Manganese Dihydrogen Phosphate: A Grounded Overview
Tracing the Roots: Historical Development
Manganese dihydrogen phosphate didn’t just pop up as a modern curiosity—its history follows the spread of chemical manufacturing and the hunger for reliable phosphates in agriculture, batteries, and technical coatings. Early studies in the late nineteenth century marked manganese phosphate’s entry into corrosion resistance research, especially in military and heavy engineering. It saw more focused work after World War II, when industrial chemistry ramped up demands for stable phosphate compounds. Electrochemistry labs, even half a century ago, experimented with manganese salts and phosphoric acid, figuring out the basic steps of synthesis. These historical benchmarks shaped today’s emphasis on careful synthesis and high-purity production, not just legacy recipes.
Product Overview
Manganese dihydrogen phosphate, known for the formula Mn(H₂PO₄)₂, serves practical needs in battery manufacturing, rust-proofing metal parts, and acting as a precursor for lithium manganese phosphate cathodes. This compound draws interest because of its blend of robust manganese capability with the functional flexibility of phosphate chemistry. People often forget the range of roles this salt plays—from soil treatments that tweak micronutrient levels to intricate parts of electrochemical energy storage. Interest from the lithium battery industry also steers investment into refining synthesis and handling, carving out a niche in a crowded chemical marketplace.
Physical & Chemical Properties
You don’t just grab a container of manganese dihydrogen phosphate and toss it around. Its powder or crystalline form takes on a faintly violet hue, thanks to manganese ions. As with other phosphate salts, it absorbs moisture from the air and dissolves well in water, liberating a mild tang that signals its acidic nature. Under heat, it decomposes, dropping off water and forming pyrophosphate and higher manganese phosphates. In a lab, these transformations leave a telltale change in color and consistency—easy to spot if you’ve spent enough time near evaporation dishes or heating mantles. Chemically, its reactivity splits between acting as a weak acid and providing manganese for redox reactions. When challenged with alkalis, the compound shifts, sometimes giving off a whiff of phosphine sulkiness.
Technical Specifications & Labeling
Industry buyers look for clear labeling on crystalline size, purity—frequently above 99% for research—and bulk density specs. Moisture content, usually under one percent in well-sealed containers, often matters for pharmaceutical and energy clients. The Material Safety Data Sheet (MSDS) insists on hazard codes, eye irritation scores, and first-aid instructions. Each drum gets a batch number, expiration date, and sometimes even a QR code for digital records. Specifications from the China National Standards GB/T or the American Chemical Society guidelines usually govern these processes, not just internal company rules. Other fields need different certification; electronics manufacturers check for trace ions like sodium, zinc, or copper below a strict threshold.
Preparation Method
Synthesis normally begins with dissolving manganese carbonate or oxide in warm phosphoric acid, driving an exothermic fizz as gas escapes. Any color change offers clues for chemists: a mild pink with slight turbidity hints at residual impurities. Operators filter the solution, cool it down, and let fine crystals form overnight. Big plants rely on batch reactors, controlling everything from stoichiometry to agitator speed and acid temperature. Washing and vacuum drying follow. I remember an old mentor shaking his head over skipped washing steps—traces of free acid can ruin downstream coatings or battery tests. Purity depends on individual care in these mundane-seeming steps, not just fancy equipment.
Chemical Reactions & Modifications
Manganese dihydrogen phosphate reacts with strong bases to yield manganese phosphate and water. Introduce it to polyatomic ions or alkali salts and watch new phosphates or mixed-metal compounds form. This behavior unlocks avenues for better battery cathodes or slow-release fertilizers. Under controlled conditions, the compound accepts mild reduction to produce mixed valence manganese species, which can support catalytic cycles or specialized pigments. In my experience, amateur experimenters sometimes stumble into noxious byproducts—modification isn’t always a friendly kitchen-table exercise. Manipulating the crystalline phase or hydration state shifts both performance characteristics and shelf life, demanding sharp attention from developers.
