Phosphoric Acid: Backbone of Industry and Everyday Life
Historical Development
Few chemicals have woven themselves so deeply into the fabric of both old and modern industry as phosphoric acid. Early mentions in chemistry trace back to the 1700s, a period marked by a growing hunger for scientific method and the first steps toward large-scale manufacturing. Chemists learned to extract phosphorus from bone ash, a practice that shaped Europe’s fertilizer revolution. As demand for food grew and arable land wore out, people saw that more yield required more nutrients. Companies scaled up, shifting from laborious bone distillation to using phosphate rock as mineral deposits were found around the world. Across generations, this one acid helped feed cities and energized such far-flung industries as metallurgy, water treatment, and carbonated drinks. Today’s world leans just as heavily on this old standby, even as scientific ideas and global supply chains keep moving ahead.
Product Overview
Phosphoric acid takes the form of a clear, syrupy liquid that can bite if splashed on skin. Factories churn out millions of tons either as food-grade or technical-grade product. The pure stuff ends up in soda cans and toothpaste tubes; the rougher kind boosts chemical reactors, metal processing baths, cleaners, and crop fields. Formulations range from pure to 85% by weight in water, creating a variety of densities and uses. Package labels flag highly concentrated forms for caution, as they can pack both heat and corrosive force. Handling the acid directly never goes without heavy gloves and goggles, but diluted, it finds its way into some surprising consumer shelves.
Physical & Chemical Properties
At room temperature, phosphoric acid flows thick as honey, heavier than water, with a boiling point near 158°C. Its hydrogen atoms make it a classic strong acid, able to strip rust off steel and etch glass. It mixes easily into water or ethanol and loathes nonpolar solvents. In pure form, it can crystallize or solidify if chilled, but warming brings it right back to liquid. This triple-pronged acid can give away one, two, or all three protons, leading to dozens of different salt forms, each finding a role in different industries. Its high affinity for sticking to other metals and minerals gives rise to both fertilizer value and tooth enamel applications.
Technical Specifications & Labeling
Standard industry specifications draw hard lines between food and technical grades. Food-grade phosphoric acid carries maximum limits on arsenic, heavy metals, and organic impurities, often measured to parts per million with analytical equipment. Technical-grade acid label includes concentration, batch number, and recommended storage range. Drum or tote containers display the United Nations “corrosive” label alongside hazard pictograms, so handlers know to prevent contact with skin or eyes. Storage is never ad hoc; steel tanks stand coated with acid-proof material to avoid leaks, and even short-term storage requires proper ventilation to handle fume build-up. Material safety data sheets must sit close at hand in every warehouse or factory.
Preparation Method
Current methods boil down to two main routes: the wet process and the thermal process. In the wet process, workers react ground phosphate rock with sulfuric acid. This yields phosphoric acid and calcium sulfate—the very gypsum that goes into drywall and cement. This process dominates agriculture, both because it’s inexpensive and because it churns out the rougher acid that crops demand. The thermal process, by contrast, burns phosphorus in dry air, then hydrates the resulting oxide to pure acid. This pricier method headlines food and pharmacy production, stripping away impurities and keeping consistency high. Neither route comes without environmental baggage. Miner’s runoff and leftover rock can taint groundwater, and factories must walk tight lines to stay within emission standards.
Chemical Reactions & Modifications
Phosphoric acid’s talent for giving up one, two, or three protons gives it real flexibility in reacting with other chemicals. It reacts with metal oxides to give orthophosphate salts—materials like ammonium and calcium phosphates. These salt forms stand at the heart of modern fertilizer recipes, bringing slow and steady nutrition to soils around the world. Acid etching of metal and teeth both draw on the power of phosphate binding. In organic synthesis, this acid can catalyze dehydration reactions, helping fine-chemists stitch together pharmaceuticals and dyes. Mixing phosphoric acid with alcohols spawns phosphate esters, surfactants, and flame retardants. Some manufacturers push research into modified polyphosphoric acids and condensed forms, aiming for new materials with heat-resistant properties or better capacity to bind metals.
