Magnesium Stearate: More Than a Pharmaceutical Additive
Historical Development
Magnesium stearate’s journey started with the early days of modern pharmacy, back when pharmacists learned that mixing agents like stearic acid with magnesium salts created something that could keep pills from sticking together. The real boom happened in the last hundred years, especially once big pharmaceutical manufacturing lines needed reliable excipients. From old apothecaries to modern tablet factories, magnesium stearate has stuck around as a staple, for good reason. My time working inside tablet production lines made it clear: you pull magnesium stearate off a formula, and all sorts of practical problems pop up—stuck punches, uneven tablet weights, lost batches. Few excipients have earned their keep through so many decades of hard, practical, trial-and-error learning.
Product Overview
Manufacturers order magnesium stearate in white, almost powdery flakes. It often goes into recipes for pills, capsules, and sometimes food, with the main job being a lubricant. The food-grade and pharma-grade differences stem from how much heavy metal and microbe contamination can be tolerated. Through lots of direct experience with production lines, I’ve seen how just a minor change—using the wrong supplier or a different grade—can cause headaches. Tablet surfaces lose their clean snap, and consistency drops. Producers care about that for more than just regulation; it hits productivity and customer trust.
Physical & Chemical Properties
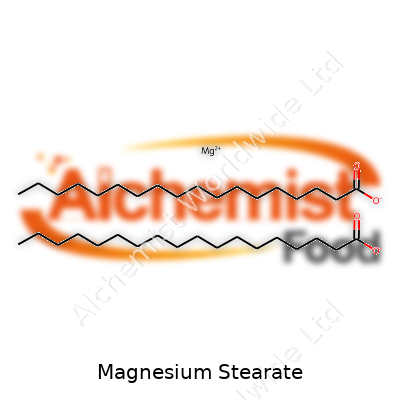
This excipient behaves like a classic greasy powder—you touch it, fingers slick up, and dust clouds show how light it is. Magnesium stearate melts at low heat, proving handy in some processes but challenging if machines heat up too much. Chemically, it has long hydrocarbon chains, which means it repels water and most polar solvents. It doesn’t dissolve much, and that’s important for how it works: the coating on other particles stays put and keeps everything from sticking. In technical terms, its chemical formula is C36H70MgO4. My own experience has shown that it keeps its form well during both hot summers and colder storage rooms, another practical detail small producers sometimes forget until they see caking or changes in flow.
Technical Specifications & Labeling
Industry-standard magnesium stearate comes with tight controls: particle size, water content, acid value, heavy metals, even microbial residue. Most product labels spell out GMP traces, possible allergens, and permitted uses by sector. Having handled incoming raw material audits, I noticed how suppliers update certificates almost weekly to align with shifting pharmacopeial standards, like USP, Ph. Eur., or JP. These changes don’t come for fun—they’re usually prompted by new research, updated toxicity limits, or regulatory pressure. An oversight, even in minor values, lands shipments in quarantine and triggers retesting.
Preparation Method
Industrial producers make magnesium stearate by combining stearic acid—coming mostly from palm or animal fat—with magnesium salts (often magnesium oxide or hydroxide). Think big jacketed reactors and careful temperature control to get things right. Everyone wants predictable purity, so feedstocks need constant monitoring for things like dioxins, pesticides, or animal byproducts. Batch records get thick fast. Shortsighted shortcuts, like not purifying the stearic acid enough, can ruin huge runs. More specialist versions, like vegetable-sourced material, popped up as vegan and halal demands increased. Careful traceability here is more than a legal checkbox; some markets simply won’t take anything with animal links.
Chemical Reactions & Modifications
In every chemistry course and factory tour I’ve taken, magnesium stearate comes across as pretty inert. High-heat or acid environments start breaking it down, leaving magnesium salts, stearic acid, and sometimes contaminants. Fancy reactors can tweak its chain length or saturated/unsaturated fat ratio, but most big producers stick to basic mods, aimed at optimizing lubricity or compressibility. Sometimes, micro-level coatings or blends with silica help formulations in high-speed tableting equipment. Custom versions end up as proprietary offerings—for folks who run lines that need anti-static grades, or for especially hard-to-compress actives. Still, most workhorse grades haven’t changed much over decades; the focus stays on consistency from batch to batch.
