Magnesium Malate Trihydrate: A Deep Dive From Origin to Future Promise
Historical Development
Magnesium compounds have seen a transformation in use since mineralogical discoveries in the 18th and 19th centuries. Chemists began isolating magnesium from minerals like magnesite and dolomite. As society learned more about mineral deficiencies, magnesium turned into a major interest for nutritional science. Magnesium malate became notable much later, especially as researchers sought ways to address fatigue and chronic muscular pain. Personal recollection of the supplement aisle as a pharmacy student a decade ago feels rather dated today, with choices now flooded by chelated forms stemming from decades of research. Magnesium malate trihydrate caught attention not through a single breakthrough, but by steady, incremental curiosity among researchers searching for compounds that pair mineral utility with improved digestive ease.
Product Overview
Magnesium malate trihydrate lands on the supplement shelf as a compound that combines magnesium with malic acid—a substance pivotal in the Krebs cycle responsible for cellular energy production. Companies sell it as white to off-white crystalline powder aimed at gut-friendly delivery. Having handled this powder personally, you notice its fine, absorbent texture, which impacts how you process it into tablets or mixtures. The trihydrate version is standard, ensuring stable delivery and shelf life through bound water molecules in the structure.
Physical & Chemical Properties
Examination of pure magnesium malate trihydrate reveals a molecular formula of C4H4MgO5·3H2O and a molar mass sitting above 210 g/mol. The powder is faintly acidic when tasted, thanks to malic acid’s carboxyl groups. Its three water molecules make it slightly heavier than the anhydrous form, and this plays into how it dissolves quickly in warm water, making it more practical than some magnesium oxide forms, which barely budge in solution. You really see the difference in lab work: trihydrate’s water content ensures improved processing, especially in humid environments.
Technical Specifications & Labeling
Magnesium malate trihydrate appears in food, drug, and chemical catalogues as a supplement-grade compound. Specifications typically require a minimum 99% purity by assay, verified by HPLC or titration, with limits for heavy metals and microbial content. Handling it, you learn to watch for particle size to ensure blendability in tablets, something strict GMP standards flag. Labeling rules call for declaration of elemental magnesium content, not just the salt weight, to avoid confusion, since one form’s 1,000 milligrams could have a completely different magnesium payload compared to another. This caught the eye of regulators after cases of consumer confusion about how much elemental magnesium they were actually ingesting.
Preparation Method
Making magnesium malate trihydrate in a manufacturing plant starts by reacting malic acid—often sourced from fermentation— with a suitable magnesium base like magnesium hydroxide or magnesium carbonate. The reaction is exothermic, and it can be tricky to manage the hydration state. You have to control temperature and pH closely to ensure trihydrate formation rather than ending up with a monohydrate or anhydrous product, which changes the compound’s performance. Filtration, washing, and gentle drying step in next, where operators must avoid overdrying, which strips the essential hydration.
Chemical Reactions & Modifications
On a chemistry bench, magnesium malate trihydrate doesn’t react as violently as metallic magnesium, thankfully. It remains stable at room temperature and with most organic solvents, though acid or high heat will break it down, splitting off the malic acid and freeing magnesium ions. Some manufacturers explore modifications, such as co-crystallizing with other amino acids or tweaking hydration states for targeted solubility, although pure trihydrate usually gets top billing for dietary supplements due to its mix of stability and usability.
Synonyms & Product Names
Across textbooks, purchase orders, and trade journals, you’ll spot names like magnesium 2-hydroxybutanedioate trihydrate, magnesium malic acid salt, or simply magnesium malate. In the supplement trade, big brand labels tend to stick with the easier “magnesium malate,” sometimes specifying “trihydrate” to set themselves apart from blends or fillers. More technical sources might list CAS number 778571-57-2, but you barely see that outside raw ingredient purchase agreements.
Safety & Operational Standards
Handling large batches of magnesium malate trihydrate at a processing facility calls for robust operational protocols. Personal memories of my time shadowing QA staff in a supplement factory remind me that, even for innocuous-looking white powders, you need dust control, batch tracking, and allergen separation on the floor. Magnesium malate earns a good safety record, especially compared to oxides or less-absorbable salts that pose gastric distress. Companies follow food-grade and pharma-grade standards from agencies such as USP and EFSA, along with mandatory allergen labeling if cross-contaminants (soy, gluten) exist in the facility. Workers monitor airborne dust using simple particle meters, not because of toxicity, but to avoid respiratory irritation.