Synonyms & Product Names
Phosphoric acid answers to more than one name. Chemists often call it orthophosphoric acid, a formal nod to the most prevalent form, H3PO4. Synonyms like phosphoric(V) acid and E338 pop up on ingredient labels or hazard sheets. Brand names stack up in both food and industrial supply, each carrying subtle differences in strength, color, and purity. Global supply reaches across markets in North America, Europe, and China, bringing unique documentation and trade naming from place to place. Shoppers reaching for cola seldom realize that “E338” on the back means phosphoric acid, the ingredient giving both tartness and preservation to their drink.
Safety & Operational Standards
Handling concentrated phosphoric acid demands more than just a steady hand. Skin or eye contact causes burns; inhaling vapors can scar airways and damage lungs. Modern warehouses carry strict spill containment protocols, acid-resistant flooring, and eye-wash stations throughout the work area. Workers suit up with gloves, chemical aprons, and full-face shields. OSHA sets clear exposure limits, while the EPA tracks environmental releases. Strict documentation trails follow every ton, starting from the shipping dock, through pipeline, to reactor vessel. Strict compliance reduces both individual risk and the danger of accidental spills turning into neighborhood crises.
Application Area
The reach of phosphoric acid never seems to end. Every year, billions of pounds enrich crops as fertilizer. Water treatment plants add controlled doses to keep pipes from corroding and to help bind heavy metals before water flows into rivers. Food manufacturers rely on food-grade acid as a tart flavoring and pH stabilizer in soft drinks, cheeses, and processed meats. Dental offices reach for it to clean and roughen enamel before putting on braces or fillings. Rust removal products put its ability to dissolve iron oxides to work, making old cars and tools look new. Metal finishers rely on it to improve paint adhesion for ships, bridges, and cars. Pharmacies add its salt forms to control acidity in medicines, suspensions, or supplements. Battery makers bring its unique properties into the lead-acid batteries that power vehicles and back up grid systems.
Research & Development
A drive to feed more people and reduce pollution shapes current research. Environmental scientists look for ways to recycle phosphates from waste streams, slashing the need for fresh mining and turning city refuse into nutrient gold for farms. Chemical engineers study new immobilization techniques for phosphates in soil, aiming for slow-release fertilizers that don’t leach into lakes or rivers. Researchers chase food purity by developing methods to remove every trace of heavy metal from finished edible acid. Battery chemists dive into phosphate modifications, trying to push energy density in modern devices while reducing flammability or toxicity. Water treatment specialists experiment with modified phosphoric acid blends that strip heavy metals efficiently while remaining safe for public consumption. Each field faces its own challenge, and steady advances demand combining old knowledge with new tools.
Toxicity Research
Every industry faces the tough problem of balancing chemical benefit with risk. Acute toxicity of phosphoric acid sits at relatively low levels compared to some harsh industrial acids, but exposure risks stack up fast with high doses or improper handling. Ingested at high concentrations, this acid damages mucous membranes in the mouth, throat, and digestive tract. Low chronic doses raise more subtle problems, from changing phosphorus-to-calcium balance in bones to eroding enamel with repeated food or beverage exposure. Research into fertilizer runoff continues, as phosphate overuse on farms leads to algae blooms and water dead zones downstream. Regulators use toxicity data to set strict limits on both occupational exposure and environmental release, while manufacturers publish thorough safety bulletins on every new product batch. New toxicity testing often comes hand-in-hand with improvements in detection technology, yielding deeper insight and safer protocols.
Future Prospects
The future story of phosphoric acid depends on both technological innovation and responsible stewardship. Agricultural scientists push to design smart fertilizers that deliver phosphorus with pinpoint precision, reducing waste while protecting natural waterways. Battery research targets new phosphate-based compounds that promise both safety and high voltage for electric cars and grid storage. Urban water systems look toward blending phosphoric acid into corrosion inhibitors that extend the lifespan of plumbed infrastructure. Policy makers debate regulations that encourage recycling phosphate from both consumer and industrial waste, transforming the acid’s life cycle. Even in the food sector, trends toward “clean label” products drive food chemists to refine acid purification steps. A hundred years from its industrial birth, phosphoric acid stands poised to keep playing a central role in manufacturing and sustainability, provided people keep learning and adapting the ways they produce, use, and recycle this resource.
