Potassium Benzoate: A Closer Look from Lab to Table
Historical Development
Potassium benzoate first turned heads back in the late 1800s. Chemists hunting for ways to keep food safer for longer ended up uncovering a preservative that proved itself in the fight against spoilage and unwanted fermentation. Producers, tired of losing entire batches to mold and bacteria, saw potassium benzoate as a relief. The compound made its way into processed foods through trial, error, and the slow march of food safety laws. Its adoption stretches across continents, from American bottling plants to European bakeries. Countries built their own rules around it, so over time, the patchwork of regulations reflected not just health research but also shifts in public confidence about chemical preservatives. Nearly everyone has eaten something protected by potassium benzoate, even if the label didn’t wave a flag about it.
Product Overview
Potassium benzoate keeps industries moving. As a salt of benzoic acid, it pushes the shelf life of sodas, fruit juices, pickles, and syrups far beyond what fresh versions can manage. The stuff doesn’t just make food last; it’s cheap to produce and beats out many alternatives at stopping yeasts and bacteria from wrecking stocks. Large food and beverage companies buy it in metric tons, mixing it into their recipes, while cosmetics manufacturers trust it to keep lotions fresh. Even animal feed suppliers lean on this preservative to keep their products from going bad before animals get fed.
Physical & Chemical Properties
Potassium benzoate looks like a white, odorless powder or crystals—fine enough to blend easily into food and drink. It dissolves in water much better than benzoic acid, giving it an edge in any recipe with liquid. With a molecular weight just shy of 200, this salt keeps its integrity up to high temperatures but breaks down in strong acids, freeing up benzoic acid, which takes the lead on microbial defense. At room temperature, this compound keeps stable almost indefinitely if stored dry. Its taste isn’t pronounced at the low levels used for preservation, so most people will never pick it out unless the dosage goes way overboard.
Technical Specifications & Labeling
Regulations tell food processors exactly how to use potassium benzoate: concentrations max out at about 0.1% by weight in products like sodas and fruit drinks. Regulatory agencies in North America, Europe, and beyond require producers to disclose the addition, using clear terms like ‘potassium benzoate’ or E212 on packaging. Production standards hinge on testing for purity, running checks for possible contaminants such as metals or residual solvents. Suppliers often issue batch documents with each shipment, laying out chemical analyses that buyers can cross-reference. For buyers, the smart approach means reading the technical datasheets carefully and checking local food laws so nothing gets flagged during import or export.
Preparation Method
The usual route starts with benzoic acid, which reacts with potassium hydroxide or potassium carbonate in water. A fizz of carbon dioxide leaves behind the potassium salt, and filtration brings out the solid. After that, producers dry and grind the crystals for shipping. Industrial production lines favor continuous reactors that keep unwanted byproducts low. Purity relies heavily on water quality and proper handling, so operations run strict quality control checks at every step. Small slip-ups in the process—like using impure reagents—show up in lab tests, so companies eager to keep contracts pay close attention to these details.
Chemical Reactions & Modifications
Potassium benzoate doesn’t stand out as a chemical workhorse, yet it opens up a few options for chemists. Exposure to strong acids in food, such as soda with a low pH, flips potassium benzoate back to benzoic acid, which then bats away bacteria and mold. Under lab conditions, the compound can take part in coupling reactions or get tweaked into esters or other benzoate salts, extending its reach beyond preservation into polymers and resins. Handling it with strong oxidizers rarely ends well—safety data sheets warn against throwing it in the mix with aggressive chemicals.
Synonyms & Product Names
Industry folks use several labels for this preservative. It goes by ‘potassium benzoate’ in the ingredient list, but E212 takes over in European Union standards. Other tags include benzoic acid, potassium salt, and food additive E212. Some brands use alternate names in their safety sheets or catalogs, such as ‘Benzoate of Potash’ or specific supplier product numbers. While scientific language doesn’t always carry over to grocery store labels, food technologists and procurement officers learn to navigate all versions during international purchasing.
Safety & Operational Standards
Potassium benzoate usually lands a spot on ‘generally recognized as safe’ lists when used in line with food regulations. Processing plants install containment so dust exposure stays low, giving workers adequate PPE to cut down on inhalation or accidental skin contact. Companies include full hazard breakdowns in their safety documentation, making sure first responders know what to do if there’s ever a leak or spill. Ongoing training helps factory crews handle, store, and dispose of this preservative within tightly defined limits, reflecting industry habits built on regulatory audits and the occasional recall scare.