Synonyms & Product Names
Labels on drums often list names like “octadecanoic acid, magnesium salt,” “magnesium distearate,” or “E470b” for those focused on food use. Food producers know it as E470b, while some pharmaceuticals say “mag stearate” or simply “stearate.” Different regions and industries apply house codes or private brand names, which can cause confusion. I’ve known more than one QA rep who got tripped up by synonyms in global supply chains—meaning even a small mistake in interpretation can mess up compliance or ingredient lists.
Safety & Operational Standards
Having spent years in regulated settings, I learned that magnesium stearate attracts close scrutiny. Its use in medicine, supplements, and food ties it to strict standards on contaminants: lead, arsenic, cadmium, and microbial cells. Producers follow ISO, GMP, and local food safety codes, which means round-the-clock documentation, release testing, and regular audits. Handling in the workplace needs strict dust controls, since too much airborne powder can cause explosions or respiratory irritation. That’s not theory; a single wrong move with a powder conveyor can set off a costly plant shutdown. Staff training goes beyond surface-level PPE—real safety comes from knowing exactly what each step exposes people to, and running frequent drills on spill response and incident containment.
Application Area
Magnesium stearate shows up almost everywhere: pharmaceuticals, food, supplements, cosmetics, paint, and plastics. Pharmaceutical factories rely on it to keep equipment running and output consistent. Food industry producers toss it into chewing gum, candies, and certain bakery items. In the supplement aisle, pretty much every tablet contains a bit. It helps powder vitamins flow evenly into dies, trims down stuck products, and keeps dosages accurate. Years working with contract manufacturers made clear that pulling magnesium stearate from the recipe almost always turned smooth production into a headache. Consumers rarely notice these invisible additives, but industry insiders know they’re essential for cost control and meeting strict acceptance levels in end products.
Research & Development
Research on magnesium stearate rarely stops. Investigators look at both practical and theoretical sides: ways to improve flow, make plant-based variants more consistent, and study interactions with new drugs. Teams keep looking for new lubricants, but magnesium stearate’s low price, easy processing, and long record make it hard to beat. In one R&D group, we tested blends with improved release for sensitive drug actives; most alternatives just couldn’t match both the easy handling and the low cost. Scientists also examine its behavior in complex mixes, especially for challenging formulations, and how particle size tweaks can fix old bottlenecks. The constant search for new suppliers that meet emerging global standards underlines the ongoing race in raw material science.
Toxicity Research
Toxicological data on magnesium stearate points to its safe status at current intake levels, with numerous safety reviews backing up its daily use. European Food Safety Authority, US FDA, and WHO all list it as low-risk, provided it stays within regulated levels. At the same time, ongoing research checks for long-term risks, especially for gut health, immune response, or chronic use in sensitive populations. Occasionally, reports link large intakes from supplements or off-label use to mild digestive upset, but documented toxicity is rare in ordinary consumers. Regulatory agencies keep tabs on manufacturing byproducts—heavy metals, residues from fatty acid sources, and cross-contaminants—so new findings on chronic exposure or impurities sometimes prompt rapid rule changes and recalls. Anyone running a plant or designing a health product ignores these risk signals at their peril.
Future Prospects
Trends shaping magnesium stearate’s future come from several directions: demand for plant-only sources, stricter purity requirements, and automation in production lines. Consumers want more transparency about origins, so batch-level traceability, vegan tags, and allergen labels are turning from extras into baseline demands. Research in nanotechnology and solid dispersion systems keeps pushing the excipient role into new territory, like personalized drug delivery and advanced nutrition. Rising environmental concerns also nudge manufacturers to refine production, cut waste, and check sustainability claims for every feedstock, down to the palm oil plantation or animal supplier level. Based on the changes I’ve watched in this sector, only those willing to adapt to tighter standards and smarter sourcing will stay in the game long-term. As long as companies and regulators keep working side by side, magnesium stearate looks ready to keep its crucial spot on the ingredient list for years to come.
What is magnesium stearate used for in supplements?
The Role of Magnesium Stearate
Magnesium stearate fills a simple need in supplement manufacturing—it helps tablets and capsules turn out right every time. I used to think its name sounded much scarier than what it is: a fine, white powder that companies blend with active ingredients. The purpose is straightforward—magnesium stearate keeps powders from clumping together and sticking to the machines that press pills.
In the world of supplement production, even a little friction can cause trouble. Machines might jam. Dosages could end up inconsistent. By mixing a pinch of magnesium stearate into the batch, the powders slide and flow easily so every tablet comes out with the intended dose. It’s a common ingredient for good reason; it just works and helps companies keep their product consistent and affordable.
Is It Safe?