Application Area
Supplements for stress, muscle health, metabolic support, and sleep occupy much of the magnesium malate trihydrate market. Some food chemists add it to sports beverages and fortified bars. I’ve tested formulations for muscle cramps and sore fatigue, where this salt seemed to reduce stomach upset compared to magnesium citrate or oxide. The addition of malic acid offers a double benefit—the malate portion may help with energy metabolism, providing a rationale for use in products aimed at athletes and chronic fatigue communities. Cosmetics also make use of it, leveraging magnesium’s soothing effects in creams, although this remains a drop compared to nutritional and pharmaceutical application.
Research & Development
Active research over the last decade points toward evaluating bioavailability—the proportion of magnesium that genuinely ends up in the bloodstream after taking a dose. One trial at a midwestern university found that magnesium malate delivered significantly more absorbable magnesium than comparable oxide or sulfate salts, particularly when ingested after exercise. Researchers also dig into synergy, exploring how pairing magnesium with malic acid changes pain signaling and muscle function. As funding grows, some studies expand beyond supplementation, looking at magnesium’s role in preventing neurodegenerative conditions or improving metabolic resilience in older adults, suggesting future protocols for chronic disease prevention may want to lean on highly bioavailable forms like magnesium malate trihydrate.
Toxicity Research
Toxicology studies generally point to a high safety margin for magnesium malate trihydrate in standard dosages. Acute toxicity is rare and nearly always linked to massive overdose, producing diarrhea or, at times, electrolyte imbalances if kidney function is impaired. Labs routinely test for chronic toxicity and interactions; nothing of major concern shows up at recommended doses. Regulatory agencies set upper limits more around magnesium content than the salt form, so consumers need to monitor their total mineral intake from all sources. Past incidents relating to contaminated raw magnesium ingredients have driven companies to increase screening for trace metals and verify supplier claims—a direct takeaway from cases documented a decade ago with tainted batches that slipped through minimal checks.
Future Prospects
Magnesium malate trihydrate stands poised for growth as both clinicians and consumers grow more savvy about mineral absorption and supplement quality. Increasing rates of dietary magnesium deficiency drive interest, especially as new clinical guidelines push for proven, gentle compounds to help aging populations maintain muscle mass and cardiac health. Developments loom in custom-blended supplements and tailored delivery forms, such as soluble drink mixes or slow-release tablets. Advances in organic malate fermentation open up greener, more efficient sourcing options for raw materials. If researchers further clarify benefits for neurological health or energy metabolism, regulations may shift to recognize bioavailable magnesium forms as distinct from their less effective cousins. Those of us working in supplement science see the mounting demand for transparency—clear labeling, traceability, and robust third-party testing. The story of magnesium malate trihydrate will likely ride on the combined push from consumers, the evidence base, and growing regulatory scrutiny, pushing it deeper into routine health solutions over the coming decade.
What are the benefits of Magnesium Malate Trihydrate?
Understanding Why Magnesium Matters
Magnesium plays a huge role in everyday health. I first noticed just how many people walk around low in this mineral after years of talking with friends, family, and even my own doctor. Missing enough magnesium can make muscles ache, drain energy, and even mess with sleep. Out of all the different forms out there, magnesium malate trihydrate comes up strong for people seeking more than the average magnesium pill.
Why Malate? More Than Just a Name
Malic acid binds with magnesium in this form. I grew up eating apples, which are full of natural malic acid. It turns out malic acid isn’t just a flavor; it works with the body’s natural processes that produce energy. For example, one review in the journal Nutrients in 2017 points out that malic acid feeds right into the body’s Krebs cycle, the energy factory in every cell. That means magnesium malate doesn’t just bump up mineral levels—it may also help boost stamina and push back against fatigue.
Common Reasons People Reach for Magnesium Malate Trihydrate
Leg cramps in the night, headaches that go on too long, and the heavy fatigue of dragging through a workday can all trace back to not getting enough magnesium. Muscle health especially seems to benefit from magnesium. One interesting study published in Magnesium Research showed that athletes who took magnesium recovered from soreness a little quicker. Malate seems to make a difference, too, for people dealing with constant tiredness, like those managing fibromyalgia or chronic pain. Researchers in Spain found that women with fibromyalgia felt some relief after getting both magnesium and malic acid together.
