where lower-impact fungicides grow in demand. Technological improvements in particle design and handling could boost its fire suppression power yet further, especially as buildings and data centers seek out greener, less corrosive safety options. In baking and food processing, product reformulation targets both lower sodium and better health, and potassium bicarbonate’s role may only expand as dietary guidelines shift. Given rising global interest in safe, renewable, and gentle chemicals, this everyday salt may yet anchor more future-facing innovations than past generations ever imagined.
What is potassium bicarbonate used for?
A Common Compound With Many Jobs
Potassium bicarbonate may sound like something you’d only hear about in chemistry class, but it shows up in spots you’d probably never expect. I’ve seen it on shelves at the garden store, and my neighbor keeps a tub in his pantry for baking. This white, odorless powder packs more punch than it first appears. Its main draw? It brings potassium to the table, and most diets fall short on that mineral these days.
Food and Kitchens: More Than Just Baking Soda’s Cousin
Baking powder often relies on potassium bicarbonate. Bakers reach for it as a leavening agent, especially those who want less sodium in their cookies and bread. Using it in place of sodium bicarbonate (baking soda) helps people with high blood pressure or those watching salt intake. The FDA recognizes potassium bicarbonate as safe for food use. It works without the bitter taste some substitutes leave behind, and its performance stays reliable across recipes. That may not seem dramatic, but healthy choices start with simple swaps like this.
Protecting Crops, Fighting Disease
I garden in my spare time, and mildew is always waiting for a rainy week to strike. Potassium bicarbonate tackles powdery mildew and black spot on fruit and vegetable plants. For organic growers and folks who stay away from harsh chemicals, this stuff checks the boxes. It wins over growers because it doesn’t stick around in the soil for long, and its effect on pests stays limited to the fungi. The Environmental Protection Agency has weighed in with strict testing to keep things safe for the environment, so it fits into sustainable farming methods better than many old-school fungicides.
Firefighting Tools
Potassium bicarbonate makes its mark in fire safety. Fire extinguishers stamped “purple-K” rely on it to knock down flammable liquid and electrical fires. In the oil and gas world or airport hangars, these extinguishers show up because the chemical smothers fire quickly and leaves little mess. It’s far more effective than sodium bicarbonate in these high-pressure settings, putting it ahead of older options for disaster response. Faster fire knock-down means smaller losses and, frankly, fewer tragedies.
Health and Home Uses
On a smaller scale, some people ask doctors about using potassium bicarbonate to treat mild acid buildup in the body, or metabolic acidosis. Medical professionals sometimes consider it for patients with low potassium, using it with regular monitoring. Keep in mind: potassium levels can affect heart function. Self-medicating with potassium salts gets risky. Always involve a doctor—too much potassium can be dangerous, especially for folks with kidney issues.
The Push For Healthier Salt Choices
Most people don’t hit the recommended daily potassium intake. High-potassium choices like potassium bicarbonate let food manufacturers tweak recipes for heart health. Swapping salty additives for potassium-based ones lowers sodium in processed foods, chipping away at chronic disease rates. Research backs up the link between more dietary potassium and lower blood pressure. For many, picking products with potassium bicarbonate instead of sodium salts serves up flavor and better nutrition, all in one bite.
Takeaways and Next Steps
In the kitchen, on the farm, in emergency kits, and maybe even in a few homes, potassium bicarbonate shows how an unassuming compound can have wide-reaching effects. As food makers, doctors, and everyday folks keep looking for ways to boost potassium and cut sodium, this ingredient deserves a spot in the public conversation. Education remains key—effects of different salts aren’t common knowledge. As with other food improvements, real change starts when people know their choices and understand what’s inside the packages on the shelves.
Is potassium bicarbonate safe to consume?
The Role of Potassium Bicarbonate in Food and Health
Potassium bicarbonate usually comes up during discussions about food additives and dietary supplements. Most know it as a powder added to help bread rise, balance pH in food processing, or keep produce fresh on store shelves. A question often follows: “Is it safe to eat?” From my background in food science and a home kitchen that experiments with all sorts of tricks, let’s dig into what using potassium bicarbonate means for health.
