Manganese Chloride: Past Insights and Current Landscape
Historical Development
Manganese chloride has been around for quite a while, shaping everything from paint to batteries. Ancient chemists processed minerals to get colorful pigments, and those first experiments brought them face to face with manganese compounds. Fast forward to the Industrial Revolution, factories started using manganese salts to improve the making of chlorine and steel, which nudged manganese chloride into wider circulation. Wheeling through the decades, manganese chloride cropped up in medicine, agriculture, and even photography. Its growth reflects the larger story of chemistry: practical applications building on pure curiosity and a bunch of trial-and-error moments.
Product Overview
Manganese chloride typically sits in the form of pale pink crystals, dissolving quite easily in water to give a clear, rose-colored solution. You’ll often see it listed as MnCl₂, which comes as a hydrate or anhydrous option. The crystalline powder turns up in labs for chemical reactions, or in factories for producing other manganese compounds. Folks in the field can spot MnCl₂ by its color and solidity, but the real work happens in its solutions, where manganese chloride acts as a source of manganese ions for everything from nutrition blends to electroplating baths.
Physical & Chemical Properties
Manganese chloride doesn’t just look pretty on a shelf. Those pinkish crystals have a melting point just over 650°C, so they don’t break down easily, which is handy in industrial settings. Manganese chloride dissolves quickly in water and a few organic solvents, forming solutions that conduct electricity. It reacts to form a variety of compounds in water, and, if heated with oxygen, it forms manganese oxides. The anhydrous form grabs water from moist air in seconds, showing how sensitive it gets to humidity. In labs, this sensitivity matters when someone’s aiming for precision.
Technical Specifications & Labeling
On chemical labels, manganese chloride turns up with CAS Number 7773-01-5. Names like “Manganese(II) chloride tetrahydrate” or “Manganous chloride” show up on barrels or bags. The labeling covers concentration, water content, and purity, which help buyers and handlers make safe decisions. Industrial manganese chloride tends to stay above 99% pure for specialty applications, while general use might run a bit lower. Transporters keep an eye on UN numbers, hazard class info, and recommendations for protection, right there on the container or paperwork, for health and regulatory reasons.
Preparation Method
Most plants prepare manganese chloride by mixing manganese dioxide with concentrated hydrochloric acid. Stir in that acid, the manganese dioxide breaks down, bubbles of chlorine gas float off, and pink MnCl₂ jumps into solution. Drying this mixture at the right temperature gives the pure salt. Crystallization kicks in to clean up impurities, and this method stays standard because it’s reliable and scales up well. Labs might use manganese carbonate or manganese metal instead, tweaking the conditions to get small, controlled amounts, suited for research or fine chemical use.
Chemical Reactions & Modifications
In labs, manganese chloride runs as a key player for making manganese-based catalysts, magnets, and pigments. Mix it with sodium carbonate, manganese carbonate drops out. Boil manganese chloride with ammonia, you’ll get manganese hydroxide, an ingredient for newer batteries. Toss it with alkali metal salts, it can become complicated manganese oxychlorides that feed other chemical reactions. Through oxidation, chemists can shift Mn²⁺ to Mn³⁺ or Mn⁴⁺, which impacts everything from water treatment to catalyst development. As a starting material, manganese chloride’s flexibility gives scientists plenty of room to explore.
Synonyms & Product Names
Manganese chloride’s aliases depend on the crowd. On academic papers, people use Manganese(II) chloride, MnCl₂, or even Manganous chloride. Industry catalogs list names like “Manganese dichloride” or “manganese chloride anhydrous/tetrahydrate.” International suppliers use EINECS, HS Codes, or local regulatory listings. This spread of names sometimes causes headaches for buyers, but everyone’s after the same pink salt in the end.
Safety & Operational Standards
Like many transition metal salts, manganese chloride has its risks. It might not taste sweet, but if someone’s skin meets it often, they risk contact dermatitis. Breathing in dust or fumes—especially during large-scale production or careless laboratory handling—can trigger respiratory issues. Workplace safety data encourages gloves, goggles, and careful ventilation, and it lists manganese chloride as a hazardous substance under GHS rules. Handling waste and rinsates brings an extra layer of caution, since manganese ions in water threaten fish and other wildlife if they slip down the drain unchecked. Companies running production floors tend to monitor airborne particles and encourage routine training, since regulatory penalties for slip-ups can be steep.