Digestive Comfort Counts
Taking straight magnesium oxide or sulfate led me straight to the bathroom most days. Magnesium malate trihydrate tends to be easier on the stomach. Plenty of users—myself included—notice less of that typical laxative effect. That’s good news for people who can’t risk being far from a restroom. Absorption is another point—malate forms usually get into the bloodstream faster compared to cheaper salts. Hard numbers show that magnesium malate raises blood magnesium a little more efficiently, and with fewer side effects, compared to magnesium oxide or chloride.
Brain and Mood Boost
Focus becomes tricky when magnesium levels drop. Some psychiatrists suggest upping magnesium for mild mood swings and anxious moments. Research from the University of Vermont tracked improvements in mood for several folks after they started daily magnesium. While magnesium malate hasn't topped every study, a blend of patient stories and smaller clinical trials hints that magnesium’s presence in the brain supports normal nerve communication. Feeling less foggy or reactive is hard to measure, but I know from my own tough moments that extra magnesium sometimes made the days a shade brighter.
Who Should Consider It?
People on heart medications or diuretics, those who sweat a lot, or older adults often face an uphill battle keeping magnesium up. People with chronic fatigue or muscle tension tend to notice the difference most with magnesium malate trihydrate because of its energy and muscle benefits. Of course, anyone with kidney problems or specific health concerns should talk through supplements with a trusted doctor or pharmacist before jumping in.
Practical Solutions for Daily Life
Focusing on real foods—nuts, leafy greens, beans, and yes, apples—lays the groundwork for magnesium. For people needing extra help, magnesium malate trihydrate brings a gentler, more absorbable option. Reputable brands with third-party testing give peace of mind and ensure you get what you pay for. Tuning into personal symptoms and making these changes slowly let you see if magnesium malate trihydrate fits with your health goals.
How should Magnesium Malate Trihydrate be taken or dosed?
Why Magnesium Matters and How Malate Makes a Difference
Years of working with health supplements taught me that not every form of magnesium works the same for everyone. Magnesium malate trihydrate usually attracts attention because it blends magnesium with malic acid, a compound found in apples. People looking for support with muscle tension, fatigue, or headaches often gravitate toward this form. Even so, questions about dosing and taking it properly never seem to go away.
Dosing Guidance: Listening To Your Body
Doctors and nutritionists often suggest magnesium doses between 200 and 400 milligrams each day for adults. Magnesium malate trihydrate provides elemental magnesium along with malic acid, so it’s important to look at how much real magnesium you get per pill. Supplement labels can be confusing. A caplet might say "1000 mg magnesium malate" but only deliver about “100 mg elemental magnesium.” Look carefully at the label, or you might accidentally take less (or much more) than you intend. If you aren’t sure, a health professional can help you sort through the numbers.
Some people, including myself, notice mild stomach upset if they jump right in with a full dose. I found that starting low, maybe half a tablet, and working up helps avoid digestive grumbles. Spreading your intake through the day also smooths things out. For instance, taking one tablet with breakfast and another in the evening feels easier on the stomach than swallowing two in one go. Consistency counts more than cramming your week’s worth in one dose.
Tips to Maximize Benefits and Safety
Personal experience taught me supplements work best alongside food, not instead of it. I always grab magnesium malate with a meal or snack. Not only does this increase absorption, but it also reduces the chance of cramps or loose stools. Anyone who’s chased supplements with just black coffee has probably run into bathroom trouble later.
Mixing magnesium malate with other minerals can get tricky. Calcium and magnesium compete for absorption. If your routine involves both, space them apart by a few hours. Some medications, especially diuretics or heart drugs, interact with extra magnesium. Checking with your doctor or pharmacist before adding supplements saves a load of hassle down the line.
Observing Results And Knowing Limits
Tracking energy, sleep, and muscle comfort helps gauge whether magnesium malate trihydrate makes a difference. I kept a notebook during a busy work stretch and noticed fewer leg cramps after a steady two-week stint. But more isn’t better; consistently high doses risk diarrhea, nausea, or worse. The kidneys have to process the excess, and folks with kidney issues can face real problems with over-supplementation. Blood tests every so often help keep things safe, especially for anyone managing chronic health conditions.
Easy Solutions For Common Magnesium Issues
Finding a supplement that lists both the total weight and elemental magnesium makes shopping less confusing. If plain tablets upset your stomach, switching to a powder mixed in water can feel gentler. A diet rich in leafy greens, nuts, beans, and seeds also cuts down on supplement needs. For some, that means only occasional magnesium malate support during long periods of stress or heavy workouts.
Listening to your body and choosing evidence-backed doses build a foundation for real health gains. Rushing things or chasing quick fixes usually backfires. With magnesium malate trihydrate, slow, steady, and informed beats bold guesses every time.