Walking down pharmacy aisles, shoppers often stop to read the labels. Magnesium stearate catches eyes because its name doesn’t sound “natural.” I’ve had friends ask if it’s a filler or something harmful. The truth is that magnesium stearate breaks down into two things your body already recognizes—magnesium and stearic acid. Both show up in foods like seeds, chocolate, and leafy greens.
The FDA and European Food Safety Authority reviewed magnesium stearate long ago and gave it a green light for use at the levels found in supplements. Experts set exposure limits that sit far above typical consumption from pills. Studies involving rats and large doses didn’t find problems. Its safety record stays strong over decades, and I haven’t seen credible research linking it to risk in healthy folks.
What About Absorption?
A popular concern crops up online: does magnesium stearate stop your body from absorbing nutrients? Some websites trade on this idea, but the science doesn’t hold up. Researchers checked how well nutrients such as vitamin B6, vitamin C, or calcium get absorbed in the presence of magnesium stearate. They found no meaningful difference. Your body seems to handle supplements with or without it about the same.
If you ever find a poorly made tablet that doesn’t dissolve, the problem comes from too much compression or a bad recipe—not magnesium stearate doing something mysterious. Most supplement makers focus on dissolvability and test their products, since a tablet that doesn’t break down wastes your money and their reputation.
Finding Other Options
The supplement industry changes fast. Some brands now advertise “magnesium stearate free” labels. That’s a marketing choice, not a proven health advantage. If you feel better avoiding it, plenty of options exist. Alternatives like rice flour or silica sometimes take magnesium stearate’s place, but they play the same role—ensuring smooth production and consistent products.
The bigger picture focuses on supplement quality. Look for companies that explain their sources and show results from independent testing. That counts for more than whether magnesium stearate or something else helps the machine keep running. If anything bothers you about an ingredient, skip to another brand. Choose what feels right, backed by solid information rather than fear.
Is magnesium stearate safe to consume?
The Basics Behind Magnesium Stearate
Walk into any pharmacy and pull a bottle of vitamins from the shelf. Flip to the ingredients, and you’ll likely see magnesium stearate on the label. It’s used as a flow agent, so powder doesn’t clump or jam in machines. Most folks never give it another thought, but some headlines spark concern about what’s really in supplements and whether magnesium stearate belongs in daily routines.
What Science Shows
Magnesium stearate is made from stearic acid (a fatty acid found in foods like beef, cocoa, and coconut oil) paired with magnesium. Both components appear naturally in meals. According to the U.S. Food and Drug Administration (FDA), magnesium stearate gets a seat at the Generally Recognized as Safe (GRAS) table. The European Food Safety Authority (EFSA) and World Health Organization (WHO) agree, noting typical supplement doses fall well below any danger zone.
My own exposure to these ingredients started years ago, working in a health store. Questions about “chemical-sounding” names popped up daily. Most concerns weren’t founded on fact, just fear of things that sound unfamiliar. I learned the value of seeking real studies. At the time, and still today, toxicology research does not flag magnesium stearate as problematic in reasonable amounts. Animal studies where rats scarfed up huge doses showed no toxic effects, and people regularly eat much smaller amounts.
Do Any Concerns Stack Up?
Critics sometimes worry about magnesium stearate as a “synthetic” additive. That term scares folks off, but here it doesn’t tell the story. The body processes magnesium stearate the same way it digests fats from food, breaking it down to stearic acid and magnesium ions. There’s no evidence it builds up or harms organs.
Some chatter on the internet blames magnesium stearate for blocking nutrient absorption. Yet studies don’t confirm this. When researchers looked at real cases, pills with magnesium stearate delivered nutrients just as well as those without. Concerns often stem from isolated lab experiments that don’t match real-life conditions. My verdict after reading through professional medical sources and handling countless supplement questions: for the average, healthy adult, this additive does not block vitamins or minerals on any meaningful scale.
Quality Counts
One issue worth noting is the source and quality of magnesium stearate. Poor manufacturing introduces risk, especially if a company cuts corners. For example, the stearic acid in magnesium stearate can come from plant or animal fats—vegan consumers might want to look for plant-sourced versions. In rare cases, contamination with harmful substances can sneak in if production is not tightly controlled. Trusted supplement brands usually offer clarity and third-party testing. I always urge people to buy from companies that post lab test results or carry seals from quality-assurance groups.