Application Area
You’ll spot manganese chloride best in metal finishing plants, where it plays a role in making steel tougher and more reliable. Nutrition supplement makers add it in trace amounts for animal feed and sometimes even for humans, since our bodies need manganese but only in tiny doses. Battery developers chase unique manganese compounds stemming from MnCl₂ to boost next-gen lithium cells. Pigment production leans on manganese chloride for pinks to browns in ceramics and dyes. In water treatment plants, manganese chloride can treat water for iron and manganese before it hits the tap. Pharmaceutical research trusts it as a reagent and building block, showing its versatility across many industries, from heavy to high-tech.
Research & Development
Workshops and conference halls keep filling with new data about manganese chloride’s chemistry. R&D staff keep tweaking manganese chloride’s formulations to fit new types of batteries, high-efficiency fertilizers, and specialty ceramics. Engineers and researchers poke at novel manganese-catalyzed reactions, hoping for breakthroughs in synthetic chemistry or green manufacturing. Universities build simulation models tracking how manganese ions interact with cell structures, feeding nutrition studies or biomedical research. As additive manufacturing and renewable energy research keep expanding, manganese chloride keeps getting new life as a building block in fresh applications.
Toxicity Research
Toxicologists dive deep into manganese chloride’s long-term impact, especially when folks work around it daily. Manganese itself causes trouble once its levels build up in the human body, possibly leading to neurological issues after years of exposure. Airborne manganese salts stand out as a greater threat than ingestion, with studies showing inhaled manganese hits the brain directly. Animal feed regulations, as a result, put strict limits in place. In aquatic systems, high concentrations hit fish and invertebrate populations hard. Scientists keep refining test methods so they can pin down exposure routes, and these results shape workplace limits and medical guidelines worldwide.
Future Prospects
Manganese chloride looks to stick around in energy storage research, especially as demand for longer-lasting, safer batteries keeps rising. Getting more from manganese chloride in nutritional science could lead to new supplements or specialized livestock feed. Researchers aim to unlock better water purification systems and fully recyclable battery components, and MnCl₂ supplies the manganese ion base for these projects. There’s growing interest in green chemistry approaches that use manganese chloride as a less toxic alternative to other transition metals. Supply chain oddities sometimes shake the market, but as the push for electric vehicles and smart devices picks up, more companies invest in reliable manganese chloride production, tracking everything from source minerals to waste and recycling streams.
What is Manganese Chloride used for?
A Handy Compound With Real-World Uses
Manganese chloride comes up often in discussions about chemical supplies or lab work. But this bright pink salt plays a much bigger role in everyday life and in some of the most important industries out there. I remember spotting jars labeled “manganese chloride” tucked away on a shelf during an undergraduate chemistry class. At the time, it just seemed like another chemical. Later on, after years in research and chatting with folks in manufacturing, I started seeing how essential this stuff really is.
Pharmaceutical Applications
The medical field puts manganese chloride to work in several ways. It’s involved in the preparation of imaging agents for Magnetic Resonance Imaging (MRI), helping doctors spot certain diseases. Manganese itself plays a role in bone formation and metabolism. Compounds like manganese chloride sometimes provide the right form of this element for nutritional supplements and other medications. Researchers keep exploring its links to brain health and enzyme function. Though more attention goes to minerals like calcium or magnesium, manganese is just as vital—without enough, the risks include bone weakness and metabolic problems.
Batteries and Energy Storage
Talk about the growth of electric vehicles or backup power, and sooner or later, the battery conversation comes up. Manganese chloride often serves as a starting material for making manganese dioxide, an essential part of alkaline and zinc-carbon batteries. Manufacturers count on the reliability of these cells in everything from children’s toys to emergency flashlights. As energy storage moves towards greener solutions, manganese-based batteries might start to play a bigger role. Companies work on ways to boost battery capacity and drop costs, and those experiments usually lead back to compounds like manganese chloride.
Water Treatment
Cities and towns want safe water. Water treatment plants turn to manganese chloride to remove iron and hydrogen sulfide, limiting bad taste and discoloration. Exposure to manganese in the right dose isn’t harmful—the danger comes from either extreme excess or total lack. Careful dosing keeps people safe and infrastructure running smoothly.