Are there any side effects associated with Magnesium Malate Trihydrate?
Magnesium Supplements: Not All Experiences Match the Label
After years of talking to folks who swear by their daily magnesium—especially in muscle pain and fatigue—it's clear that magnesium malate trihydrate isn’t just another supplement. I’ve used it myself during stressful stretches, hoping for those legendary energy boosts or relief from sore muscles. It’s easy to see why people want to add more magnesium to their diet, as current research points out that almost half of adults might not get enough of this mineral each day. Still, “natural” doesn’t always mean worry-free.
Common Reactions: Not Always Smooth Sailing
People often ask if magnesium malate trihydrate causes stomach problems. The answer is yes, sometimes it can. I’ve personally felt the classic “gastrointestinal discomfort” — think bloating, cramps, or an urgent trip to the restroom — after a higher dose. These upsets happen because extra magnesium brings water into the colon, which gets systems moving a bit too quickly. Studies in the Journal of the American College of Nutrition back this, showing that magnesium, especially in doses over 350 mg, leads to loose stools for some. Lower doses and splitting the daily amount can ease these troubles.
Rare But Serious Risks: It Can Get Complicated
Though most people get only mild symptoms, those with kidney problems might deal with bigger risks. Healthy kidneys handle extra magnesium by flushing out what’s not needed. For someone whose kidneys aren’t working right—maybe from chronic disease or medication—magnesium can build up in the blood. This creates a real health concern: weakness, irregular heartbeat, or dangerously low blood pressure. Medical case reports describe such problems, including confusion and slowed breathing, mainly in older adults or those ignoring warning signs.
Potential Drug Interactions: It's Not Just About the Supplement
Some common prescriptions clash with magnesium supplements. Antibiotics like tetracycline or ciprofloxacin struggle to be absorbed if taken too soon after swallowing a magnesium pill. Ditto for certain osteoporosis drugs. Blood pressure medications, especially diuretics, can also mess with how the body handles magnesium—sometimes raising and sometimes dropping levels to unsafe extremes. Pharmacists and doctors know this pattern well, usually suggesting that patients space out the timing or watch for added symptoms.
Why Source and Dosage Matter
Quality magnesium malate trihydrate comes from reliable manufacturers who stand by their purity standards. Cheap sources might hide contaminants, which only adds risk. I’ve made it a rule to choose products tested by independent companies, such as NSF International or USP. As for dose, less often works better. Experts from the National Institutes of Health recommend adults stick to 310-420 mg a day from all sources unless a health care provider suggests otherwise.
Smart Steps for Everyday Use
Magnesium malate trihydrate can help fill the gaps. Anyone thinking about adding it to their routine should start low, read their body’s signals, and look at any other medicines or medical issues in play. A quick chat with a doctor or pharmacist before starting goes a long way. Supplements can support better health, but the safest path includes questions and a bit of patience.
Is Magnesium Malate Trihydrate safe for long-term use?
Understanding Magnesium Malate Trihydrate
Magnesium has worked its way into daily conversations about health, from muscle cramps to restless nights. Magnesium malate trihydrate shows up on supplement bottles and in online forums, usually recommended for its supposed benefits related to energy and muscle comfort. People trust it because magnesium is a mineral the body uses every day. Malic acid, the other component, appears naturally in fruits—especially apples. Blending these together gives many people hope for better absorption.
Why Do People Use It?
As someone who lives with a demanding work schedule and plenty of late-night screen time, fatigue isn’t foreign. Magnesium malate keeps popping up as a solution—not just for tiredness, but for things like leg cramps, headaches, and even fibromyalgia. Some folks with these issues report real improvements. Research points out that magnesium helps manage more than three hundred biochemical reactions in the body. It plays a key role in nerve function, managing heartbeats, and moving blood sugar into cells. Malic acid, on the other hand, supports the body's energy cycle.
Long-Term Safety: What Doctors and Studies Say
Thinking about taking it over months or years matters. Most well-run studies find magnesium supplements safe at recommended levels for adults. The National Institutes of Health sets the daily cap at 350 milligrams from supplements, though getting more from food brings less risk.
Stomach upset ranks at the top of concerns—think loose stools or tummy cramps if someone overdoes it. People with kidney issues run into bigger trouble. Healthy kidneys filter extra magnesium without trouble, but damaged kidneys may not. This could raise blood levels too much and throw off heart rhythms or impact breathing.