Most people already eat small amounts of potassium bicarbonate without noticing. Bakeries and soda manufacturers have relied on it for years. In the world of natural alternatives, it’s often chosen instead of baking soda (sodium bicarbonate), especially where low sodium diets matter. According to the U.S. Food and Drug Administration, potassium bicarbonate falls under the “Generally Recognized as Safe” (GRAS) category. For the average person eating a standard diet, the tiny sprinkle in processed foods won’t cause problems.
Potassium and the Body: Not Just a Food Additive
Potassium plays a huge role in keeping the heart and muscles working. The Centers for Disease Control and Prevention recommend adults get about 2,600–3,400 milligrams daily, depending on age, weight, and sex. Potassium bicarbonate serves as one way to add more potassium, especially for those watching their sodium. Some use it as a salt substitute, hoping to lower blood pressure or manage heart risks tied to too much salt. Research backs up potassium for balancing electrolytes and counteracting bloating from salty foods.
Concerns and Caveats: Who Should Be Cautious?
For most healthy folks, eating foods with potassium bicarbonate poses little concern. Things change with kidney disease or certain heart conditions. When kidneys can’t flush out excess potassium, levels can spike, causing confusion, irregular heartbeat, or worse. According to Harvard Health, people with kidney disease, those taking blood pressure pills called ACE inhibitors or certain water pills, or anyone with adrenal issues should not add extra potassium without checking with a doctor. At high doses, potassium bicarbonate might upset the stomach, leading to cramps or diarrhea. I’ve learned from relatives managing kidney issues that reading labels and limiting these additives really matters for them.
How to Use It Safely
People who want to use potassium bicarbonate in cooking or baking can do so in small amounts. One practical solution is switching half the baking soda in recipes for potassium bicarbonate, lowering sodium without losing the chemical reaction needed for leavening. Sports drink enthusiasts sometimes add it to homemade hydration formulas to aid recovery. For those with high blood pressure, talking with a healthcare provider before using it regularly makes sense—testing potassium levels in blood is pretty simple and can prevent surprises.
Diet diversity helps keep potassium levels in a healthy range. Leafy greens, bananas, beans, and avocados supply plenty of potassium without resorting to processed food additives. Labels make it easy to track what goes into packaged food—even home bakers can pick additives that meet their needs and comfort level. Decisions around food safety and personal health should rely on a mix of science, listening to your body, and honest conversations with primary care doctors.
How do you store potassium bicarbonate?
A Common Chemical With Some Simple Demands
Potassium bicarbonate shows up across industries, from fire extinguishers to baking. At home, it keeps wine making less acidic and soil healthier. Plenty of gardeners and food pros swear by it, but its usefulness sometimes hides a basic truth: careless storage turns good powder clumpy or useless.
Moisture: The Real Enemy
Anyone who’s opened a bag of baking powder on a humid day knows how quickly fine powders clump. Potassium bicarbonate acts the same way—exposing it to moisture can set off a chemical reaction that ruins its usefulness. I once saw a food safety technician toss out a nearly full drum because someone hadn’t screwed the lid on tight. The powder felt almost like clay.
Science backs up these worries. Potassium bicarbonate absorbs moisture from the air and starts to break down, especially if humidity stays above 50%. This lets carbon dioxide gas escape, messes up weight measurements, and shortens shelf life. So storing it bone dry—think well-sealed containers in low-humidity rooms—matters if you want the chemical to work as it should.
Why Air-Tight Containers Stay King
Lots of folks use whatever’s handy: plastic tubs, glass jars, even Ziplock bags. Some containers promise to be airtight, but it pays to test a few out. In my kitchen, Mason jars with rubber gaskets beat out cheap plastic every time—the seal stands up to repeated opening and closing, and glass never reacts with the chemical.
For labs or industry, heavy-duty plastic drums with gasketed lids offer bulk protection. Labels mean a lot here. Too many times, someone has grabbed the wrong powder because the label faded or got smeared. Permanent marker fades; adhesive labels can peel, especially if handling wears them down. Laminated or printed labels fix that problem.