What are the main uses of phosphoric acid?
Essential Role in Agriculture
Every time I visit the local agricultural supply store, I spot sacks labeled with “P” for phosphate fertilizer. What most people don’t realize is that this “P” traces back to phosphoric acid. Crops need phosphorus almost as much as sunlight and water. By converting phosphate rock to phosphoric acid, companies give farmers a reliable way to keep their soil productive. Without this boost, corn yields in the Midwest and rice fields in Asia would not meet demand. Phosphoric acid stands behind the world’s ability to feed billions.
Food and Beverage Industry Contributions
Anyone who grew up reaching for soda knows its bracing tang. That flavor owes a lot to phosphoric acid, a secret ingredient in many cola drinks. It balances sweetness, heightens flavors, and keeps the drink shelf-stable. Some processed cheeses, jams, and canned foods also rely on it to adjust acidity. The US Food and Drug Administration classifies phosphoric acid as “generally recognized as safe” in regulated amounts, though dietitians remind us that too much phosphate from sodas can bump calcium out of bones over time. Moderation matters.
Key Ingredient in Manufacturing
Out in the factory, phosphoric acid gets put to all sorts of work. Metal finishers clean iron and steel with it before painting—phosphoric acid cuts through rust, leaving behind a more paint-friendly surface. In electronics, workers use it to etch microchips, a step in nearly every smartphone’s journey. On construction sites, concrete admixtures sometimes use a dash of it to fine-tune setting times. The results shape bridges, buildings, and the circuits inside digital gadgets.
Role in Cleaning Products
Everyday cleaning supplies sometimes carry phosphoric acid, especially specialized ones for removing hard water stains and limescale. Anyone scrubbing out a toilet bowl or restoring a stained porcelain sink may reach for a cleaner with a little bit of it mixed in. The acid tackles stubborn mineral deposits far better than basic soaps. Still, I’ve always told friends and family to rinse thoroughly, as too much contact can rough up plumbing or harm water sources downstream.
Dental and Medical Uses
At the dental office, there’s a blue gel the hygienist applies before sealing or filling a tooth. That gel contains phosphoric acid, strong enough to roughen the enamel just enough for the new filling to grip properly. Dentists count on its safety in small, controlled doses. Within medicine, researchers look at phosphoric acid for certain lab reactions and as a pH buffer in formulations, though alternatives sometimes do the job just as well.
Balancing Benefits and Risks
Across all of these industries, phosphoric acid enables both basic comforts and advances. Farmers keep fields green, manufacturers make parts smooth or precise, and households rely on its strength. Yet, it comes with responsibilities. Waterways can suffer when phosphorus runoff from farms gets too high, so adoption of buffer strips and careful timing of fertilizer application helps limit loss. The food industry always faces questions about phosphate levels, reminding us to stay aware of what goes into our bodies, not just what comes off factory lines.
Phosphoric acid holds a low profile outside industrial circles, yet its touch is everywhere—on farms, in classrooms, beneath our sinks, and inside our cups. It’s woven into the world’s routines, quietly supporting progress, but always deserving of careful use.
Is phosphoric acid safe for food and beverage applications?
Understanding Phosphoric Acid in Our Diet
Phosphoric acid turns up in all kinds of drinks and processed foods. Open a can of cola and you’ll spot it on the label. Bakers use it to balance dough pH. Cheese processors lean on it to tweak flavor and consistency. Its sour kick balances sweet, masks bitterness, and helps products stay shelf-stable. The U.S. Food and Drug Administration recognizes it as generally safe at regulated amounts, putting it in the same pool as other everyday food additives.
The Science Around Health and Safety
Everyday people usually come across this acid most in sodas. A typical cola contains about 50 to 70 mg in one can. Studies show a link between heavy consumption of these drinks and lower bone density. The catch: research suggests the main culprit could be replacing milk or water with soda, so folks miss out on calcium and get too much sugar. A 2014 study in the American Journal of Clinical Nutrition notes frequent cola drinkers, especially women, face a higher risk of weaker bones. It’s the whole dietary picture that matters, not just phosphoric acid alone.