Application Area
Food and drink industries count on potassium benzoate as a pick against spoilage. Soft drinks and fruit juices, acidic enough for the preservative to work, headline the list; condiments and pickles, prone to fermentation, follow close behind. Winemakers and brewers sometimes use it as a backup preservative to keep yeast activity under control after fermentation. Outside food, this compound appears in cough syrups, oral care products, and even the odd personal care lotion or shampoo. Some industrial water treatments ride on potassium benzoate’s antifungal clout as well. In most uses, the aim isn't to dramatically change taste or feel, just to keep products in circulation longer without opening the door to microscopic freeloaders.
Research & Development
Recent R&D focuses on cleaner production, new blends, and understanding the compound’s exact path through the body after ingestion. Food scientists looking for alternatives keep an eye on potassium benzoate’s interplay with other common preservatives—mixing it with sorbates, for example, often delivers broader protection using less material. Consumers ask tougher questions, pushing producers to show more detail about ingredient sourcing and traceability, which leads to improved analytical methods for detecting potassium benzoate at lower levels in finished products. Studies exploring novel encapsulation techniques hope to slow down the preservative’s release or mask its presence for even longer shelf life or improved flavor experience. Academic labs occasionally dig into the compound’s interaction with new food processing methods, such as high pressure or pulsed light, aiming for products that look and taste fresh but still last as long on the shelf.
Toxicity Research
Countless safety reviews, including work from the USFDA, EFSA, and WHO, pin safe intake at a level far higher than any normal diet provides. Researchers pay close attention to what happens if potassium benzoate bumps into ascorbic acid (vitamin C), since under the right conditions, they can form trace benzene—a compound nobody wants in their food. Oversight agencies set tight benzene thresholds, watching for issues with recipes combining the two ingredients, especially in products with long shelf lives. Animal studies indicate low acute toxicity, with modest intake showing no significant health effects. Potential risks, like allergic reactions, rarely surface in consumers. The science world circles around ongoing studies with children and long-term consumption, especially since beverage habits keep changing across generations.
Future Prospects
The road ahead for potassium benzoate ties into the growing demand for transparency and gentler preservation strategies. Food processors and ingredient companies balance using time-tested additives and answering skepticism from shoppers weary of ‘chemical-sounding’ names. Alternatives with cleaner labels crop up, but new regulations and market shifts keep potassium benzoate in the running—especially for producers chasing global markets where cold chain logistics lag behind. Researchers explore how packaging innovations and reformulation can stretch product life without any preservative, but shelf life improvements and cost pressures often bring the conversation back to tested compounds like potassium benzoate. Solutions could come from finding ways to reduce total preservative levels, combining ingredients smarter, or communicating substantively about safety and sourcing to win back trust where it’s dipped.
What is Potassium Benzoate used for?
Why You Keep Seeing Potassium Benzoate on Labels
Walk into any grocery store, flip a soda can or salad dressing, and the ingredient list might mention potassium benzoate. This compound shows up in everything from diet soft drinks to pickles, and for folks who try to keep tabs on what’s in their food, it’s worth understanding why companies keep adding it.
In my years growing up—especially during long, hot summers—there was almost always a jug of homemade lemonade chilling in the fridge, no preservatives added. But most store-bought bottles don’t get that luxury. Drinks sit for weeks on shelves, and that's where potassium benzoate comes into play. This additive keeps drinks, jams, fruit syrups, and some salad dressings from going bad. Bacteria and mold like sugary or acidic environments, and potassium benzoate helps prevent them from turning your soda into a science experiment.
Protection Against Spoilage
Foodborne illness always hovers in the background of mass food production, so manufacturers lean on solutions that address those risks. Potassium benzoate steps in as a sturdy guard because it works well in acidic conditions, like sodas or fruit products. According to the FDA, potassium benzoate has earned the green light for use, provided concentrations in food don’t go above 0.1%. That amount helps slow down the growth of unwanted microbes without affecting taste, when used responsibly. You end up with safer products that can last until you’re ready to open them.
I’ve seen firsthand, especially in family pantries, how quick perishables can turn when left unchecked during the summer. Food waste is no joke. Preservatives like this compound cut down on spoilage. In a world where one third of all food grown ends up tossed, this role matters, especially for folks living farther from cities or supermarkets.