Synonyms & Product Names
Many suppliers call this compound manganese(II) dihydrogen phosphate, though you’ll spot manganese acid phosphate or just MnP2 in technical shorthand. In Asia, labels occasionally bear the name 'manganese biphosphoric acid salt.' Standardized CAS labeling—often 18718-07-5—takes the guesswork out of procurement. Commercial brands sometimes stick to a 'phosphate of manganese' or go with a trade name like 'PhosManganese DHP.' A chemist might roll their eyes at creative label flair, since the core identity stays unchanged. Whether in agricultural catalogs or specialty materials listings, consistent naming supports safe handling and research.
Safety & Operational Standards
Regulations from OSHA, REACH, and GHS shape safe handling, from wearing N95 dust masks when weighing powder to using closed systems in preparative work. Manganese compounds pose inhalation risks and can agitate airways; phosphate dust sometimes leads to mild skin rashes. Training staff to recognize exposure symptoms, keeping emergency eyewash stations nearby, and following spill cleanup rules make a difference. Chemical plants build pressure-relief systems and plan for secondary containment—nobody wants to scrub phosphate slurry from storm drains. Internal audits monitor records on stock levels and shelf times, since degraded product can trigger unexpected risks. In practice, workers develop a feel for safe manipulation. I’ve seen old techs spot a breached drum from a faint sour odor before digital sensors caught a warning.
Application Area
Clients in battery development buy manganese dihydrogen phosphate to feed into lithium manganese phosphate cathodes, where it provides a stable manganese source and supports energy efficiency. Metal finishing shops treat steel or aluminum with phosphate coatings formed in situ, fighting rust in automotive and defense parts. Farms could integrate small doses as a micronutrient—sometimes called secondary fertilizer—boosting crop reliability on manganese-poor soils. In water treatment, the compound adsorbs heavy metals or sets up redox buffering, although it demands careful dosage. Anyone in academic chemistry can cite uses as a pH buffer or reagent for complex inorganic synthesis. All these fields overlap less than most textbooks suggest—each brings challenges from dosing scale to regulatory review.
Research & Development
University and industrial labs keep pushing manganese dihydrogen phosphate into new forms and hybrid materials. Battery researchers, spurred by electric vehicles, are optimizing its particle size and doping profile to push performance beyond older cathode salts. Materials scientists test it as an anticorrosion base for concrete bars, searching for enough adhesion without blocking flexibility. Granular forms target slow-release fertilization, factoring in climate change and precision agriculture. Funding ebbs and flows, often tied to how hot the energy storage market gets or how fertilizer prices shake out across Asia and South America. Collaborative patents pop up, drawing on cross-discipline teams who merge synthesis routes with advanced analytics like XRD and FTIR—no single lab can crack all hurdles alone.
Toxicity Research
Toxicologists flag manganese exposure for potential neurological effects; even moderate dust inhalation in confined spaces can provoke symptoms if careless. Some phosphate salts aggravate iron absorption, complicating nutritional profiles for animal feed. Water studies occasionally detect trace manganese compounds near mining or phosphate-using industries—too much can trigger fish kills or human health advisories. Animal models and human trials suggest acute toxicity remains low under controlled exposure, but long-term ingestion or chronic airborne exposure draws stricter scrutiny from environmental regulators every year. Industry veterans recall policy changes that shifted safety limits, sometimes overnight, as new data emerged. Personal experience has shown that training, environmental monitoring, and transparent reporting seem to curb most risks before they spiral.