Chemistry in the Classroom and Industry
Walk into any college chemistry lab and you might spot this compound. Teachers use manganese chloride in simple experiments to help students understand redox reactions. Its vivid pink color makes tests for metal ions easy to see and learn from. Outside universities, chemical industries use it as a catalyst or a precursor to other specialty chemicals. Whether creating fertilisers or specialty coatings, this material shows up behind the scenes.
Potential Challenges and Responsible Use
No story about chemicals should skip the question of safety. Inhaling dust or ingesting too much manganese chloride can cause health problems. Factories and labs must follow safety guidelines, storing and disposing of the substance properly. The answer lies in training workers, giving access to protective equipment, and keeping clear records of use and exposure. That’s the best way to serve both the industry and the families who live nearby.
Looking Ahead
It’s easy to overlook basic salts like manganese chloride because they lack the glamour of cutting-edge tech. Dig a little deeper, though, and you find them helping keep medical diagnostics sharp, batteries reliable, and water clean. Sometimes, the materials that feel ordinary turn out to be the real backbone of progress.
Is Manganese Chloride hazardous to health?
Everyday Encounters with Manganese Chloride
Manganese chloride pops up in labs, industrial settings, and sometimes even in agriculture. Most people might not run into it shopping for groceries, but workers in factories or scientists in chemistry labs almost certainly will. I've seen it in classrooms during chemical demonstrations – a pinkish salt sitting among other colorful powders. It looks harmless. But like many chemicals, it’s got a side that demands respect.
Manganese itself plays a key role in our bodies. It turns up in nuts, whole grains, and leafy vegetables. Our nerves and bones rely on small amounts. The catch lies in the dose and the form. The manganese in food acts differently from compound forms like manganese chloride. It’s a bit like the difference between the salt on your table and the chlorine in your swimming pool – related, but definitely not the same experience.
What Happens with Exposure
Handling manganese chloride in a laboratory or industrial environment, I noticed many rules around its use. Safety glasses, gloves, and well-ventilated rooms become essential. There’s a good reason for that. The dust or fumes, if inhaled, can irritate the nose and throat. Long-term or heavy exposure, even from touching without protection, might bring headaches, nausea, or worse – signs of poisoning.
Breathing high levels of manganese compounds for months or years turns into a genuine health issue. In some factories where manganese dust drifts through the air, workers have developed manganism. It’s a neurological disorder, with symptoms a lot like Parkinson’s disease. That’s not something you want to mess with. The fact that the body can clear out only a certain amount of manganese before it builds up highlights why exposure limits matter.
How Safety Rules Make a Difference
Industries using manganese chloride keep strict guidelines thanks to research and some tough stories from the past. The U.S. Occupational Safety and Health Administration (OSHA) puts exposure limits in place for a reason. Scientists have linked high levels of manganese in workplace air to memory loss and movement troubles. The Centers for Disease Control and Prevention (CDC) points out that touching or eating small amounts rarely brings harm, but breathing it regularly can lead to serious issues.
Household risks stay low unless someone’s misusing products with manganese chloride. The trouble shows up in jobs where powders get sifted, liquids are poured, and cleanup isn’t done carefully. Masks, proper storage, and even training sessions for workers go a long way. These measures matter. I’ve watched workplace injuries drop when employers handed out better safety gear and more training on chemical hazards.
Better Choices for the Future
No one wants to give up the benefits manganese compounds provide to various industries, but keeping exposure in check remains crucial. Substituting safer chemicals brings benefits when possible. Where manganese chloride is needed, investing in proper protective equipment and air ventilation keeps workers stronger and healthier. Encouraging medical check-ups for people exposed on the job can catch small problems before they grow. My experience tells me that information, vigilance, and equipment often spell the difference between an ordinary day at work and a health crisis. We don’t chase fear; we trust knowledge, steady routines, and the simple act of looking out for one another in the workplace.
What is the chemical formula of Manganese Chloride?
Understanding What Manganese Chloride Really Means
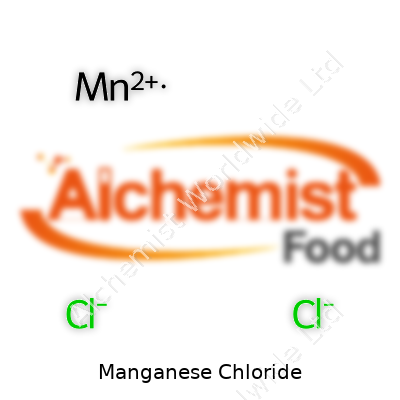
Manganese chloride sits inside the family of inorganic compounds that find their way into chemistry classrooms and industrial plants alike. The chemical formula happens to be MnCl2. That’s two chloride ions attached to one manganese ion. It’s a straightforward mixture at first glance, but it comes with its share of scientific and real-world value. The real interest doesn’t just lie in the string of letters and numbers — there’s a story behind how such a compound gets used, why it matters, and what it can teach beyond the classroom test.