Other concerns touch on kidney health. Researchers at Tufts University found an increase in kidney changes when people drank two or more colas per day. These are observational findings, and scientists haven’t pinned the blame only on phosphoric acid since sugar, caffeine, and other cola ingredients might also play a role. For most people drinking soft drinks occasionally, the science does not show a real danger.
Why It Got So Widely Used
Phosphoric acid helps companies create the consistent taste shoppers expect—think cola’s tanginess or the subtle sharpness in some cheeses. It’s cost effective and dependable compared to natural acids. Most folks don’t eat much natural phosphoric acid otherwise; it exists naturally in certain meats, beans, and dairy, though in forms the gut breaks down differently. The synthetic version matches food safety standards and doesn’t add unwanted flavors, which put it high on the list for food and beverage makers.
Weighing the Risks and Benefits
The acid isn’t a nutrient; it brings no direct health benefit. The main concern comes from patterns of overconsumption, mostly in the form of sweetened drinks. The Center for Science in the Public Interest points out how easy it is for kids and teens to exceed healthy levels just by swapping water or milk for soft drinks. Diabetes, tooth decay, and heart disease concerns all circle soda’s sugar content, not phosphoric acid itself, but acids can chip away at tooth enamel if sipping becomes a habit.
Anyone worried about bones or kidneys will find more benefit looking at their total diet. Getting calcium from milk, leafy greens, or fortified foods helps support bones, even if drinks or foods contain a small amount of phosphoric acid. Swapping out daily cola habits for water with lemon or seltzer brings real benefits.
Food Choices and Consumer Power
People can read labels, stay informed, and make food choices based on their own health needs. Food and beverage companies respond to feedback, so the market now offers low-acid or acid-free sodas and snacks. Focusing on whole fruits, vegetables, and minimally processed foods often limits unnecessary acid intake.
The bottom line hits home for anyone: moderation matters more than fear. Understanding why companies use phosphoric acid and where it shows up allows consumers to decide what’s right for their own tables.
What is the concentration or grade of phosphoric acid available?
The Numbers on the Label
Step into any hardware store or scroll through industrial suppliers and you’ll spot phosphoric acid labeled by percentage. For most folks outside specialized labs, the stuff on the shelf hits about 85% concentration. That’s the high-strength grade. It pours clear and syrupy, packing a punch for metal cleaning, rust removal, and some food processing needs. Diluted versions show up in everything from soft drinks to fertilizer plants, sliding down to 10-75% depending on use. Lower concentrations tend to go into consumer products, like cola, where the acid adjusts flavor and acts as a preservative.
No Guesswork Behind the Grades
Manufacturers spent decades figuring out the right concentrations for safety, efficiency, and price. An 85% solution balances chemical activity without making handling too risky or eating up extra shipping costs. Go higher, and dangers leap — acid that concentrated burns skin and corrodes through common materials. Go lower, you need bigger volumes and storage for the same results. This careful balance shapes the kind of bottling you see in pharmacies or hardware aisles. Quality control becomes key, since slight changes in strength can throw processes out of whack.
Purity Isn’t Just a Buzzword
Purity links directly to concentration, especially in food and pharmaceutical grades. Impurities like arsenic or heavy metals should not sneak their way into your cola or dental rinse. Processes like thermal extraction and solvent purification let suppliers hit purity targets, sometimes labeled “food grade” or “technical grade.” Food grade often stays under 0.001% impurity content, since health regulations take no prisoners with safety. Technical grade gets the nod for fertilizer or metalwork, where tiny contaminants won’t ruin the job but could pose a problem if regulations get tighter. Anyone dealing with the production side—farmers, food makers, manufacturers—ends up trusting these details to meet both health and business standards.
The Hidden Stakes: Storage and Handling
From experience, one of the biggest slip-ups comes from treating all phosphoric acid the same. Storage rules should change based on strength. Acid at 85% eats away at cheaper plastics, so storage tanks need resistant linings. Commercial cleaning operations have strict training for employees, because burns from concentrated acid stick around for months. Rooms need ventilation, spill containment becomes routine, and everyone reaches for serious gloves. Diluted acid looks tame, but it still demands respect. Even in schools, regulations cover everything from container labeling to eye-wash stations. These are not abstract fears—emergency rooms see burns from home users who underestimated a cleaning product’s label. Education on proper handling matters, and clear instructions lower accident rates.
