Manganese Carbonate: The Realities Behind a Quietly Essential Compound
Historical Development
Manganese carbonate has roots that stretch back to the nineteenth century, not emerging as a glamorous discovery, but as a byproduct of mining and ore processing. Early chemists looking for ways to isolate and purify manganese often found themselves staring down pink and brown earth—mineral samples like rhodochrosite. Over time, its importance grew. Once the steel industry started relying on manganese to combat brittleness, chemists stitched new applications together. From observation and necessity, workers realized this little-known compound could serve in dyes and fertilizers, not just as a passing step toward producing pure manganese metal. It’s easy to think of the periodic table as a closed book, but the historical path shows how much real-world demand and basic needs keep reviving old compounds in new forms.
Product Overview
Manganese carbonate looks modest—a pale pink to light brown powder, not something that grabs attention in a storeroom. It doesn't fetch headlines, yet it makes its mark in fertilizers, ceramics, animal feed, pigments, and even electronics. Each bag or drum calls for careful handling and right placement. Industries turn to manganese carbonate for specific manganese tweaks in formulas, since it dissolves in most acids, making it more predictable than some harder minerals. The agricultural sector, in particular, pushes for micronutrient additives that help stronger crop yields. From the pharmacist’s shelf to the ceramic glaze studio, folks rely on reliable, easy-to-handle manganese sources to keep processes simple and repeatable.
Physical & Chemical Properties
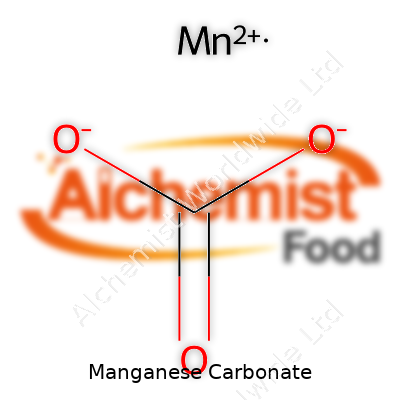
Chemistry buffs will recognize manganese carbonate by its chemical formula, MnCO3. The appearance often reeks of understatement: a powder, neither sparkling nor slick, sticking easily to gloves or bins. It won’t melt in heat but starts to break down into manganese oxide and carbon dioxide at around 200 degrees Celsius, squeezing out the carbon part and releasing a gas. Water leaves it pretty much alone. Acids, on the other hand, open it up quickly. That behavior underpins a lot of its industrial usefulness, making it a versatile starting material for manganese-based reactions. It weighs about 3.1 grams per cubic centimeter and doesn’t burn, so fire risk stays low during storage.
Technical Specifications & Labeling
Labels on manganese carbonate drums or sacks spell out more than composition—purity matters a lot, especially in high-end uses like battery manufacturing or pharmaceuticals. Purity often sits above 98% for technical and analytical grade material, where even slivers of other metals can mess up sensitive results or end-products. Particle size gets measured in microns because clumping or dustiness affects both mixing and reaction speed. Safety labeling covers more than the basics. Requirements press for hazard warnings about dust inhalation, reactivity with strong acids, and environmental handling instructions, especially for big users in fertilizer production. Handling guides often bulk up the rules with reminders on personal protective gear, enclosed transfers, and quick clean-up of spills to keep workplace health solid.
Preparation Method
Most manganese carbonate comes from either mining rhodochrosite or cooking up a reaction between manganese sulfate and sodium carbonate. Some of the big industrial producers lean on a simple precipitation process where manganese sulfate—pulled straight from ore—gets neutralized with soda ash. The resulting pale fluff settles out, then filtration, washing, and drying follow. If you dig deep into specialty powder supply, workers shift to recycling manganese-rich battery waste or chemical process byproducts, a practice that’s growing as environmental regulations put the brakes on mining waste. Each method, whether resource-heavy or batch-scaled, circles back to the need for clean, decontaminated starting materials so the finished powder meets strict chemical and safety specifications.