Light and Heat: Friends to Avoid
Potassium bicarbonate breaks down faster in heat and direct sunlight. Even sitting next to a sunny window takes its toll. I’ve seen fire extinguisher refills kept in sunlit back rooms lose their punch months early. Rooms set at stable, moderate temperatures—cool, but not freezing—give a longer shelf life. Storage near ovens, boilers, or sunny walls adds extra risk.
Safe Distances Mean Fewer Hazards
This powder isn’t toxic, but mixing it up with acids triggers messy reactions—think fizz and foam. Storing chemicals with a buffer between them prevents accidents. Separate shelves or cabinets, just like in chemistry labs, cut down on cross-contamination. Fire codes also expect tight chemical management—insurers and regulators won’t accept slack storage, so having practices in place also supports peace of mind.
Practical Habits for Everyday Use
No one likes running out or finding spoiled stock. Keeping only as much as you’ll use, rotating supplies, and labeling with open dates helps. In my experience, relying on clear jars and visible expiration dates avoids headaches, whether you’re fighting kitchen moisture or industrial slip-ups. If powder acts strange—lumpy, discolored, or foul-smelling—it’s safest to start fresh.
Final Thoughts
Smart storage keeps potassium bicarbonate ready to do its job, whether that’s neutralizing acids, stopping fires, or making food taste right. A few extra minutes sealing up a jar, labeling, and picking the right shelf goes a lot further than most folks think.
What are the side effects of potassium bicarbonate?
Potassium Bicarbonate Isn’t Just a Baking Ingredient
It’s easy to glance at the label on an antacid or a dietary supplement and shrug off potassium bicarbonate as some background additive. In truth, real people put these compounds into their bodies, sometimes daily, either to treat low potassium, calm acid reflux, or protect their bones. Its presence across so many over-the-counter products makes understanding side effects not just a medical chore, but common sense for anyone who cares about their own health.
Too Much Potassium Can Disrupt the Heart
Healthy kidneys do a great job balancing potassium levels. Problems often begin when kidneys struggle or when someone already has another medical issue like diabetes or heart disease. If the body can’t get rid of surplus potassium, the level in the blood can sneak up. At a certain point, the heart’s own electrical signals get jammed. This can trigger skipped beats, dangerous heart rhythms, or, in the worst cases, cardiac arrest. The US Food and Drug Administration repeatedly traces hospitalizations back to high potassium intake from supplements—facts that keep popping up in medical publications and poison control reports.
Upset Stomach and Digestion Problems Appear First
Over the years, many people experience stomach cramps or nausea soon after they swallow potassium bicarbonate tablets. I’ve sat through plenty of conversations with folks who joke about these tablets feeling “like a rock” in their gut. Physicians even suggest taking potassium supplements with meals not just because it helps absorption, but because fewer people feel ill this way. Reports of vomiting, diarrhea, and loose stools follow closely behind—none of which are subtle, and all can easily derail a daily routine.
Bicarbonate Brings Its Own Set of Risks
Bicarbonate, as part of baking soda and antacids, can cause the blood to veer toward being too alkaline, a condition called metabolic alkalosis. Some symptoms are tricky: muscle twitching, tingling in the fingers, or persistent headaches. When left unchecked, this chemical shift creates a mess of complications—muscle weakness pops up, breathing may slow, and there’s a real risk of confusion or even seizures. Studies inside hospitals highlight older adults and those with kidney issues as especially vulnerable. The advice from dietitians and doctors matches my own experience in talking with patients: monitor symptoms and tell someone quickly if new ones show up.
Allergies and Medication Conflicts
Allergic reactions, though rare, get reported. The more common stories feature rashes, swelling, shortness of breath, or a scratchy throat after taking a supplement. Another real worry: drug interactions. Common drugs for high blood pressure or heart problems—ACE inhibitors and potassium-sparing diuretics—boost potassium, too. No warning label on a supplement bottle replaces the wisdom of a pharmacist or a doctor who draws from years of real-world cases. Conversations with healthcare providers stay crucial, especially with complex medication lists.