Practical Guidance
Folks with allergies or dietary restrictions should scan their multivitamin labels and ask the manufacturer about sources. Otherwise, evidence shows magnesium stearate is safe for most people. The best thing is to pay attention to dosage instructions and trust brands focused on transparency. Supplements—or any packaged items—work best as part of a balanced lifestyle, not as magic bullets. Panic rarely has much to offer, but solid information does.
Does magnesium stearate cause any side effects?
Spotlight on a Common Ingredient
Magnesium stearate comes up a lot in nutrition and pharmacy circles. Walk into any pharmacy and most likely any vitamin, painkiller, or supplement bottle you pick up contains this fine, white powder. Manufacturers often use it as a flow agent. Its job is to keep individual ingredients from sticking together so pills come out of the machine smooth, consistent and easy to swallow.
Safety Worries from Consumers
Most of the time, people just accept what's in their medicine cabinet. Yet magnesium stearate keeps popping up in questions about supplement safety. Some folks link it to digestion problems, while others voice bigger concerns about long-term health. My own inbox fills with questions from friends who’ve seen chatter about “toxic fillers.” It wouldn’t be fair to dismiss these concerns with a wave of the hand.
The bulk of scientific research, though, doesn’t support claims that magnesium stearate harms health at the doses found in supplements and medicines. The U.S. Food and Drug Administration gives it a “Generally Recognized as Safe” status. That designation isn’t handed out casually. Studies in animals have used much higher amounts than anyone would get through normal supplement use, often without adverse effects.
Digestive Complaints and Rare Reactions
Some people do notice stomach upset or diarrhea after taking pills that use this additive. These issues can come from several sources, not just magnesium stearate. The chalky texture, aftertaste, or hard-to-swallow size of big tablets play a role, too. Food allergies rarely relate to it — magnesium stearate comes from either vegetable or animal fat, so the real allergy risk mostly depends on the original source material (for example, lactose in those with dairy sensitivities).
For those with fat malabsorption issues, like people who live with certain pancreatic conditions, the stearic acid backbone in magnesium stearate might pose a challenge. Still, for most people with healthy digestion, the body processes and excretes the tiny amounts cleanly.
Misinformation Spreads Faster Than Facts
The internet elevates worries fast. Some websites claim magnesium stearate blocks nutrient absorption, or suppresses T cell function in the immune system. Researchers have dug into these claims. Peer-reviewed papers show nutrients in pills still get into the body as they should. Any immune studies involve mixing isolated cells in labs with huge levels of the compound — far from what a daily multivitamin delivers.
The worry over “hydrogenated oils” sometimes tied to magnesium stearate leads to confusion with food industry fats. While some forms once used hydrogenation, most supplement makers now use palm oil or other safe sources for production, after consumer outcry over trans fats.
Making Smarter Choices as a Consumer
Folks who want to play it safe have options. Many companies offer “additive-free” vitamins or label the origin of magnesium stearate clearly. Some even provide third-party testing results, which shows respect for customer worries. Instead of quitting supplements outright over a single ingredient, reaching out to the brand for sourcing details gives peace of mind. Health always benefits when consumers ask questions and get straight answers.
Confusion around food and medicine ingredients will always exist. Magnesium stearate, used in small amounts, doesn’t deserve its bad-guy image for most healthy people. Anyone who deals with allergies, absorption problems, or chronic illness can always speak to a pharmacist or doctor about what to avoid — and which pills have the cleanest ingredient lists.
Is magnesium stearate derived from animal or plant sources?
Unpacking the Origins of Magnesium Stearate
Magnesium stearate pops up in many supplements and medications as a common additive. It helps keep powders from sticking together and makes those tablets easy to swallow. Some folks get concerned about where it actually comes from: animal or plant? The answer can get a little tangled, which might surprise anyone checking the ingredient list, especially if you follow a vegetarian, vegan, or religious diet.
How it's Made: The Source Dilemma
Manufacturers make magnesium stearate by combining magnesium with stearic acid. Stearic acid happens naturally in both animal fats and vegetable oils. Think lard, tallow, palm oil, or cocoa butter. If you’re eating mostly plants or you avoid animal products for cultural or health reasons, that may matter a lot in your shopping choices.
Over the years, I’ve noticed most big supplement makers use vegetable sources as their base, especially those targeting the vegetarian or vegan crowd. They often list “vegetable magnesium stearate” or “derived from palm oil” on labels. Still, some companies don’t specify the source at all. That leaves you guessing or reaching out to the manufacturer for a clear answer, which can feel frustrating if you’re trying to follow personal or religious dietary rules.