Chemical Reactions & Modifications
Manganese carbonate doesn’t like to stand still; it’s a springboard for a whole family of manganese compounds. Strong acids turn it quickly into manganese(II) salts and foam up bubbles of carbon dioxide. Cook the powder hot enough and it drops its carbonate, turning into manganese(II) oxide—an important pigment and catalyst. Researchers in battery science play with skipping between carbonate and oxide forms to fine-tune performance in lithium-ion cathodes. Some processes use manganese carbonate as a feeder for organometallic syntheses, where it brings a more controllable source of manganese ions than irregular ore powders or oxides. Chemists have shifted away from high-carbonate waste routes now that regulation punishes dirty effluent and nagging activism holds the industry’s feet to the fire.
Synonyms & Product Names
In trade and science, you’ll hear manganese carbonate called manganous carbonate, or even E170(ii) in food circles. Some suppliers slap on trade names like "RhodoCarb" or "Managanese Superfeed." Those names don’t change the chemistry but help buyers navigate a crowded supply market, where generic product numbers sometimes cause confusion in ordering. Food and pharma grades add another layer, since regulators keep a long list of acceptable names. Some buyers even call it "manganese ore powder," especially if sourcing from less-refined mineral streams, but this invites trouble, since ore powders may bundle in unwanted trace metals.
Safety & Operational Standards
Strict rules shape both workplace safety and environmental protection—regulatory groups like OSHA, REACH, and national agencies have written these requirements over years of observation and, frankly, workplace accidents. Manganese carbonate dust can build up in the air, posing inhalation risks to workers. Chronic overexposure to airborne manganese links to neurological symptoms, a real concern in processing plants without good ventilation or dust suppression. Facilities regularly have to keep particle counts down, supply respiratory masks, and automate transfers. Disposal of material and residues can’t go straight to landfill; waste streams must pass screening to prevent heavy metal leaching, especially in agricultural districts. Staff training can’t lag behind—new hires shouldn’t walk into a storage area blind, and repeat drills reinforce habits against spills or improper mixing. Packaging also requires clear hazard pictograms and traceable lot numbers, addressing recalls or incident investigations much faster.
Application Area
You find manganese carbonate feeding into agriculture, where it shores up micronutrient levels for plant growth—something overlooked until soil science started pointing out "hidden hunger" in crops. Feed manufacturers blend small, measured portions into animal supplements to correct livestock deficiencies, since livestock absorb manganese more effectively from this salt than many others. The pigment world taps it for brown and pink hues in ceramic glazes and paints. Electronics research has borrowed it for precursor chemistry, especially for batteries and supercapacitors. Pharmaceuticals call for almost clinical purity, using it to create medicinal preparations that address dietary deficiencies. Water treatment sectors quietly add columns of manganese carbonate media to filter and treat contaminants in municipal purification plants.
Research & Development
Research labs chase better ways to refine, recycle, and reuse manganese carbonate, often under pressure from rising mineral costs and waste management headaches. Battery researchers tune manganese carbonate’s role in next-generation cathode materials, working out doping and calcination tricks that produce better charge cycles and longer lifespans for lithium or sodium-ion cells. Agricultural scientists compare uptake in different soil types, finding ways to cut application rates without sacrificing crop yields—precision farming hinges on those data points. Biochemists test ways to convert manganese carbonate into new coordination compounds, with potential therapeutic uses or advanced catalysts. Grants flow toward "greener" synthesis, hoping to cut fossil raw materials out of the loop while keeping yields up and impurities down. Conferences buzz with updates about nano-scale processing and biotechnological routes that could cut costs and tame pollution.
Toxicity Research
Despite its usefulness, manganese carbonate poses challenges in health and environmental safety that researchers can’t brush aside. Toxicology studies track how inhaled or ingested manganese can over-accumulate in the human brain, triggering symptoms similar to Parkinson’s disease in severe cases. Factory workers, if not given proper protection, can face higher risk—a fact shown in both old and recent occupational health reviews. Agro-environmental monitoring programs watch for manganese build-up in water supplies, since runoff from treated fields can climb above safe thresholds, especially when improper dosing occurs. Toxicologists today push for long-term, low-dose studies, since acute poisonings rarely tell the whole story. Animal studies provide early warning, but human epidemiology remains crucial, especially among exposed populations near mines or fertilizer plants. This research keeps driving new rules, lower emissions, and safer workplace limits.