What Makes Standardization Key
Relying on trusted suppliers for phosphoric acid reduces headaches down the road. Substandard or off-spec acid can ruin a production run or jeopardize food safety. Regulatory bodies like the FDA and EPA monitor concentrations and contaminant levels, pushing the industry toward safe practices. As more sectors lean on precise chemistry—think battery makers or microchip cleaning—these standards grow even tighter. A few years back, deviations in acid quality triggered recalls and supply chain headaches across several industries. Industry and regulators both know it’s cheaper to get things right the first time.
Better Outcomes Through Training and Transparency
More isn’t always better. Handling pure acid at home due to price or a recommendation from a friend leads to more risk than reward. People in the industry often talk about disaster stories—tank leaks, chemical burns—or ruined products from a misread label. Clear communication about what the numbers mean, what grade you’re buying, and how to handle it safely solves problems before they start. Most folks want results, not hospital visits or ruined equipment. Anyone using phosphoric acid, from hobbyist brewers to heavy industry, benefits from good labeling, smart storage, and strong supplier relationships. Experience on the ground keeps proving that one mistake with concentration costs far more than getting it right up front.
How should phosphoric acid be stored and handled?
Understanding the Risks
Phosphoric acid plays a big role in industry, especially in fertilizers, food, and cleaning products. Its high acidity comes with real hazards. My time around industrial chemicals taught me respect for substances like this. Even a small splash can burn your skin or ruin your favorite pair of boots. Breathing the fumes never ends well. The acid eats away at metal if left unchecked and reacts badly with common materials like bases or metals that release hydrogen gas.
Choosing the Right Container
Factories don’t use just any drum or tank. Polyethylene and fiberglass-reinforced plastic tanks resist corrosion. Some grades of stainless steel also work, but not all. Regular steel falls apart fast, corroded by the acid. Even the smallest container needs a sturdy, acid-resistant lid and a tight seal. I’ve seen damage caused by cheap caps or ill-fitting covers—the result never gets forgotten. Any damage in storage tanks can cost millions in lost product or environmental cleanup.
Smart Storage Conditions
A good storage area stands away from high-traffic zones. Stay far from sources of heat or ignition since phosphoric acid releases toxic fumes once hot. I always look for proper ventilation, and, if possible, a containment system: a simple concrete barrier can help if a leak happens. Floors coated in acid-resistant paint keep the risks down. People often overlook labeling—labels must stay clear and easy to read. Whether in a massive industrial tank or a small workplace drum, nobody ever benefited from guessing what’s inside. Safety data sheets should sit close at hand.
Practical Handling Tips
Personal protective equipment is no joke. Gloves that resist acids, safety goggles, and face shields frequently spare workers from medical emergencies. I’ve found that every crew needs training—not a short talk at the start of a shift, but hands-on guidance. Spills happen quickly and unpredictably. A well-prepared team knows exactly what to do: neutralize with soda ash or lime, and collect the remains safely. Never pour water into the acid. Instead, add acid to water slowly, avoiding splatters and violent reactions.
Looking Out for Each Other
Complacency invites accidents. Regular inspections catch leaks and corrosion before disaster strikes. Any sign of damage on the tank or drum means it gets removed from service. No one should ignore small warnings—tiny leaks always grow. Record keeping seems tedious until something goes wrong, but logbooks built strong safety cultures at sites where I worked.
Improving Safety Over Time
Companies have started using automated systems that limit human contact with hazardous substances. Double-checking valves and fittings, using remote sensors for leaks, and monitoring room air quality all raise safety. Updating safety protocols over time gives the whole crew confidence. Sharing stories about near-misses, or better practices picked up from other sites, can save lives and protect property.
The Bigger Picture
Treating phosphoric acid with care isn’t just company policy. Mistakes hurt people, threaten the environment, and ruin reputations. The lessons learned in industrial settings apply anywhere this acid gets used, including water treatment or agriculture. A culture of respect for dangerous chemicals never came from one rulebook. It grows out of shared experience and a willingness to look out for each other.
What safety precautions should be taken when using phosphoric acid?