Industry Practices and Consumer Confidence
People have a right to know what’s going into their bodies. I’ve seen confusion turn into mistrust because companies don’t always label things clearly. It’s common for people to give up on a supplement just because they don’t want to risk an animal-based ingredient sliding into their daily routine. This isn’t just about diet—it’s about culture, faith, allergies, and ethical choices.
Labeling practices and transparency vary, depending on local laws and market demand. In the United States, the FDA doesn’t require companies to mention where magnesium stearate comes from. In the EU, labeling rules push companies to be a little more descriptive but can still leave room for interpretation.
Real-World Health Implications
Some online debates question the safety of magnesium stearate itself, worrying about potential health risks. Research from trusted sources like the National Institutes of Health and Food and Drug Administration shows typical amounts found in food and supplements are considered safe. The bigger concern for most people isn’t about the ingredient being unsafe, but about it not matching their values or dietary restrictions.
Solutions: Clarity and Choice
Transparency solves a lot of these problems. Companies gain trust by clearly stating where they get their ingredients. Simple labels, customer-friendly websites, and responsive customer service teams make that difference. I’ve contacted supplement brands before, and the most straightforward answers always made me feel better about my choices. Some companies even get certifications from groups like the Vegan Society to show their product’s origin, which removes the guesswork for the customer.
People care about these details—they want to align what they consume with their personal beliefs and dietary needs. If you find yourself in doubt, reach out to the manufacturer or choose brands that proudly share their sourcing stories.
Can magnesium stearate affect the absorption of nutrients or medications?
The Role of Magnesium Stearate in Tablets
Walk into any pharmacy or health store, and most bottles on the shelf list magnesium stearate among their ingredients. Manufacturers favor this compound because its slippery qualities help powder flow through machines during production. It prevents pills from sticking and makes them easier to swallow. It pops up in everything from vitamins to prescription medications, so naturally, concerns about its effect on absorption come up often.
Concerns and Claims From the Supplement Community
Some health blogs, practitioners, and supplement companies say magnesium stearate might coat a pill so thoroughly that the body cannot access the nutrients inside. They argue it may slow or block absorption, especially in people with sensitive digestive systems. These claims stir anxiety, especially in people who already struggle with nutrient deficiencies or must take critical medications.
Fact-Checking the Fears: What Research Says
Scientific studies examining magnesium stearate suggest a different picture. Most of the research finds no solid evidence that normal amounts interfere with how the digestive tract absorbs nutrients or medicine. An often-cited paper in Pharmaceutical Technology reported that tablets containing magnesium stearate typically dissolve just as well as those without it. For most people, the digestive juices and movement in the gut break down the coating quickly enough. Medications still do their job, and supplements release their contents so the body can use them.
Still, the scientific world keeps studying every angle, especially for populations with digestive challenges. People with short bowel syndrome or very reduced stomach acid might see slower breakdown, but so far, proven harm remains elusive. The U.S. Food and Drug Administration considers magnesium stearate safe in the doses found in food and medicine.
Personal Concerns: Experience Matters
Over the years working in health reporting, I’ve spoken to many supplement users who believe they “feel better” with pill brands advertising no magnesium stearate. Their anecdotes vary—faster symptom relief, fewer digestive complaints. Sometimes, this reflects differences in the active ingredients or simple placebo effect. At the same time, people with allergies or compromised digestion notice problems with any additive, not just magnesium stearate. The power of individual experience shouldn’t be denied, but the evidence on this particular ingredient doesn’t point to widespread risk.
Why the Debate Persists
This story continues in part because of mistrust toward “fillers” and modern manufacturing. Many folks want to avoid anything that’s not strictly necessary in their pills. Misinformation spreads fast, especially when health choices feel overwhelming and personal. Social media and supplements sold as “pure” or “additive-free” amplify the message that all extra ingredients are bad. Some companies stand out by touting “magnesium stearate-free,” tapping into these worries even as evidence suggests little reason for alarm.