Looking at Potential Health Concerns
Questions keep popping up about artificial preservatives, and potassium benzoate isn’t immune from scrutiny. Some believe it hurts health if overconsumed. The main worry happens when it gets mixed with certain ingredients, like ascorbic acid (vitamin C). Under high heat or with long storage, that mix may produce small amounts of benzene, a chemical no one wants to drink. The good news: companies have stepped up, reformulating recipes and closely watching benzene levels. Checks run by agencies like the Food and Drug Administration and World Health Organization show that, in ordinary diets, potassium benzoate doesn’t come close to reaching unsafe amounts.
Are There Alternatives or Solutions?
If you’re trying to steer clear of added preservatives, fresh foods and homemade options dodge most additives. A shift toward cold-pressed juices or fermented favorites, where old-school methods keep things safe, gives people choices. For those in the food business, transparency helps—labeling clearly, limiting preservative levels, and keeping production clean. If trust gets built, consumers can make informed calls.
Potassium benzoate won’t steal the spotlight like organic farming or fancy superfoods, but it plays a familiar role in today’s food supply. Safe in the right amounts, useful for cutting down waste, and with ongoing oversight, it keeps showing up for a reason. Everyone benefits from knowing what goes into the food on their plate.
Is Potassium Benzoate safe to consume?
Understanding the Source
Shoppers often pause once they catch a glimpse of potassium benzoate listed among the fine print of a food label. The name sounds more like a lab experiment than a pantry staple. Despite the unsettling vibe, potassium benzoate gets added to sodas, pickles, jams, and salad dressings. Companies choose it because it stops the growth of mold, yeast, and some bacteria. Potassium benzoate basically holds the line on food spoilage.
The Science Behind the Use
Research helps ease the nerves. The FDA has given potassium benzoate the green light, calling it “generally recognized as safe” at certain limits. The safety level sticks at up to 0.1% of the food by weight. On one hand, tests show it’s not prone to build up in the body. The liver quickly breaks it down, and kidneys flush it out. Large health studies, including data from the World Health Organization, haven’t tied potassium benzoate to cancer or major diseases—at least at the low levels you see in sodas or canned tomatoes.
Why Potassium Benzoate Draws Concerns
Some online forums spark alarm by connecting potassium benzoate with benzene, a known carcinogen. Here’s the lay of the land: potassium benzoate can break down to form benzene, but only under specific conditions, such as with heat and light in the presence of vitamin C (ascorbic acid). The FDA and food scientists noticed this issue back in the early 2000s and asked companies to swap out problematic formulas. Spot checks keep happening, and so far, tested products don’t carry benzene levels that approach danger. It’s understandable to feel suspicious about preservatives, given the chemical drama, but those tests help put the issue in context.
My Take From Real Experience
I remember working in kitchens where big tubs of a preservative kept salad dressing fresh through long and humid summers. Without additives, that dressing might turn sour in a week. I’ve yet to see a friend or family member get sick from potato chips or cola that list potassium benzoate. Still, anyone with a sensitive palate or allergies tends to watch labels carefully. Artificial preservatives rarely add nutritional value but make a big difference in convenience, reducing waste, and lowering costs. More than a few cash-strapped college students depend on shelf-stable food to make their budget last until payday.
Watching Labels and Supporting Change
Plenty of folks still prefer simple ingredient lists. Some small-scale producers have ditched potassium benzoate and switched to vinegar, citric acid, or even refrigeration. These methods work in small batches, though scaling them gets tricky and more expensive. If you want to cut down your exposure, stick to fresh or frozen foods when possible. Reading ingredients doesn’t hurt, especially when feeding small kids, since research on the effects in developing bodies continues to unfold.
Looking at the Bigger Picture
Potassium benzoate helps solve real problems caused by spoilage—food waste, foodborne illness, and rising grocery bills. Food science keeps tweaking processes to make additives safer or to find alternatives. Pushing for more research just means we keep learning—an open mind anchored in facts handles most food fears better than rumors. Moderation matters. Avoiding panic, asking questions, and staying alert matters far more for smart eating than memorizing chemical names or cutting out whole food groups because of a scary-sounding word.
What foods contain Potassium Benzoate?
Where Potassium Benzoate Shows Up
Potassium benzoate pops up in foods many people eat every week, sometimes without thinking much about it. On the back of soda bottles, fruit juice boxes, or even jars of pickles, you might spot its name deep in the fine print. Companies add potassium benzoate to food and drinks as a preservative, mostly to prevent spoilage and keep things fresh. This additive gets used because it stops bacteria and mold from growing in acidic foods.