Practical Steps for Safer Use
Many problems with potassium bicarbonate start simple: using more than prescribed, skipping lab checks, or forgetting to update doctors about new supplements. Clinics everywhere push routine blood tests for anyone taking potassium. Technology helps—apps and reminders keep track of doses. Even better, talking regularly with the pharmacy team closes knowledge gaps. Smart supplement use, solid communication, and steady follow-up make all the difference in keeping side effects in check.
Can potassium bicarbonate be used as a baking substitute?
Baking and Leavening: The Science in Your Kitchen
Pretty much every baker, whether professional or home-based, has scrambled for baking powder or soda at some point. It’s only natural to start eyeing the other white powders in the pantry when you’re in a pinch. Potassium bicarbonate often gets a curious look, especially as folks look for sodium-free options. The real question is—does it do the trick in cookies, cakes, and quick breads?
What Potassium Bicarbonate Actually Does
If you look at the chemistry, potassium bicarbonate does the heavy lifting as a leavening agent much like familiar baking soda (sodium bicarbonate). Mix it with an acid—think yogurt, vinegar, lemon juice—and it fizzes up, releasing carbon dioxide that helps the dough or batter rise. You end up with fluffier bakes, just like with traditional soda. Diabetics and people keeping an eye on blood pressure often welcome potassium-based products since they dodge some of the pitfalls of high sodium.
Health and Dietary Choice
Many packaging labels highlight “sodium-free” as a desirable trait these days. A lot of folks with hypertension or heart disease get told by their doctors to cut back on sodium, and swapping out regular baking soda for potassium bicarbonate keeps baking on the table. The American Heart Association points to excess sodium as a risk factor for high blood pressure, so options that skirt around this mineral hold real value, especially for aging adults.
Baking Results: Similar, But Not the Same
From my own trial runs and several cookbooks full of notes, potassium bicarbonate generally stands in for baking soda at about a one-to-one ratio. Some sources recommend bumping it up just a bit, since it’s a touch less alkaline. There’s a difference in taste—potassium adds a very slight bitterness or metallic edge if overused, which shows up more in delicate pastries and less in bold-flavored baked goods like spiced muffins or whole grain bread.
The other thing to watch is the saltiness. Swapping sodium out means that some familiar flavors shift. To compensate, bakers often increase regular table salt just a bit, enough to round out the taste profile. You’re not doubling up—just paying attention to what’s missing once sodium steps out of the spotlight.
Cost, Availability, and Safety
Potassium bicarbonate doesn’t get the same shelf space as baking soda at most big-name supermarkets. Health stores and online retailers stock it, but it definitely costs more. Folks on a low-budget baking schedule don’t always want to make the switch.
There’s one more thing—potassium isn’t something everyone can pack into their diet without thinking. Renal patients and those with certain heart conditions get strict potassium limits for their own safety. Anyone who needs to monitor mineral intake should double-check labels and consider medical guidance before making major substitutions.
Solutions for the Home Baker
For daily baking needs, potassium bicarbonate pulls its weight as a sodium-free leavening alternative. It fills an important gap for health-conscious bakers. If taste shifts or texture issues turn up, blend it with familiar recipes and adjust acid and salt levels until you nail the right balance. Consult a registered dietitian if you’re managing a health condition. Science backs up the swap, and personal trials bring the finishing touch, making every loaf and cookie truly your own.