The Importance of Getting Formulas Right
Accuracy in chemical formulas doesn’t just keep chemistry teachers happy. Mixing up one number, or even a single letter, means risking accidents in research or mistakes in manufacturing. In the workplace, chemists and lab workers count on MnCl2 for reliable reactions, especially when creating specialty alloys, dyes, or working through water treatment processes. Using the wrong formula wastes supplies, raises expenses, and weakens trust in results. I remember a lab session in college when a mislabeled compound led the whole group down the wrong path, blowing an afternoon and frustrating everyone. That memory sticks with me: precision isn’t just about being picky; it’s about saving time and even ensuring safety.
Everyday Ties: More Than Just Letters and Numbers
You might not pick up a bottle labeled “MnCl2” at the pharmacy or grocery store, but its chain reaction weaves quietly through many products. Manganese serves as a key trace element in animal nutrition. Chlorides support many industrial reactions, including textile dyeing and disinfectant production. When these two meet, the result stands out for its role in research and specialty processing. Manufacturing teams rely on consistent chemical quality. Schools and universities use it to introduce students to the building blocks of ionic chemistry. A faulty label or mishandled formula doesn’t just delay a lesson — it could cause lab mishaps or damage expensive equipment.
How Chemistry Education Could Improve
Back in high school, chemical formulas felt like codes cooked up to stump students. Later, I realized much of the confusion grew from dry explanations and not enough context. More lab time, hands-on experiments, and real-world stories could close that gap. Imagine learners seeing the effects a single compound can have in everything from vitamins to metal alloys. A high school teacher of mine brought in a piece of steel treated with manganese chloride, showing how a chemical formula on paper connects to the feel of metal in your palm. Those kinds of experiences stick.
Looking Toward Better Safety and Efficiency
Chemical safety relies on more than just rules printed on lab walls. Workers need clear information, correct labeling, and enough training to spot a mistake before it spreads. Some facilities adopt color-coded systems or regular cross-checks, making errors less likely. Suppliers can double down on packaging clarity, so anyone pulling a container off the shelf understands right away what’s inside. In the bigger picture, updating standards for both education and industry can keep slip-ups rare and make labs safer for everyone involved.
The Bottom Line for Everyone
Manganese chloride — formula MnCl2 — shows up all over chemistry, but its true weight comes from the people who use it. Whether mixing solutions in a lab or tracing minerals in a factory, accuracy keeps everyone on track. Better training, clear labeling, and bringing chemical lessons out of textbooks and into real life can make even the most basic compounds more meaningful and useful.
How should Manganese Chloride be stored?
Getting the Basics Right
Storing manganese chloride calls for a lot more than a spot on a shelf. From the first time I worked with this compound in a college chemistry lab, I noticed its tendency to pick up water from the air and clump up. That lesson stuck with me. Anyone who leaves it in an open jar soon learns it turns from crisp crystals to a sticky mess. Humidity ruins the whole batch, and nobody wants to run an experiment with compromised chemicals.
Avoiding Moisture Trouble
Manganese chloride loves soaking up moisture. I store it in tight, sealed containers and always reach for glass jars with sturdy rubber gaskets. Forget regular plastic snap-on lids—they let air sneak in after a day or two. Some folks trust desiccators, and with good reason. My experience proves a desiccator loaded with fresh silica gel keeps things dry even in a damp basement lab after heavy summer rains.
No Sun, No Fun for Chemical Storage
Direct sunlight can cause some chemicals to degrade, and manganese chloride is no exception. The reddish-pink crystals might look nice in the sun, but chemical changes begin. Keep the container inside a cupboard or drawer, away from any window. Labs I worked in stick to simple labeling, clear dating, and keeping all light-sensitive chemicals together in a darker cabinet.