Future Prospects
Looking forward, manganese carbonate will keep adapting to new demands. As battery manufacturers hunt for local sources of manganese, pressure builds to recycle scrap and post-consumer material, giving new life to waste battery streams. Advanced ceramics could use tailormade manganese carbonate grains for custom glazes, pushing both color range and mechanical properties. Soil scientists harness better geochemical mapping to pinpoint exactly where crops or pastures respond to targeted applications, cutting down raw material waste and runoff in agriculture. Tighter legal standards for workplace exposure and environmental discharge guarantee more research dollars for process improvement and risk reduction. Digitizing supply chain tracking, from mine to user, could shut the door on rogue sources and grade mislabeling. Biotechnology joins the mix as enzyme-catalyzed routes challenge old chemical synthesis, eyeing plant-based or waste-derived starting points. The entire industry faces a balancing act—providing enough high-purity product while acknowledging its health and ecological baggage. As these challenges press in, those working in research, regulation, and production will shape whether manganese carbonate stays a background player or pushes into the spotlight as cleaner tech and agriculture demand smarter, safer chemistry.
What are the main uses of Manganese Carbonate?
Power Behind Plant Fertilizers
Imagine a tomato plant stubbornly yellowing, leaves limp even with plenty of water and sunlight. Over the years gardening, I’ve learned that not every sickly plant suffers from bugs—nutrient deficiencies also play a role. Manganese carbonate shows up as a critical nutrient fix for crops needing a boost. Farmers sprinkle it in fertilizer blends to help plants create chlorophyll and grow taller, greener, and faster. Without enough manganese, cereal crops stunt and leaves develop odd spots. Knowing this, producers use manganese carbonate to balance soils, fueling global wheat, rice, and corn fields. The result: bigger harvests and less food insecurity. Reliable harvests add real stability to rural economies.
Paints and Pigments That Stick
A walk down any hardware aisle, and you’ll spot buckets of paints in rich red, brown, and pink. That depth of color doesn’t appear out of nowhere—manufacturers rely on manganese carbonate as a pigment ingredient in ceramics and paints. Artists and builders have trusted manganese-based colors for generations, thankful for their opacity and lasting tones. What seems like a simple undercoat in wall paint or detail in pottery quietly depends on this mineral. These products bring character and durability to rooms, commercial buildings, and even city art installations.
Medicines and Supplements
Growing up, I watched relatives take vitamin pills like clockwork—tiny insurance policies against weak bones and slow healing. Few realize that certain manganese supplements begin with manganese carbonate. It’s processed into medicines that help the body produce collagen and absorb calcium, defending against bone problems like osteoporosis. Doctors suggest manganese as part of a broader mineral routine, especially where malnutrition is a concern. That puts this quiet mineral in a starring role for seniors and folks with dietary gaps, especially in places where fresh vegetables can be hard to find.
Electronics and Technology
Phones, laptops, and electric cars use rechargeable batteries that store more power every year. Most people won’t see the chemistry inside these gadgets but manganese shows up again, this time as a key ingredient in making lithium-ion batteries. Producers use manganese carbonate in the early stages, purifying it to add strength and reliability to battery cells. Without this mineral, electric vehicles and portable gadgets simply wouldn’t run as long, making modern convenience less practical. Battery improvements trickle through the supply chain, allowing us to work from remote areas and drive cleaner vehicles.
Risks and Responsible Use
Not every use of manganese carbonate comes without concern. In fertilizer factories, dust and small particles can cause lung issues among workers if not managed well. Researchers note that chronic exposure in mining towns sometimes leads to nervous system disorders. Farms also risk adding too much to fields, upsetting soil chemistry and water safety for communities downstream. Factories must keep close track of the way they process and store this compound. Investing in good ventilation, personal protective gear, and regular soil testing cuts down on these problems.