Understanding Why Precautions Matter
No one forgets their first introduction to strong chemicals. Back in school, I remember how a few careless seconds in chemistry lab meant a ruined sleeve and red skin for days. Phosphoric acid isn’t something to shrug off. Often found in fertilizer plants and food processing rooms, it’s common to misjudge how much damage a splash can cause. Factories that use it for rust removal or soft drink production see it as an everyday tool, but safety sometimes slips down the to-do list.
What Happened After a Splash
Someone working at a bottling plant told me about a time they skipped their gloves to quickly swap a filter. Sweat built up, impatience won, and the result was a chemical burn on their hand that required weeks of bandages. It wasn’t just painful, but cut into pay. That’s just skin deep. Breathing in mist or fumes without protection stings the lungs, leaves the throat raw, and sometimes trips up your breathing hours after leaving work. Eyes take even more punishment. Even a small drop in the eye risks permanent injury.
Simple Actions, Big Difference
Start with the basics: gloves resistant to chemicals should stay within arms’ reach. Good quality nitrile or neoprene gloves do the trick. Keep safety glasses or, better yet, a face shield on the bench. In a cramped workspace or one with splashing risks, aprons that don’t soak up liquids save a lot of trouble. Wearing long sleeves and closed shoes lets you avoid nasty surprises around ankles or wrists.
Ventilation deserves real respect too. Fumes seem harmless in the open air, but inside tight rooms, they concentrate quickly. I’ve seen folks tape open a window or run an old fan, but it works much better to use a proper exhaust hood. Old ventilators sometimes sputter out, so routine checks matter just as much as just having one plugged in.
Label, Store, and Know Your Stuff
Safe behavior always starts with clear labels. In some busy warehouses, workers deal with dozens of cleaning or processing chemicals. Accidental mix-ups bring real risk—phosphoric acid reacts, sometimes violently, with ammonia cleaners, causing toxic vapor. Lock storage cabinets keep the curious and clueless away from big mistakes. I remember one repair tech who left a container in an unmarked locker, which led to a minor panic before someone noticed in time.
Spill kits need to be real and accessible. A kit with proper absorption pads, neutralizers, and gloves makes all the difference during a spill. It’s no good to remember there used to be a kit once someone’s already searching with burning skin. In one factory I visited, the manager tied the kit to each production line and checked it before any shift could start their work. No one there risked running empty-handed.
Training Changes Everything
Walk into a place where folks know their chemicals, and you spot it right away. Workers show up to safety training, and management answers questions instead of brushing them off. Safety data sheets aren’t buried at the bottom of a drawer—they hang above sinks and next to exit doors. Accidents still happen, but the response comes quicker and smarter. In jobs dealing with phosphoric acid, the best practices turn into muscle memory over months of careful repetition.
Solutions and Habits
Real safety comes from daily habits, not just an annual training lecture. Gloves, goggles, and the right apron belong in your workday, not stuffed in a closet. Check ventilators, replace soaked-up chemical kits, and keep labels clear. Anyone working near phosphoric acid—seasoned techs, weekend warriors, managers—owes it to themselves and their crew to treat these basics as non-negotiable. Injuries cost time, money, and sometimes a permanent mark on health. A bit of attention beats regret every single time.