A Cool Place Is a Safe Place
I learned the hard way that leaving a jar of manganese chloride near radiators shortens its shelf life. The heat speeds up the chemical’s reaction with ambient moisture, and in extreme scenarios, can intensify its oxidation. Room temperature works best. If the storage area swings hot and cold, that’s inviting problems. My advice is to choose a spot with stable temperature—nothing fancy, just a corner of a climate-controlled room always stays under 25°C.
Safety Is Non-Negotiable
This stuff isn’t edible—it’s toxic. I always keep it away from any food prep areas, well out of reach of kids or pets. My old high school used lockable cabinets for chemicals like these and checked logbooks every week. Gloves and goggles aren’t optional; they go on before the container comes out. If someone gets careless and a spill happens, don’t sweep it up with bare hands. Toss on gloves, grab a dustpan, and wipe the area with damp paper towels. Dispose of all contaminated items in clearly marked hazardous waste bins.
Labeling and Inventory: No Shortcuts
Mark every container with the name, date received, and any hazard codes. Cross-check the shelf regularly and rotate the stock so the oldest goes first. In my experience, forgetting dates means running the risk of discovering mystery jars years later. Digital inventory systems help, but a handwritten log on a clipboard near the storage shelf gets the job done and takes only a few seconds to update.
Disposal and Environmental Care
Old manganese chloride should never end up down the drain. Local authorities offer clear guidelines. I bring my wastes in sealed bags to the closest hazardous waste facility and keep printed receipts for good recordkeeping. Paying attention to disposal rules protects not just your health, but local wildlife and water supplies as well.
Routine Prevents Mishaps
Safe storage doesn’t run on autopilot. Each month, I do a quick sweep, check for any leaks, cracks, or faded labels, and replace desiccant packets if they’re no longer blue. Staying ahead of problems sharpens chemical safety habits and keeps surprises out of the lab. A little vigilance goes a long way.
What is the appearance and solubility of Manganese Chloride?
A Closer Look at Manganese Chloride
Manganese chloride pops up in classrooms and labs, but unless you know chemistry well, it’s not something you see every day. Stored in containers across university shelves, the stuff rarely grabs attention at first glance. In its most used form, manganese(II) chloride usually appears as pale pink crystals. Sometimes it looks almost red or even slightly purple, depending on the humidity or purity. Touching those crystals leaves a salt-like residue that clings to the skin, almost like the dust from sidewalk chalk. This color gives away its identity much more reliably than a label on the bottle.
Manganese chloride takes on a whole new personality depending on where you see it. As a solid, it’s fragile and crumbly, sometimes turning a little sticky if the room’s damp. I remember opening an old sample in the back of a school cabinet, and the crystals had practically fused from absorbing moisture in the air.
Mixing with Water: Solubility in Real Terms
Once those pink crystals hit water, they don’t hang around for long. Manganese chloride dissolves quickly, and you end up with a clear, light pink solution. It doesn’t need hot water—the process works fine at room temperature. After a few stirs, there’s rarely any residue left at the bottom. Not all salts behave so well, but manganese chloride rarely puts up a fight, even in cold tap water. Its high solubility in water explains why so many industries pick it for quick reactions.
Older chemistry books usually rank manganese chloride as highly soluble, reporting up to 72 grams dissolving in 100 grams of water at 20°C. In my own work, I’ve seen slightly less if the sample was older or a little dirty, but the high solubility means you don’t need fancy equipment to get it all into solution. It has its limits in alcohols and other non-water solvents—stick with water if you want all of it to dissolve at once.
Many research projects and industrial applications lean on this trait. Making catalysts, testing for trace metals, or even producing dry cell batteries, workers need a reliable way to get manganese ions into a system. If a dissolved source works best, this chemical is hard to beat.
Why Appearance and Solubility Matter
Color and solubility might sound trivial outside a lab, but they tell you a lot about how safely and reliably you can use the material. For example, the deep pink hue signals contamination or too much moisture, sometimes flagging storage issues long before paperwork or safety data sheets catch up. Fast and easy dissolution saves time and reduces the risk of undissolved lumps screwing up an experiment or reaction.
In the classroom, these properties also let teachers safely demo reactions without struggling to prepare the solution. In industry, predictable solubility helps keep costs down and processes smoother. Workers avoid time wasted stirring and filtering out grit—a bonus for both safety and efficiency.