Soda and Soft Drinks
Any trip down the soda aisle will show just how often manufacturers use potassium benzoate. Soft drinks, especially colas, citrus sodas, and diet carbonated drinks, use this preservative to fight off unwanted germs. Many soda companies combine it with another preservative, sodium benzoate. These two work well in anything with a lower pH. While this ingredient keeps sodas shelf-stable, its use has come under scrutiny as experts question how much humans should be consuming.
Fruit Juices and Juice Drinks
Packaged fruit juices, fruit punches, and juice blends often list potassium benzoate on their ingredient panels. Both chilled and shelf-stable juices rely on it, especially if they do not contain enough natural acid or sugar to stay fresh on their own. Even “all natural” juice drinks sometimes include this preservative, especially if the product needs to last on a store shelf for months. Lemonade, limeade, and orange-flavored drinks seem to use it regularly.
Pickles, Relishes, and Vinegar-Based Condiments
Look at a jar of bright green pickles or a container of relish, and potassium benzoate might be holding back yeast growth. Vinegar-based condiments like certain salad dressings and hot sauces rely on it too. This compound helps keep crunchy pickles flavorful and vivid in color long after leaving the factory. Many small brands avoid chemical preservatives, but you’ll see big national brands use potassium benzoate for consistency and shelf life.
Salad Dressings and Syrups
Bottled salad dressings and ready-to-serve syrups line supermarket shelves for months, so potassium benzoate often keeps these products tasting their best. Honey-based sauces, syrups for pancakes, and some fruit toppings use it for microbial control. Sugar might preserve some products, but dressings low in sugar or with lots of water often need a little extra help from this preservative.
Understanding the Health Angle
Potassium benzoate remains safe under current guidelines set by health agencies, but not everyone feels comfortable with preservatives. Some studies explore links between benzoates and allergic reactions or hyperactivity in certain people, particularly children. Most regulatory reviews find the amounts used in foods do not threaten health for most people. All the same, reading ingredient labels brings more awareness about what goes in our bodies.
Choosing Foods with Fewer Additives
People wanting to avoid potassium benzoate have a few options. Choosing whole fruits over packaged juice shrinks preservative intake. Select local pickles or small-batch dressings that focus on simple, traditional ingredients. Try sparkling water or make flavored drinks at home instead of buying factory-made sodas. By looking for clear, simple labels, people can lower their exposure if they wish, all while enjoying foods that taste good and support well-being.
Are there any side effects of Potassium Benzoate?
Understanding Potassium Benzoate
Potassium benzoate pops up in plenty of food and drinks, mostly to keep them fresh for longer. You’ll spot it in sodas, fruit juices, pickles, even some condiments at the grocery store. Manufacturers like using it because it does a good job. Not every ingredient with a long scientific name needs to be scary, but it pays to see what happens when you eat these chemicals, especially over time.
Possible Side Effects for Sensitive Folks
Eating or drinking stuff with potassium benzoate rarely causes problems for most people in small amounts. Bodies break it down, and kidneys flush it out without much fuss. For people with certain allergies or asthma, though, things get tricky. There’s some evidence linking benzoates to allergic reactions— think hives, itching, or trouble breathing—especially among kids already sensitive to aspirin or who have chronic asthma. A lot comes down to a person’s makeup and how much exposure builds up over the years.
Stomach issues can show up, usually if you are hitting large quantities. Headaches and mouth irritation aren’t reported all the time, but they may happen to some unlucky people. The U.S. Food and Drug Administration, along with the European Food Safety Authority, considers potassium benzoate “generally recognized as safe” so long as food makers stick to set limits. The problem isn’t usually with the small amounts in a single can of soda, but with habits. Diets loaded with preserved foods add up.
Concerns About Benzene Formation
Benzene gets folks talking and not in a good way. Potassium benzoate can react with ascorbic acid, which is vitamin C, in the presence of heat or light. This combination sometimes pops up in soft drinks. Benzene has been called out by scientists as a cancer risk with long-term exposure. Health agencies set maximum levels allowed in food, and drink companies tweak recipes to avoid this chemical reaction as best they can. Still, folks who often reach for sweet, carbonated drinks should probably pay attention.
Looking at the Bigger Picture
The modern diet has shifted toward more packaged, long-lasting foods, and with that, more exposure to ingredients like potassium benzoate. The tough part? Many people reach for these foods out of convenience, budget, and habit. Folks with chronic illnesses, older adults, and children might be more sensitive to any accumulation. This doesn’t mean panic, just some awareness goes a long way.