Smart Solutions and Approaches
People trying to make the best choices can get lost in the noise. Label reading helps, but context matters more. If you’ve noticed issues every time you take a certain brand or type of supplement, switching brands or choosing powder form can help clarify if magnesium stearate is the cause. Healthcare providers and pharmacists can help sort out real interactions with medicines or medical conditions. Instead of avoiding an ingredient just because it’s unfamiliar, ask about what matters most—the active ingredient, the dose, and your personal health picture.
| Names | |
| Preferred IUPAC name | magnesium octadecanoate |
| Other names |
Octadecanoic acid, magnesium salt Stearic acid magnesium salt Magnesium dioctadecanoate |
| Pronunciation | /maɡˈniːziəm ˈstɪəreɪt/ |
| Preferred IUPAC name | Magnesium octadecanoate |
| Other names |
Octadecanoic acid, magnesium salt Stearic acid magnesium salt Magnesium distearate E572 |
| Pronunciation | /maɡˈniːziəm ˈstɪəreɪt/ |
| Identifiers | |
| CAS Number | 557-04-0 |
| Beilstein Reference | 3940864 |
| ChEBI | CHEBI:6849 |
| ChEMBL | CHEMBL1200872 |
| ChemSpider | 10147 |
| DrugBank | DB01377 |
| ECHA InfoCard | 01aa0eaf-db0b-42e6-9ab6-fbc94af7e9bb |
| EC Number | 205-769-8 |
| Gmelin Reference | 17471 |
| KEGG | C01769 |
| MeSH | D008271 |
| PubChem CID | 11176 |
| RTECS number | OMU1880000 |
| UNII | 7OXO66RJP2 |
| UN number | UN1418 |
| CompTox Dashboard (EPA) | DTXSID2020853 |
| CAS Number | 557-04-0 |
| Beilstein Reference | 3857846 |
| ChEBI | CHEBI:31824 |
| ChEMBL | CHEMBL1201208 |
| ChemSpider | 73074 |
| DrugBank | DB01378 |
| ECHA InfoCard | 01b822ec-358a-4c2b-92f4-c3fd7c3c6d23 |
| EC Number | EC 209-150-3 |
| Gmelin Reference | 17673 |
| KEGG | C01941 |
| MeSH | D008268 |
| PubChem CID | 11176 |
| RTECS number | OM5950000 |
| UNII | 70097M6I30 |
| UN number | UN- Magnesium Stearate: "UN1418 |
| CompTox Dashboard (EPA) | DBLT000052 |
| Properties | |
| Chemical formula | C36H70MgO4 |
| Molar mass | 591.27 g/mol |
| Appearance | White, fine, powder |
| Odor | Odorless |
| Density | 1.09 g/cm3 |
| Solubility in water | Insoluble |
| log P | 5.8 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa > 20 |
| Basicity (pKb) | pKb ≈ 5.2 |
| Magnetic susceptibility (χ) | -20.7×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.38 |
| Dipole moment | 0 D |
| Chemical formula | C36H70MgO4 |
| Molar mass | 591.24 g/mol |
| Appearance | White, fine, powder |
| Odor | odorless |
| Density | 1.09 g/cm3 |
| Solubility in water | Insoluble |
| log P | logP = 13.1 |
| Vapor pressure | Negligible |
| Basicity (pKb) | 7.6 |
| Magnetic susceptibility (χ) | -8.9×10⁻⁶ |
| Refractive index (nD) | 1.43 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 496 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -1474.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -37.4 kJ/g |
| Std molar entropy (S⦵298) | 472.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1310.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -16180 kJ/mol |
| Pharmacology | |
| ATC code | A07AA02 |
| ATC code | A07AA02 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Dust may cause mechanical eye irritation. May cause skin and respiratory tract irritation. |
| GHS labelling | GHS labelling for Magnesium Stearate: "Not classified as hazardous according to GHS. |
| Pictograms | GHS07, GHS08 |
| Signal word | No signal word |
| Hazard statements | No known significant effects or critical hazards. |
| Precautionary statements | P264, P270, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Autoignition temperature | 400°C |
| Lethal dose or concentration | LD50 (Oral, Rat): > 10,000 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 10 g/kg (oral, rat) |
| NIOSH | WN4725000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Magnesium Stearate: "15 mg/m³ (total dust), 5 mg/m³ (respirable fraction) as 8-hour TWA (OSHA) |
| REL (Recommended) | 2 mg/m³ |
| IDLH (Immediate danger) | No IDLH established |
| GHS labelling | GHS: Not classified as hazardous according to GHS |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Autoignition temperature | 410 °C (770 °F) |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 (oral, rat): > 10,000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): >10,000 mg/kg |
| NIOSH | WN0325000 |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 5 mg/kg bw |
| Related compounds | |
| Related compounds |
Calcium stearate Zinc stearate Stearic acid Magnesium palmitate |
| Related compounds |
Calcium stearate Zinc stearate Magnesium palmitate Magnesium laurate Sodium stearate |