Future Prospects
A growing electric vehicle sector, climate-resilient farming, and global health priorities all pull manganese dihydrogen phosphate into the foreground. Green chemistry and circular economy programs motivate manufacturers to refine recovery methods and recycle effluent, not just dump spent phosphate. Academic labs continue to build partnerships with scale-up centers, closing the gap between bench-top synthesis and commercial reactors. Demand from battery and specialty chemical producers grows, but clients insist on tighter specs and expanded toxicity studies. Regulatory change, especially around heavy metal runoff and greenhouse gas reporting, will demand new compliance measures and creative research. From what I’ve seen, people who pay attention to details—not just bottom lines—are best placed to help this compound find safer, more effective roles in tomorrow’s industrial world.
What is Manganese Dihydrogen Phosphate used for?
A Closer Look at Purpose and Practical Value
Manganese dihydrogen phosphate often flies under the radar in conversations about chemicals, yet its impact remains significant. If you’ve heard about it, odds are the talk circled around batteries, fertilizer, or metal treatment. Those uses have found their way into many products, tasks, and even the food grown on fields across the world. The basic idea revolves around this compound’s ability to deliver both phosphate and manganese in a form plants and industrial processes can readily use.
Fertilizer: Feeding the Food Chain
Many years working close to agriculture taught me just how much crop growth relies on both trace minerals and phosphate. Manganese may not headline major fertilizer labels, but it quietly boosts plant metabolism, nutrient absorption, and enzyme function. Legumes, cereals, and leafy vegetables, in particular, pull more weight from soil when manganese levels stack up right. Dihydrogen phosphate, as the carrier, helps move these nutrients straight into plants, reducing the chance for runoff and waste. According to the International Fertilizer Association, micronutrients like manganese prevent yield loss that hits difficult-to-replace food supplies.
Batteries: Pushing Beyond Old Limits
Many conversations focus on lithium or nickel when talking about rechargeable batteries. Manganese-based compounds offer real value as well. Manganese dihydrogen phosphate often features in research for cathodes. Compared to other mixes, it stands out for cost and safety. A Forbes report from last year spotlighted the potential for manganese in making batteries less reliant on more expensive or difficult-to-source metals. Experienced engineers know a wide resource base bodes well for price and manufacturing stability. For electric vehicles and energy storage, these factors matter far more than hype or headlines.
Corrosion Protection: Extending the Life of Tools and Machines
Many in the metalworking field use manganese dihydrogen phosphate during phosphating, a finishing process. This process lays down a protective layer on steel and iron, slowing rust and wear. Every farmer or mechanic knows replacement for worn-out tools cuts into tight budgets, especially in tough seasons. The rough texture created by the phosphate layer also gives paints and coatings better grip, so touch-ups and repairs stick longer. This isn’t about fancy chemistry but plain value – tools lasting another season or machine parts surviving hard workday after day.
What Stands in the Way, and Where Solutions Lie
Access and knowledge still block progress. Many growers don’t realize soil might miss vital trace elements. Teaching farmers the full benefits of micronutrients, including manganese, helps squeeze more food from shrinking arable land. In industry, cleaner production methods and waste controls could secure the future for compounds like manganese dihydrogen phosphate. Tighter rules from environmental agencies will nudge manufacturers to reduce harmful outputs and reclaim materials. Partnerships between researchers, producers, and users bring innovation within reach—saving both costs and resources.
These are not small matters. Through both food security and stable technology, manganese dihydrogen phosphate links chemical know-how to practical daily needs. It deserves more than just a line in a list of industrial salts. Its influence quietly shapes buses, bread, and bolts, keeping life moving.
What is the chemical formula of Manganese Dihydrogen Phosphate?
Breaking Down Manganese Dihydrogen Phosphate
Manganese dihydrogen phosphate, recognized in scientific circles as Mn(H2PO4)2, gives us a window into both chemistry’s simplicity and complexity. Recognition of this formula comes from real hands-on research in laboratories and studies in applied science. Spending time around wet benches and university classrooms, you see firsthand why getting a formula right isn’t just an academic exercise. A misplaced hydrogen or swapped subscript can trip up a synthesis or give the wrong properties in a fertilizer blend, paint, or corrosion inhibitor.