Side effects aren't common, but the trick is balance. Fresh produce, home-cooked meals, and fewer processed snacks can dodge a lot of additives. If you deal with allergies or asthma, read ingredient lists a little closer and listen to your body’s signals. Sticking to moderation avoids problems most of the time.
Possible Solutions and Smarter Choices
Clearer labeling would help people avoid potassium benzoate if they want to. Supporting research into new ways to keep foods fresh without raising health flags would also benefit everyone. Parents, in particular, should watch for behavioral or allergy-like reactions in their kids after trying new foods or drinks made with this preservative. Cleaning up household diets is an easy first step.
Change often starts at home. Cooking meals from scratch, limiting sodas and packaged sweets, and making time for regular grocery shopping keep most risks in check. Potassium benzoate serves a purpose, but it’s smart to know where it hides and keep an eye on your own and your family’s reactions.
Is Potassium Benzoate the same as sodium benzoate?
What Are These Additives?
Potassium benzoate and sodium benzoate show up on food labels all over the grocery store. Their main job? Fight off mold, yeast, and bacteria to keep foods fresh. They slip into everything from soft drinks to salad dressings. At first glance, they look like twins—each comes from benzoic acid, a weak acid present in some fruits such as cranberries. The difference lies in the mineral attached. Potassium benzoate has potassium; sodium benzoate sports sodium. That seems minor. But that one swap carries real meaning for health-conscious folks and anyone advised to mind their sodium intake.
Does Swapping Sodium for Potassium Matter?
Many people today worry about eating too much sodium. Doctors and nutrition experts point to the steady climb in blood pressure and heart disease happening in communities with high-sodium diets. Sodium benzoate, by virtue of its name, tacks a little more sodium onto your daily total. That might seem small—after all, sodium benzoate gets added in milligram amounts—but it adds up, especially if you lean on processed foods.
Potassium acts differently. Nutritionists often say folks don’t get enough of it. Eating foods with potassium benzoate corners a bit more of that essential mineral, which can help balance blood pressure. The Centers for Disease Control and Prevention backs up the push for lower sodium and higher potassium in diets. The switch from sodium benzoate to potassium benzoate fits into that shift, though food chemistry and taste must hold up in the process.
Health Concerns Surrounding Benzoates
Both potassium benzoate and sodium benzoate go through decades of safety reviews. The U.S. Food and Drug Administration labels them as “generally recognized as safe” when used as approved. The problems show up when they bump into certain ingredients. In acidic environments, like sodas, these benzoates can interact with vitamin C (ascorbic acid) and create benzene. Benzene ranks among known carcinogens, though regular soft drink tests often reveal amounts well below concerning levels. Still, food manufacturers work to keep these combinations in check and test for benzene formation in products before they hit shelves. Having a watchdog looking out for this is important because customers don’t always know how ingredients mix behind the scenes.
Why Some Food Makers Pick Potassium Benzoate
Beverage producers often pick potassium benzoate for drinks aimed at folks who want “low sodium” or “no added sodium” claims. Products designed for people with hypertension or other salt-sensitive issues sometimes cut the sodium wherever possible. Here, potassium benzoate offers a tool for food scientists to extend shelf life without giving up taste. Customers expecting a certain flavor profile or color stability in their foods aren’t always keen on big changes, so the option to swap a preservative without upending the product helps companies keep loyal customers happy. I’ve seen companies reformulate old favorites in search of a cleaner or more health-forward label and potassium benzoate often marks a step in that journey.
Is One “Better” Than the Other?
No one-size-fits-all answer works here. For most healthy adults, the small amount of either preservative won’t tip them into danger. Potassium benzoate’s lower sodium profile makes it a smart choice for those watching salt. Still, not everyone can load up on potassium, especially folks with kidney problems. The lessons from nutrition research suggest that reading labels and paying attention—especially if your diet leans on lots of processed snacks or sodas—matters more than fixating on a single preservative. Those living with specific health challenges should listen to their care teams about what fits best.
Improvements on the Horizon
Companies in the food industry face growing pressure to use simpler, more transparent ingredients. Some begin turning to natural options, like vinegar or rosemary extracts, where possible. Chemical preservatives keep food affordable and safe for longer periods. Until all people can lean fully on natural preservation, safe, regulated use of potassium or sodium benzoate sticks around. Shoppers who care about these details can vote with their wallets, encourage clear labeling, and keep an eye out for new preservation solutions down the road.