Moving Forward: Smarter Handling
Everyone—from big mining companies to organic gardeners—plays a part in how this mineral shapes our world. Using it safely calls for solid training, modern equipment, and regular health checks for workers. Research teams look for ways to recycle manganese from batteries, lowering the pressure on mines. Community outreach and smart labeling mean food producers and the public spot trouble sooner, keeping both people and landscapes safe for seasons to come.
Is Manganese Carbonate hazardous or toxic?
Looking Closer at a Common Compound
Manganese carbonate isn’t a household name, but it pops up in industries you wouldn’t expect. It feeds into agriculture, ceramics, and even the coatings on steel. Folks in these businesses handle bags of the stuff, often without giving much thought to the risks. Most days, people want to know: is it safe, or should they worry about exposure?
The Real Risk Lies in How You Handle It
No chemical feels totally risk-free. Manganese carbonate, like many powdered minerals, comes with its own set of cautions. I have worked with agriculture suppliers who add manganese carbonate to fertilizers. Every time someone opens a bag and particles puff into the air, there’s a whiff of dust that goes right up the nose or settles on skin. That seems minor, but over time and with repeated contact, inhaling dust or getting lots of it into your body raises a red flag.
Manganese itself fuels healthy metabolism in tiny amounts. But as with most metals, problems start building when the body collects too much. Chronic exposure can mess with your nervous system. The Centers for Disease Control and Prevention (CDC) notes that workers exposed to high levels for months or years sometimes show symptoms a lot like Parkinson’s disease: muscle stiffness, trouble walking, and mood changes. What worries safety pros isn’t a single encounter — it’s steady exposure without the right gear.
Environmental and Handling Concerns
Plants need manganese. That’s why it crops up in fertilizer mixes. Farmers get higher yields, but run-off can push metals into water systems if not managed well. Regulators, from the Environmental Protection Agency (EPA) to local water authorities, track manganese runoff and its build-up in the soil. If this keeps happening, manganese levels swell, hurting freshwater creatures and entering drinking water.
Storing this compound properly changes everything. Most businesses keep it in sealed, labeled containers, away from acid or wet conditions. Spills should get cleaned up with care to avoid scraping up the powder and sending it airborne; using a mask and gloves cuts down the risk.
How to Stay Safe – The Practical Fixes
Over time, old habits stick, but improving safety takes simple steps. A basic dust mask, goggles, and gloves block most contact risks in daily handling. Good ventilation stirs fresh air through workrooms, scattering dust clouds and making them less intense.
Training matters. I’ve seen shifts in attitudes at facilities that run regular health and safety sessions on chemical exposure. People remember what’s at stake and pay more attention when lifting bags or sweeping up powder. Companies that keep health records and encourage check-ups tend to spot early warning signs, protecting their crew over the long haul.
Moving Forward with Awareness
It takes time to build better practices, but the science is clear. Manganese carbonate helps crops and supports many industries, yet its potential hazards can’t get brushed aside. The key rests in recognizing the dangers, teaching safe handling, and paying attention to signs of exposure—at work and in the environment. In the end, staying informed brings the biggest long-term benefit.
What is the chemical formula and appearance of Manganese Carbonate?
Straight Facts: What Is Manganese Carbonate?
Manganese carbonate carries the unmistakable formula MnCO3. The makeup itself looks simple—a manganese ion bound tightly to a carbonate group. Although it sounds basic, don’t let the letters fool you. This compound shows up in real-world settings with unique visual character and plenty of practical use.
The Look and Feel
If you’ve worked in chemistry labs, you probably remember the calm, chalky appearance of manganese carbonate. This isn’t a dazzling or shiny material—what greets the eye is a very pale pink, sometimes almost tan or rose-colored powder. The color comes from the manganese, a metal known for producing all sorts of shades in minerals.
Hold a handful, and it stays fine, practically silky. It doesn’t clump easily when dry, unless it’s exposed to high humidity. The powder doesn’t show much odor either, and it stays stable at room temperature as long as you keep it dry and shielded from acids or direct moisture.
Beyond the Textbooks: Why It Matters
In my time working with trace minerals, I noticed how often people overlook these pale powders. Yet manganese carbonate serves as a steady source of manganese for everything from ceramics to plant fertilizers. Its muted pink tone gives a useful sign: the material hasn’t picked up iron impurities, which often sneak into other manganese minerals and cause a rusty tint.