Why Mn(H2PO4)2 Matters
Manganese brings value for more than one reason. In soil science, for example, it acts as a vital micronutrient. Plants feeling a shortage show weak growth and yellowing leaves. The dihydrogen phosphate part supplies phosphorus, another must-have for any plant looking to thrive. Together in this formula, manganese dihydrogen phosphate steps up as a dual-nutrient option, something growers notice when they want to patch up deficiencies quickly. Based on field reports and peer-reviewed studies, finding effective fertilizers often involves blending elements with precision—here, the chemistry has to stack up.
Fact-Checking the Basics
Writing the formula out: Manganese brings a +2 charge, with the symbol Mn2+. Dihydrogen phosphate gives H2PO4-. You need two H2PO4- ions for every Mn2+ to balance the charges, leading to Mn(H2PO4)2. This isn’t just trivia; formulas dictate how these substances dissolve, react, and interact with other materials in real life. Someone working in water treatment or battery chemistry quickly realizes the importance of getting this detail correct. Some research from groups working on energy storage highlights how the arrangement of ions in such compounds helps determine their conductivity or chemical stability.
Application and Impact
Applications in agriculture and materials science depend on knowledge like this. Looking at published fieldwork, the use of manganese dihydrogen phosphate supports efforts to boost plant growth, especially in places hit by micronutrient shortages. In corrosion science, phosphate treatment strategies often factor in manganese’s ability to shield metals from rust, with the Mn(H2PO4)2 structure contributing significantly to protective coatings. These real-world ties bring credibility and trust to those handling or recommending it.
Addressing Challenges in Practical Usage
I’ve seen colleagues struggle with the safe storage and mixing of such phosphates. Handling powders that release dust demands basic safety steps, from gloves to ventilation. Teams in manufacturing and labs share lessons about preventing moisture uptake or accidental combination with incompatible chemicals—mistakes here can cost both time and resources. Industry groups and government safety boards recommend robust training and clear labeling to reduce risks. That practical wisdom, found in manuals and worker testimonials, goes a long way in keeping operations running smoothly without incidents.
Looking Forward
Scientists and engineers keep digging into manganese dihydrogen phosphate’s properties. Ongoing research explores tweaks to enhance delivery methods in soil and new uses in specialty coatings. As demand for precise nutrients and advanced materials rises, understanding this chemical formula—Mn(H2PO4)2—remains key for safety, efficiency, and innovation across many fields. For anyone dealing with chemistry day in and day out, it’s information worth knowing by heart.
Is Manganese Dihydrogen Phosphate safe to handle?
Everyday Contact with Chemical Compounds
Most people rarely think about the different substances behind fertilizers, batteries, or ceramics. Manganese dihydrogen phosphate is among those chemicals that pop up in industrial plants and research labs, but it isn’t a compound anyone would come across during a trip to the grocery store. The scientific name might sound intimidating, so it makes sense to ask if this stuff could harm you. I’ve spent enough time looking into chemical safety sheets and talking to lab workers to know: “safe” doesn’t mean “harmless.” Plenty of perfectly ordinary chemicals, even salt and baking soda, become risky in certain settings.
Direct Risks and Exposure Details
Manganese dihydrogen phosphate has several uses—from battery technology to agricultural products. Touching a small amount won’t put someone in immediate danger, but contact deserves respect. Dust from powders can irritate eyes, throat, or skin. Repeated exposure to manganese compounds sometimes leads to serious health concerns. Breathing in fine particles over time damages nerves and causes symptoms that show up years later. I once chatted with a former lab technician whose job involved weighing metal salts. Even though her job never made her sick, her employer still insisted on gloves and masks in case of accidental spills and airborne dust. It only takes one day without a mask or goggles for small exposures to add up.