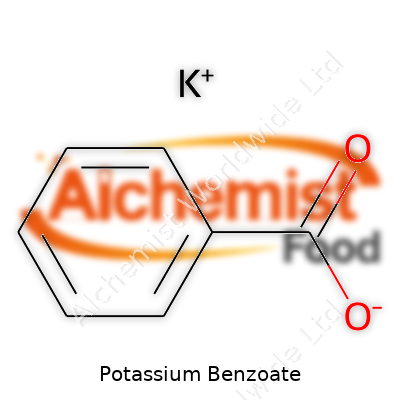
| Names | |
| Preferred IUPAC name | Potassium benzenecarboxylate |
| Other names |
Benzoic acid potassium salt E212 Potassium salt of benzoic acid |
| Pronunciation | /pəˈtæsiəm ˈbɛnzəʊ.eɪt/ |
| Preferred IUPAC name | Potassium benzoate |
| Other names |
Benzoic acid, potassium salt E212 Potassium benzenecarboxylate |
| Pronunciation | /pəˈtæsiəm ˈbɛnzoʊ.eɪt/ |
| Identifiers | |
| CAS Number | 582-25-2 |
| Beilstein Reference | 1206810 |
| ChEBI | CHEBI:77564 |
| ChEMBL | CHEMBL1356 |
| ChemSpider | '8613' |
| DrugBank | DB06710 |
| ECHA InfoCard | 100.028.252 |
| EC Number | 211-072-2 |
| Gmelin Reference | 17190 |
| KEGG | C01787 |
| MeSH | D017681 |
| PubChem CID | 5178 |
| RTECS number | DH6650000 |
| UNII | OY599E2GPI |
| UN number | UN1431 |
| CAS Number | 582-25-2 |
| Beilstein Reference | 1207934 |
| ChEBI | CHEBI:32044 |
| ChEMBL | CHEMBL1356 |
| ChemSpider | 5285 |
| DrugBank | DB11150 |
| ECHA InfoCard | 100.960.081 |
| EC Number | 211-082-2 |
| Gmelin Reference | 7142 |
| KEGG | C07125 |
| MeSH | D017708 |
| PubChem CID | 23681699 |
| RTECS number | DH6650000 |
| UNII | X7T129JQ5U |
| UN number | UN1431 |
| Properties | |
| Chemical formula | C7H5KO2 |
| Molar mass | 142.19 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.5 g/cm3 |
| Solubility in water | Soluble |
| log P | -2.5 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.2 |
| Basicity (pKb) | 11.0 |
| Magnetic susceptibility (χ) | +40.0e-6 cm³/mol |
| Refractive index (nD) | 1.530 |
| Dipole moment | 2.65 D |
| Chemical formula | C7H5KO2 |
| Molar mass | 160.21 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.5 g/cm3 |
| Solubility in water | 55.8 g/100 mL (25 °C) |
| log P | -2.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10 |
| Basicity (pKb) | 8.35 |
| Magnetic susceptibility (χ) | -34.0e-6 cm³/mol |
| Refractive index (nD) | 1.492 |
| Dipole moment | 2.45 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 155.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -561.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -385.0 kJ/mol |
| Std molar entropy (S⦵298) | 155.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -601.5 kJ/mol |
| Pharmacology | |
| ATC code | A01AB02 |
| ATC code | A01AB21 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "May cause respiratory irritation. Causes serious eye irritation. |
| Precautionary statements | Wash thoroughly after handling. Do not eat, drink or smoke when using this product. |
| NFPA 704 (fire diamond) | 2-0-0-✨ |
| Flash point | > 100 °C (212 °F) |
| Lethal dose or concentration | LD50 (oral, rat): 4,070 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Potassium Benzoate: "4,100 mg/kg (rat, oral) |
| NIOSH | SN4530000 |
| PEL (Permissible) | 0.1% |
| REL (Recommended) | 3,000 mg |
| IDLH (Immediate danger) | Not established |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | Wash hands thoroughly after handling. Avoid release to the environment. |
| NFPA 704 (fire diamond) | 2-1-0 |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 (oral, rat): 4,070 mg/kg |
| LD50 (median dose) | LD50 (median dose): 4,070 mg/kg (rat, oral) |
| NIOSH | RN8190000 |
| PEL (Permissible) | 5 mg/kg |
| REL (Recommended) | 300 mg/kg |
| Related compounds | |
| Related compounds |
Benzoic acid Sodium benzoate Calcium benzoate Potassium salicylate |
| Related compounds |
Benzoic acid Sodium benzoate Calcium benzoate Potassium sorbate Sodium salicylate |