Manganese itself, delivered through this compound, backs up several industries. Battery manufacturing relies on manganese for making cathode materials. Agriculture uses this powder to correct soil manganese shortages and boost plant health. Production of ceramics and some specialty glazes benefit from both the chemistry and the subtle pink coloration. The appearance of the raw material tells you something about the end result—fewer impurities in your starting material mean more reliable results later.
From Personal Experience
Working in both education and industrial settings, I’ve seen confusion spark when someone expects a metal to look metallic. Manganese carbonate throws that expectation out the window. Many people picture shiny, solid metal, but most pure manganese compounds don’t look like coins or pipes. The soft, almost cloudy pink of manganese carbonate makes it feel almost gentle—a note worth sharing if you’re teaching chemistry or inspecting raw mineral deliveries.
Sometimes, questions pop up about material quality or purity—especially when batches shift in shade. Over the years, manufacturers and lab managers have told me that color alone often signals whether you’re working with the real deal or a contaminated batch. If you hoped for a bleach-white powder, you’d be let down; if it’s brown or rusty, someone’s probably cut corners or let the sample oxidize.
Health and safety guidelines also matter. Dust management becomes important because inhaling fine powders, even from minerals seen as safe, can create health risks over time. Good protocols use enclosed handling and basic protective gear, adding a layer of respect for what might seem like “just” a pale mineral powder.
Looking for Solutions
Purity can make or break a project. For those sourcing manganese carbonate, sticking with reputable suppliers and insisting on batch testing helps catch impurities early. Education in schools and factories alike can emphasize not only the formula but also the importance of how it looks. As more people pay attention to subtle signs like powder color and texture, industries and classrooms both can stay on top of material quality—keeping projects and research moving forward with confidence.
How should Manganese Carbonate be stored and handled?
Why It Matters
Many people walk past chemical storage rooms every day without thinking much about what’s really inside. In that collection, you might find manganese carbonate—a pinkish powder with more uses than people realize. Farmers, ceramic artists, battery makers, and water treatment workers depend on it. Still, handling it gets overlooked because it sits on shelves without drama. That low profile hides real risks. My years working in labs have shown me how easy it is to let your guard down with common chemicals. It only takes one careless move to put health and safety at risk.
Moisture and Air Can Change Everything
One thing that trips up a lot of folks is thinking manganese carbonate can sit anywhere because it “looks safe.” It reacts with acids to form toxic gases and can turn brown if exposed to air and moisture for too long. This isn’t theoretical: I’ve seen oxidized product turn into a useless mess, costing money and time. Damp rooms or broken containers don’t just ruin its quality—they invite bigger hazards. A dry, cool, well-ventilated spot keeps things stable. If a bag rips or gets wet, you’ll wish you’d sealed it tight or used airtight jars from the beginning.
Don’t Treat It Like Just Another Powder
In most shops and labs, powders pile up. Staff scoop and stir without thinking about what might go airborne. Breathing in manganese dust isn’t safe. The metal can settle in your lungs and build up in your body over time, causing nerve and brain problems—facts the CDC and EPA document clearly. I’ve watched colleagues ignore dust masks and wind up coughing for hours after a spill. The rules exist for a reason: always use a dust mask or, even better, a respirator, especially if pouring large batches or cleaning up spills. People tend to relax rules after years with no accidents, but that’s when slips happen.
Label Everything Like Someone’s Life Depends on It
Complacency makes mislabeling easy, and in shared workspaces, that habit brings danger. I’ve opened jars labeled “blush” in ceramics studios to find heavy metal powders with no warning notes. It’s not just an inconvenience—it can lead to accidents. From my own experience, a day’s work gets turned upside down when a container goes missing or gets mixed up. Always label containers with clear names, hazard warnings, and safety notes. Workers down the line will thank you, and you avoid fines from inspections.