Safety Protocols Under Scrutiny
OSHA, NIOSH, and similar authorities monitor manganese exposure. They don’t list manganese dihydrogen phosphate as one of the wildest hazards, but anyone in a workplace should stick to the guidance for manganese in general. Most labs use closed containers and fume hoods for handling powders. Access to running water and eyewash stations makes a big difference if someone spills or touches their face by accident. I remember a classmate who wiped her eye before washing her hands after mixing metal salts. Five minutes later, her eye burned and swelled—luckily, quick rinsing prevented trouble.
Addressing Environmental Impact
Careless disposal creates risk not just for workers but for everyone. Dumping manganese compounds in regular trash or pouring them down a drain lets them find their way to soil or water. High levels of manganese in drinking water affects brain health, especially for children. Some towns have already dealt with contaminated wells that required costly cleanup. Safe handling at the job site protects the wider community too.
Solid Steps Toward Safer Handling
Training matters more than blinking lights or warning signs. Before using manganese dihydrogen phosphate, learn what the material looks like, how it behaves, and what emergency measures to take. Stores of the compound should stay shut tight, labeled, and out of reach for anyone not trained to use them. Workers benefit from gloves and dust masks—simple, cheap, and sometimes lifesaving. Plenty of accidents happen when people think they “know what they’re doing” and skip basic precautions. I’ve seen even veteran lab workers get careless when things feel routine. A culture of respect for chemicals—asking questions, double-checking information, helping coworkers notice mistakes—keeps everyone safer and accidents rare.
How should Manganese Dihydrogen Phosphate be stored?
The Real Risks You Can’t Ignore
Manganese dihydrogen phosphate plays a crucial role in battery manufacturing, agriculture, and chemical labs. As with most chemical compounds, the storage method makes all the difference between safe handling and a disaster waiting to happen. From years working in maintenance and supply rooms, it becomes clear shortcuts in chemical storage come back to bite you hard. Leaks, odd odors, or even a subtle change in appearance all signal storage failures that nobody wants to deal with.
Humidity: The Silent Enemy
This phosphate behaves best in a dry environment. Moisture creeping in sets off clumping, weird changes in color or consistency, and even reduces its shelf life. I remember a warehouse losing an entire shipment after someone left a door cracked open over a humid weekend. The humidity in the air soaked right in. For anyone handling this compound, simple humidity control using solid sealed containers and a cool, ventilated area takes precedence. Silica gel packets never hurt for insurance.
Label Everything — No Exceptions
Unexpected surprises hide in unlabelled containers. Over the years, I’ve seen more than one chemist curse their luck after grabbing the wrong jar. Every container should wear a clear, chemical-resistant label that shows the name, concentration, hazard pictograms, and the date received. Hazmat response teams cite poor labeling as a key reason for accidents. A little diligence here means peace of mind later.
Avoid the Sun’s Wrath
Direct sunlight acts like an accelerant for unwanted reactions, or at the least, it heats things up beyond what’s safe. Shelving set away from windows, in a shaded part of the storage area, keeps the heat away. Years ago, a shipment stored by a sunny window gave off a strange odor and lost its crisp, free-flowing form almost overnight. Out of the sun, you lower the risks.
Don’t Let Reactives Mingle
This phosphate can react with various chemicals, following the age-old rule: don’t store oxidizers, reducing agents, or acids right next to each other. Segregation using dedicated shelving or even bins marked with color codes makes a real difference. After seeing spills caught early by simple shelf dividers, I know it’s not extra work – it’s common sense that may save the entire stockroom.
Safety Above All
No one plans for spills, but preparation beats scrambling. Chemical storage guidelines from organizations like OSHA or the CDC recommend spill kits, gloves, dust masks, and even eye-wash stations nearby. People forget: cleaning up after a spill without the right gear risks both health and an expensive cleanup.
Keep Inventory Tight
Chemicals left forgotten in the back of the closet develop issues, or labels rub off. I run through my inventory every six months, check for expiry, and inspect packaging. Facilities that stick to a regular audit avoid more problems. Good record-keeping keeps people accountable and prevents surprises down the line.