Room for Improvement
Plenty of labs could pay more attention to proper storage—moist air can turn crystals into a sticky mess, leading to measurement errors. Airtight containers and drying agents help, but consistent staff training makes a real difference. Also, simple labels on jars showing a color reference can quickly flag any drift from that telltale pink, heading off bigger problems before they start.
Manganese chloride’s everyday qualities highlight how basic chemistry knowledge translates into safety, savings, and smoother work in settings big and small.
| Names | |
| Preferred IUPAC name | Manganese(II) chloride |
| Other names |
Manganese(II) chloride Manganous chloride |
| Pronunciation | /ˈmæŋ.ɡəˌniːz ˈklɔːˌraɪd/ |
| Preferred IUPAC name | Manganese(II) chloride |
| Other names |
Manganese(II) chloride Manganous chloride Manganese dichloride |
| Pronunciation | /ˈmæŋ.ɡəˌniːz ˈklɔː.raɪd/ |
| Identifiers | |
| CAS Number | 7773-01-5 |
| Beilstein Reference | 1202464 |
| ChEBI | CHEBI:66361 |
| ChEMBL | CHEMBL3304696 |
| ChemSpider | 48811 |
| DrugBank | DB11110 |
| ECHA InfoCard | 100.033.888 |
| EC Number | 231-869-6 |
| Gmelin Reference | Gmelin Reference: 1747 |
| KEGG | C01739 |
| MeSH | D008353 |
| PubChem CID | 24580 |
| RTECS number | MN3750000 |
| UNII | 8OC22C1B99 |
| UN number | UN2817 |
| CompTox Dashboard (EPA) | DTXSID7020826 |
| CAS Number | 7773-01-5 |
| Beilstein Reference | 3589796 |
| ChEBI | CHEBI:31344 |
| ChEMBL | CHEMBL1287549 |
| ChemSpider | 56451 |
| DrugBank | DB11245 |
| ECHA InfoCard | 100.028.786 |
| EC Number | 231-869-6 |
| Gmelin Reference | Gmelin Reference: 17008 |
| KEGG | C07396 |
| MeSH | D008357 |
| PubChem CID | 24580 |
| RTECS number | MN9350000 |
| UNII | V9G29RU8M9 |
| UN number | UN2817 |
| Properties | |
| Chemical formula | MnCl2 |
| Molar mass | 125.84 g/mol |
| Appearance | Pink crystalline solid |
| Odor | Odorless |
| Density | 2.977 g/cm³ |
| Solubility in water | Soluble |
| log P | -2.00 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.3 |
| Basicity (pKb) | 7.34 |
| Magnetic susceptibility (χ) | +10600.0e-6 cm³/mol |
| Refractive index (nD) | 1.705 |
| Dipole moment | 0 Debye |
| Chemical formula | MnCl2 |
| Molar mass | 125.84 g/mol |
| Appearance | Pink crystalline solid |
| Odor | Odorless |
| Density | 2.977 g/cm³ |
| Solubility in water | Soluble |
| log P | -3.55 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.30 |
| Basicity (pKb) | 6.62 |
| Magnetic susceptibility (χ) | +2.1·10⁻⁵ |
| Refractive index (nD) | 1.600 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 187.9 J⁄(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | -641 kJ/mol |
| Std molar entropy (S⦵298) | 176.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -641 kJ/mol |
| Pharmacology | |
| ATC code | A12CC01 |
| ATC code | A12CC02 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P330, P391, P501 |
| NFPA 704 (fire diamond) | 2-0-1 |
| Lethal dose or concentration | LD50 oral rat 1,480 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 420 mg/kg |
| NIOSH | Manganese Chloride: NIOSH IDLH = 500 mg Mn/m3 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Manganese Chloride: 5 mg/m³ |
| REL (Recommended) | 10 mg/kg |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P280, P273, P305+P351+P338, P310 |
| Lethal dose or concentration | LD₅₀ Oral - Rat - 420 mg/kg |
| LD50 (median dose) | 418 mg/kg (rat, oral) |
| NIOSH | MNCOC |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Manganese Chloride: "5 mg/m³ (as Mn), OSHA PEL (ceiling) |
| REL (Recommended) | 100 |
| Related compounds | |
| Related compounds |
Manganese(II) sulfate Manganese(II) acetate Manganese(II) nitrate Iron(II) chloride Cobalt(II) chloride |
| Related compounds |
Manganese(II) oxide Manganese(II) sulfate Iron(II) chloride Cobalt(II) chloride |