Training and Knowledge Cannot Be Optional
No one understands every chemical by instinct. Even in workplaces with experienced staff, new hires often get a half-hour walkthrough and a thick manual they rarely read. Experienced workers sometimes skip refreshers, assuming they remember everything. That’s a setup for problems. Regular training sessions—quick, practical, and honest—keep safety at the front of everyone’s mind. Demonstrations go further than video presentations, in my view. Asking around, most accidents happen with folks who thought “just this once” would be fine.
Storage Isn’t Just a Policy—It’s a Habit
Safe handling comes from good habits: tight seals, clean hands, well-ventilated rooms, and proper disposal. Getting lazy once turns into shortcuts. In my career, I’ve seen what happens when procedures become suggestions. Manganese carbonate looks harmless in a jar, but treating it with respect protects everyone—from the person unpacking deliveries to the one scrubbing up after work.
What are the specifications and purity levels available for Manganese Carbonate?
Why Quality and Purity Aren’t Just Numbers
Manganese carbonate isn’t just another industrial chemical tucked away in a catalog. This pale pink powder shapes more than its color suggests: it goes into fertilizers, ceramic glazes, batteries, animal feed, and even plays a role in pharmaceuticals. The importance of manganese carbonate starts with its specifications, and these numbers drive product choice in the real world.
Purity Levels: From Technical Grade to High Purity
If you’ve spent any time sourcing chemicals, you know there’s no “one size fits all.” For manganese carbonate, the scale runs from technical grade, where purity sits around 80-85%, up to high purity and reagent grades, which hit 98-99.9%. Purity means much more than avoiding a slight tint or haze – it keeps crops healthy, prevents ceramic flaws, and avoids introducing unwanted metals into pharmaceutical or animal feed applications.
Most fertilizer applications accept material that contains at least 80% manganese carbonate, but for ceramics, pigment work, or battery manufacturing, closer to 98% purity becomes critical. Every fraction of a percent of impurity, particularly iron or heavy metals, can alter electrical properties and crop safety or create quality headaches when regulatory standards get strict.
Understanding Specifications Beyond the Percentage
Quality assessment goes beyond one big number. Here’s what regularly shows up on a proper tech sheet for manganese carbonate:
- Manganese (Mn) Content: Expressed as MnCO3 or as elemental manganese, usually the anchor of the assay.
- Insoluble Residue: Indicates how much stuff just doesn’t dissolve; high leftovers cause issues in processing.
- Loss on Ignition: Shows water or organic material present; high numbers hint at contamination or instability.
- Heavy Metal Limits: Look for specific callouts around iron, arsenic, lead, and cadmium—safety and performance often ride on these ppm levels.
- Moisture: Water content tells you if you’re buying dry powder or excess weight.
- Physical Form: Most manganese carbonate appears as a fine powder, with typical particle sizes under 45 microns. For feed use, some suppliers offer coarser forms or granules to improve mixing and reduce dust.
Experience With Supply Chains: The Gaps Between Grades
Anyone who’s switched between suppliers or countries learns quickly that stated purity doesn’t always tell the full story. I’ve worked with fertilizer producers frustrated by caking or inconsistent granulation—problems traced back to varying insolubles or unpredictable particle sizes, not always caught in routine spec checks. In the battery space, manganese carbonate with trace metals beyond tight tolerances caused unpredictable cell behavior, undercutting efficiency claims.
The lesson: read the certificate of analysis every time, ask for actual batch data, and don’t rely on blanket specs. Trusted suppliers run regular testing—sometimes that means third-party labs backing up what the manufacturer claims.
Finding the Right Purity for the Job
Most industries set their own minimums for manganese content and maximums for contaminants. For specialty ceramics or electronics work, nothing beats an up-to-date analysis and reference samples before committing to major lots. Animal feed producers tend to pay more attention to bioavailability and the absence of heavy metals since both can affect animal health and compliance with food-chain rules.