Practical Storage, Lasting Solutions
Common sense, consistency, and attention to detail go a long way. Take humidity out of the equation, label everything, stick to cool and dry places, avoid sun, and store away from incompatible compounds. The habits built around safe storage reflect a respect for both the material and the people handling it. It’s about prevention, not just compliance.
What are the physical properties of Manganese Dihydrogen Phosphate?
Solid, Ordinary…with a Scientific Kick
You won’t find manganese dihydrogen phosphate putting on any fancy shows. At its core, this is a salt that comes out as a solid. Most people looking at it would see something that appears as a pale pink or purple powder or crystals. The color doesn’t come from a dye; manganese adds that gentle hue all on its own. Years back in a college lab, I watched as a glass beaker filled with the compound changed from colorless to a soft blush after proper mixing—the color shift felt like a kind of chemistry magic, but there was nothing dramatic about it.
Solubility and Its Real Role
Drop some in water, and manganese dihydrogen phosphate doesn’t stay together for long. It dissolves fairly well, especially in warm water. This makes it a practical pick for liquid fertilizer mixes and certain battery preparations where straightforward mixing is a bonus. Try the same trick with ethanol or acetone, and you won’t get much—you’re left with a stubborn powder at the bottom of your flask. Chemists who make rural soil blends like this, since you can lace irrigation water with it and trust it to spread nutrients around a field without clogging nozzles or hoses.
How It Handles Heat and Air
Heat this salt, and it keeps its structure up to a modest temperature, somewhere under 180°C. That means you won’t catch it melting in the sun or getting volatile during shipping. Higher temps spell breakdown; the compound gives up its water and changes to a different substance. Expose it to air, and you won’t see it reacting quickly with oxygen or moisture, so it sticks around on your shelf. I’ve seen samples in glass jars stay just as pink and free-flowing months after opening them, as long as the storage space stays dry.
Grain and Texture—More Than Cosmetic
Many commercial batches feel a bit gritty, like fine sand, or sometimes clump if they draw a little moisture from the air. This rough texture influences how the material spreads in blends or granulates with other powders, which matters if you want exact dosing in tablets or fertilizer granules. Folks working in agriculture or battery plants care about this more than you’d think. Gritty, clumpy powder doesn’t flow smoothly through machines; the smoother stuff works better, costs less to run, and leads to fewer headaches for plant operators.
Weight That Pulls Its Load
Manganese dihydrogen phosphate is not especially dense; its crystals stack in a way that leaves a bit of air here and there. Measure out a cup, and it won’t be as heavy as iron salts or other dense minerals. For shipping, this trait keeps freight costs in check. Bulk handlers and warehouse workers find it less trouble to shift sacks of it around compared to heavier mineral salts.
Practical Concerns and Fixes
One issue crops up often: powder caking during humid months. Clumping makes it a pain to use, especially in fertilizer hoppers or chemical reactors. Producers sometimes add flow agents or seal bags with extra care to help. Improving the grain shape at the manufacturing stage—think rounder, drier crystals—goes further to reduce problems. Those who rely on manganese dihydrogen phosphate, whether farming, repairing soil, or building batteries, rarely want surprises. Keeping a close eye on these traits helps make sure every batch works just as the last one did.