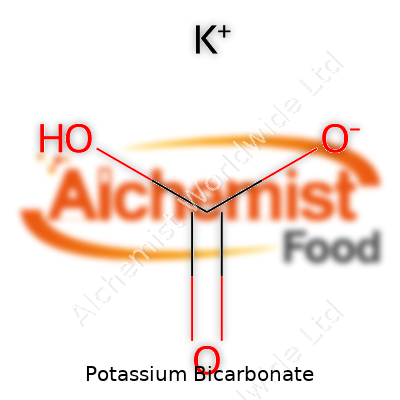
| Names | |
| Preferred IUPAC name | Potassium hydrogencarbonate |
| Other names |
Potassium hydrogen carbonate E501(ii) KHCO3 |
| Pronunciation | /pəˌtæsiəm baɪˈkɑːbənət/ |
| Preferred IUPAC name | Potassium hydrogencarbonate |
| Other names |
Potassium acid carbonate Potassium hydrogen carbonate E501(ii) KHCO₃ |
| Pronunciation | /poʊˌtæsiəm baɪˈkɑːrbəneɪt/ |
| Identifiers | |
| CAS Number | 298-14-6 |
| Beilstein Reference | 358916 |
| ChEBI | CHEBI:132678 |
| ChEMBL | CHEMBL1201201 |
| ChemSpider | 72863 |
| DrugBank | DB09420 |
| ECHA InfoCard | 03e6c7be-d855-427d-ae87-2e8362a9d7fc |
| EC Number | 290-350-9 |
| Gmelin Reference | 78038 |
| KEGG | C06469 |
| MeSH | D017687 |
| PubChem CID | 516892 |
| RTECS number | TT2975000 |
| UNII | VZ8Z878B3G |
| UN number | UN 2984 |
| CAS Number | 298-14-6 |
| Beilstein Reference | 320269 |
| ChEBI | CHEBI:132676 |
| ChEMBL | CHEMBL1201473 |
| ChemSpider | 54868 |
| DrugBank | DB11097 |
| ECHA InfoCard | ECHA InfoCard: 03-2119956402-49-0000 |
| EC Number | 204-589-4 |
| Gmelin Reference | Gmelin Reference: 25557 |
| KEGG | C18606 |
| MeSH | D011187 |
| PubChem CID | 516892 |
| RTECS number | TZ7800000 |
| UNII | VZ8Z890U78 |
| UN number | UN3352 |
| Properties | |
| Chemical formula | KHCO3 |
| Molar mass | 100.115 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.17 g/cm³ |
| Solubility in water | 22.4 g/100 mL (20 °C) |
| log P | -0.8 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10.33 |
| Basicity (pKb) | 8.3 |
| Magnetic susceptibility (χ) | 'Slightly diamagnetic' |
| Refractive index (nD) | 1.410 |
| Dipole moment | 2.39 D |
| Chemical formula | KHCO3 |
| Molar mass | 100.115 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.17 g/cm³ |
| Solubility in water | 74.4 g/100 mL (20 °C) |
| log P | “-0.77” |
| Vapor pressure | Vapor pressure: Negligible |
| Acidity (pKa) | pKa 10.3 |
| Basicity (pKb) | 8.3 |
| Magnetic susceptibility (χ) | Magnetic susceptibility (χ): −40.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | “1.412” |
| Dipole moment | 6.48 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 102.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -948.58 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -882.6 kJ/mol |
| Std molar entropy (S⦵298) | 146.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -811 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -882.6 kJ/mol |
| Pharmacology | |
| ATC code | A12BA02 |
| ATC code | A12BA02 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory irritation. |
| GHS labelling | GHS07; Warning; H319 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: "Store in a dry place. Avoid breathing dust. Wash thoroughly after handling. Wear protective gloves/eye protection. |
| NFPA 704 (fire diamond) | 2-0-1 |
| Autoignition temperature | > 400 °C |
| Lethal dose or concentration | LD50 oral rat 2820 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 2825 mg/kg |
| NIOSH | TTQ39440K0 |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 600 mg |
| Main hazards | May cause mild skin and eye irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | Store in a dry place. Store in a closed container. Avoid breathing dust. Wash hands thoroughly after handling. |
| NFPA 704 (fire diamond) | 1-0-1 |
| Lethal dose or concentration | LD50 (oral, rat): 2820 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 2820 mg/kg |
| NIOSH | SA165 |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 3000 mg |
| Related compounds | |
| Related compounds |
Potassium carbonate Sodium bicarbonate Ammonium bicarbonate Potassium chloride |
| Related compounds |
Potassium carbonate Sodium bicarbonate Potassium chloride |