| Names | |
| Preferred IUPAC name | trihydroxidooxidophosphorus |
| Other names |
Orthophosphoric acid E338 Phosphoric(V) acid o-Phosphoric acid Acidum phosphoricum Phosphorsäure |
| Pronunciation | /fɒsˈfɒr.ɪk ˈæs.ɪd/ |
| Preferred IUPAC name | phosphoric(V) acid |
| Other names |
Orthophosphoric acid E338 Phosphoric(V) acid Acid of phosphorus Monophosphoric acid |
| Pronunciation | /fɒsˈfɒr.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 7664-38-2 |
| Beilstein Reference | 3563783 |
| ChEBI | CHEBI:43481 |
| ChEMBL | CHEMBL1425 |
| ChemSpider | 15928 |
| DrugBank | DB04264 |
| ECHA InfoCard | DTXSID0020837 |
| EC Number | 231-633-2 |
| Gmelin Reference | 827 |
| KEGG | C00009 |
| MeSH | D010728 |
| PubChem CID | 1004 |
| RTECS number | TB6300000 |
| UNII | NP40P0205T |
| UN number | 1805 |
| CompTox Dashboard (EPA) | DTXSID7024356 |
| CAS Number | 7664-38-2 |
| Beilstein Reference | 3563698 |
| ChEBI | CHEBI:43481 |
| ChEMBL | CHEMBL134907 |
| ChemSpider | 579 |
| DrugBank | DB03760 |
| ECHA InfoCard | ECHA InfoCard: 018afc9e-6d1a-48d6-8e5c-3d68b5d195e9 |
| EC Number | 231-633-2 |
| Gmelin Reference | 569 |
| KEGG | C01033 |
| MeSH | D010718 |
| PubChem CID | 1004 |
| RTECS number | TB6300000 |
| UNII | VP55JX927K |
| UN number | UN1805 |
| Properties | |
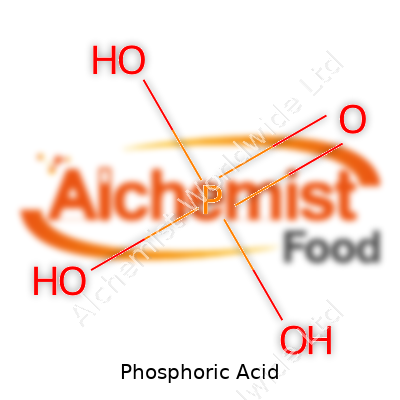
| Chemical formula | H₃PO₄ |
| Molar mass | 98 g/mol |
| Appearance | Clear, colorless, odorless liquid |
| Odor | Odorless |
| Density | 1.685 g/cm³ |
| Solubility in water | Miscible |
| log P | -2.39 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | 2.16, 7.21, 12.32 |
| Basicity (pKb) | 1.10 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.433 |
| Viscosity | 23 cP |
| Dipole moment | 1.13 D |
| Chemical formula | H₃PO₄ |
| Molar mass | 98 g/mol |
| Appearance | Colorless, transparent, syrupy liquid |
| Odor | Odorless |
| Density | 1.87 g/cm³ |
| Solubility in water | Miscible |
| log P | -0.99 |
| Vapor pressure | <0.01 mmHg (20 °C) |
| Acidity (pKa) | 2.16, 7.21, 12.32 |
| Basicity (pKb) | 1.30 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.433 |
| Viscosity | Viscosity: 26.8 mPa·s (20 °C, 85%) |
| Dipole moment | 1.13 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 110.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1279.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1281 kJ/mol |
| Std molar entropy (S⦵298) | 197.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −1271 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1281 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | A09AB12 |
| ATC code | A09AB12 |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H290, H314 |
| Precautionary statements | P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 |
| Autoignition temperature | 450 °C |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 oral rat: 1,530 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1,530 mg/kg |
| NIOSH | MN1400000 |
| PEL (Permissible) | 1 mg/m3 |
| REL (Recommended) | 30 mg/L |
| IDLH (Immediate danger) | 1000 mg/m3 |
| Main hazards | Corrosive, causes severe skin burns and eye damage, may be harmful if swallowed or inhaled |
| GHS labelling | Danger. Hazard statements: Causes severe skin burns and eye damage. May cause respiratory irritation. |
| Pictograms | GHS05 |
| Signal word | Danger |
| Hazard statements | H290, H314 |
| Precautionary statements | P280, P305+P351+P338, P310, P234, P390, P406 |
| Autoignition temperature | 450 °C (842 °F) |
| Lethal dose or concentration | LD₅₀ oral rat: 1,530 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 1,530 mg/kg |
| NIOSH | MN1400000 |
| PEL (Permissible) | 1 mg/m³ |
| REL (Recommended) | 30-80 mg/L |
| IDLH (Immediate danger) | 1000 mg/m3 |
| Related compounds | |
| Related compounds |
Thiophosphoric acid Phosphorous acid Hypophosphorous acid Polyphosphoric acids Organic phosphoric acids |
| Related compounds |
Phosphorous acid Phosphates Pyrophosphoric acid Polyphosphoric acids Orthophosphates |