| Names | |
| Preferred IUPAC name | dihydroxidooxidomanganese;phosphonic acid |
| Other names |
Manganese(II) dihydrogen phosphate Manganous dihydrogen phosphate |
| Pronunciation | /ˈmæŋɡəniːz daɪˈhaɪdrədʒən ˈfəʊsfeɪt/ |
| Preferred IUPAC name | manganese(II) dihydrogen phosphate |
| Other names |
Manganese(II) dihydrogen phosphate Phosphoric acid, manganese(2+) salt (2:1) Manganese(2+) phosphate (1:2) Manganese bis(dihydrogen phosphate) |
| Pronunciation | /ˈmæŋɡəniːz daɪˈhaɪdrədʒən ˈfəʊsfeɪt/ |
| Identifiers | |
| CAS Number | 10101-66-3 |
| Beilstein Reference | 3618733 |
| ChEBI | CHEBI:63005 |
| ChEMBL | CHEMBL1201562 |
| ChemSpider | 18642173 |
| DrugBank | DB11257 |
| ECHA InfoCard | 03f783c4-6ff7-443d-8a24-9d5a5b13b68e |
| EC Number | 01-2119457558-22-0000 |
| Gmelin Reference | 57847 |
| KEGG | C18652 |
| MeSH | D052967 |
| PubChem CID | 16211554 |
| RTECS number | TB8609000 |
| UNII | J5547V6S22 |
| UN number | UN2813 |
| CAS Number | 18718-07-5 |
| Beilstein Reference | 1460686 |
| ChEBI | CHEBI:14316 |
| ChEMBL | CHEMBL1233003 |
| ChemSpider | 10210994 |
| DrugBank | DB14540 |
| ECHA InfoCard | ECHA InfoCard: 100.035.441 |
| EC Number | 231-846-0 |
| Gmelin Reference | 31983 |
| KEGG | C18507 |
| MeSH | D048869 |
| PubChem CID | 162112 |
| RTECS number | UU8225000 |
| UNII | 31ZOC1XD8Z |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID3025140 |
| Properties | |
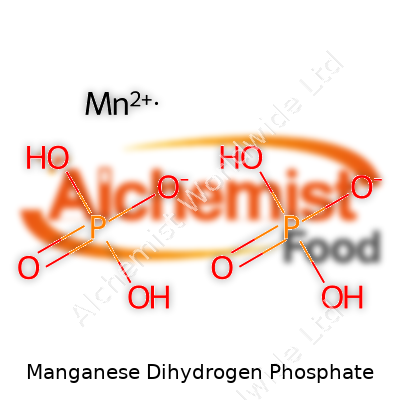
| Chemical formula | Mn(H₂PO₄)₂ |
| Molar mass | 222.92 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.53 g/cm³ |
| Solubility in water | Soluble |
| log P | -4.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 1.6 |
| Basicity (pKb) | 6.37 |
| Magnetic susceptibility (χ) | +1420.0e-6 cm³/mol |
| Refractive index (nD) | 1.509 |
| Dipole moment | 0 D |
| Chemical formula | Mn(H₂PO₄)₂ |
| Molar mass | 222.94 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.47 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -4.5 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 1.6 |
| Basicity (pKb) | 6.37 |
| Magnetic susceptibility (χ) | +825.0e-6 cm³/mol |
| Refractive index (nD) | 1.691 |
| Dipole moment | 3.79 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 108.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1896 kJ/mol |
| Std molar entropy (S⦵298) | 111 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1894.7 kJ/mol |
| Pharmacology | |
| ATC code | A12CC05 |
| ATC code | A12CC05 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P280, P301+P312, P305+P351+P338, P337+P313, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (oral, rat) |
| NIOSH | MU8225000 |
| PEL (Permissible) | 5 mg/m3 |
| REL (Recommended) | 10 mg Mn/m³ (as Mn) |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H272, H302, H318 |
| Precautionary statements | P264, P280, P301+P312, P305+P351+P338, P337+P313, P501 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Lethal dose or concentration | LD50 (oral, rat): > 2000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): >2000 mg/kg |
| NIOSH | TB8500000 |
| PEL (Permissible) | 5 mg/m3 |
| REL (Recommended) | 60 mg |
| IDLH (Immediate danger) | IDLH: Not listed |
| Related compounds | |
| Related compounds |
Manganese(II) phosphate Iron(II) dihydrogen phosphate Cobalt(II) dihydrogen phosphate Nickel(II) dihydrogen phosphate |
| Related compounds |
Iron(II) phosphate Zinc phosphate |