Where regulations keep tightening—think of the food, pharma, and battery sectors—producers invest in better refining and certification to hit rising benchmarks. Greater scrutiny means cleaner, safer products, but it also makes transparency and traceability just as valuable as the numbers printed on the bag.
| Names | |
| Preferred IUPAC name | Manganese(II) carbonate |
| Other names |
Manganous carbonate Manganese(II) carbonate Manganese mono carbonate |
| Pronunciation | /ˌmæŋ.ɡəˈniːz ˈkɑː.bə.neɪt/ |
| Preferred IUPAC name | Manganese(II) carbonate |
| Other names |
Manganous carbonate Manganese(II) carbonate Carbonic acid, manganese(2+) salt |
| Pronunciation | /ˈmæŋɡəniːz ˈkɑːbənət/ |
| Identifiers | |
| CAS Number | 598-62-9 |
| Beilstein Reference | 3589836 |
| ChEBI | CHEBI:31563 |
| ChEMBL | CHEMBL1201434 |
| ChemSpider | 72155 |
| DrugBank | DB11110 |
| ECHA InfoCard | 100.028.289 |
| EC Number | 4.2.99.3 |
| Gmelin Reference | Gmelin Reference: 14154 |
| KEGG | C01754 |
| MeSH | D008351 |
| PubChem CID | 10100138 |
| RTECS number | OP9625000 |
| UNII | 79JZU3T01H |
| UN number | UN3077 |
| CAS Number | 598-62-9 |
| Beilstein Reference | Beilstein Reference: 1907446 |
| ChEBI | CHEBI:1312 |
| ChEMBL | CHEMBL1200932 |
| ChemSpider | 64210 |
| DrugBank | DB14531 |
| ECHA InfoCard | ECHA InfoCard: 100.007.297 |
| EC Number | 4.2.99.2 |
| Gmelin Reference | Gmelin Reference: 13208 |
| KEGG | C00808 |
| MeSH | D008361 |
| PubChem CID | 10129908 |
| RTECS number | OP9625000 |
| UNII | E8K1386C4Z |
| UN number | UN3077 |
| Properties | |
| Chemical formula | MnCO3 |
| Molar mass | 114.95 g/mol |
| Appearance | Pinkish to pale brown powder |
| Odor | Odorless |
| Density | 3.12 g/cm³ |
| Solubility in water | Insoluble |
| log P | -13.15 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 5.4 |
| Basicity (pKb) | 10.40 |
| Magnetic susceptibility (χ) | +1300·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.60 |
| Dipole moment | 0.0 D |
| Chemical formula | MnCO3 |
| Molar mass | 114.95 g/mol |
| Appearance | Pinkish white powder |
| Odor | Odorless |
| Density | 3.12 g/cm³ |
| Solubility in water | Insoluble |
| log P | -13.15 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.0 (for H2CO3, as MnCO3 is a salt of a weak acid and a weak base) |
| Basicity (pKb) | 8.21 |
| Magnetic susceptibility (χ) | +5350e-6 cm³/mol |
| Refractive index (nD) | 1.618 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 81.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -814 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -814.1 kJ/mol |
| Std molar entropy (S⦵298) | MnCO3: 65.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -814.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -814.0 kJ/mol |
| Pharmacology | |
| ATC code | A12CC01 |
| ATC code | A12CC01 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin and eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0-W |
| Lethal dose or concentration | LD50 oral rat 4820 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 5,750 mg/kg |
| NIOSH | MNCO3 |
| PEL (Permissible) | 5 mg/m3 |
| REL (Recommended) | 200 mg/kg |
| IDLH (Immediate danger) | Unknown |
| Main hazards | May cause irritation to eyes, skin, and respiratory tract; harmful if swallowed or inhaled. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P260, P280, P302+P352, P304+P340, P312, P314, P501 |
| NFPA 704 (fire diamond) | 1-1-0-N |
| Lethal dose or concentration | LD50 oral rat 4820 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 4820 mg/kg |
| NIOSH | SD2350000 |
| PEL (Permissible) | 5 mg/m3 |
| REL (Recommended) | 197 mg/day (as manganese) |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Manganese(II) chloride Manganese(II) sulfate Manganese(II) oxide Manganese(III) oxide |
| Related compounds |
Manganese(II) oxide Manganese(II) chloride Manganese(II) sulfate Iron(II) carbonate Calcium carbonate |