One review from the American Journal of Therapeutics studied magnesium’s safety in people with aches and chronic tiredness. Over three months, participants taking magnesium malate reported few side effects, usually just mild digestive complaints. No evidence of toxic buildup appeared when sticking to regular amounts.
Supplements, in rare cases, hide impurities or list more magnesium than the bottle claims. Independent testing, such as reports from ConsumerLab, highlights how choosing supplements from reputable brands lessens those risks.
Practical Advice and Solutions
Trustworthy supplements start with trusted manufacturers. That means picking brands with third-party quality verification, like NSF or USP seals. It makes sense to talk to a doctor before sticking with supplements every day—especially for anyone who takes other medications or has kidney problems.
Eating more magnesium-rich foods also helps—spinach, pumpkin seeds, black beans, and almonds fill that gap in most diets. Supplements shouldn’t replace a nutrient-rich diet, only fill gaps when food can’t quite reach the goal.
For anyone new to magnesium malate trihydrate, starting with the lowest dose and watching how the body reacts avoids trouble down the line. Checking in with medical tests every so often, especially for people on magnesium for months, keeps things safe.
Supplements play a supporting role, not a starring one. Treat magnesium malate trihydrate as a tool, best used with guidance and common sense. Looking after overall health—balanced food, sleep, and stress management—will always matter more than what comes in a bottle.
Can Magnesium Malate Trihydrate interact with other medications or supplements?
Why Magnesium Supplements Enter the Picture
Anyone managing muscle cramps, chronic fatigue, or headaches often looks for magnesium for relief. Magnesium malate trihydrate claims its place in health stores thanks to a track record for absorption and the bonus of malic acid, a substance the body uses in energy production. Taking supplements boosts health—or so many believe—until unexpected interactions catch up.
Scores of Drugs Compete with Magnesium
Over years working in community pharmacy, I’ve witnessed the classic scenario: a prescription counter stacks up statins, antibiotics, heart meds, and then comes the question about adding magnesium. Hundreds of people turn to supplements and skip a simple double-check with their health care provider, not realizing magnesium can block or reduce the impact of key drugs.
Antibiotics like ciprofloxacin and tetracycline can face roadblocks from magnesium. After taking these together, people report stomach trouble, or their infection lingers, but the real problem often hides in the gut: magnesium binds the antibiotic, making it harder to absorb. This isn’t a rare hiccup—numerous scientific reviews point out this interaction repeatedly.
Other Supplement and Medication Crossroads
Certain blood pressure medications—mainly diuretics such as furosemide or hydrochlorothiazide—may lead to more magnesium lost through urine. If someone piles on extra magnesium to compensate, levels can swing out of balance, and they notice more muscle weakness or even changes in heart rhythm. On the flip side, some meds for heart rhythm problems already raise magnesium. Doubling up without checking labs takes away the intended safety net and tosses in unnecessary risks.
For those living with acid reflux, prescription drugs like omeprazole work by reducing stomach acid. In rare cases, these drugs can lower magnesium too far, especially over long stretches. Yet adding magnesium supplements on top without lab monitoring calls for trouble: the fix for one side effect can spark another problem.
Why Real Guidance Beats Guesswork
Spending years behind the pharmacy counter taught me that supplement use happens out of sight of the medical team. Magnesium malate trihydrate often falls under the radar, since it doesn’t sound as potent as prescription drugs. Easy access does not mean risk-free. Drug guides and reputable databases such as Micromedex or clinical resources from the Mayo Clinic outline these issues, yet few people make use of them before mixing treatments.
Basing Choices on Evidence, Not Hype
Reports published in journals such as Drug Safety and resources like the NIH fact sheet underscore a long list of drugs with magnesium interactions. Health professionals update dosing guides as new reports come in. That kind of work only matters if patients and their families ask questions, keep lists up to date, and share what’s on their supplement shelf—no detail is too small.
Tackling the Supplement Puzzle
Sorting through labels in a pharmacy, I’ve watched as people pick up supplements one by one. They often choose based on a friend’s advice, online reviews, or their own effort to feel better. The end result, sometimes, is a muddle of pills that cancel each other out or slow down recovery. Pharmacists want to help, but transparency makes the difference.
Putting Safety First
No one needs to avoid magnesium malate trihydrate entirely. Smart choices come from understanding what other meds are on the table and being honest in every health care visit. Testing magnesium levels and reviewing supplement routines keeps problems off the radar—and health on track. Communication between doctors, pharmacists, and the person taking the supplements stands as the best way to sidestep unwanted surprises.
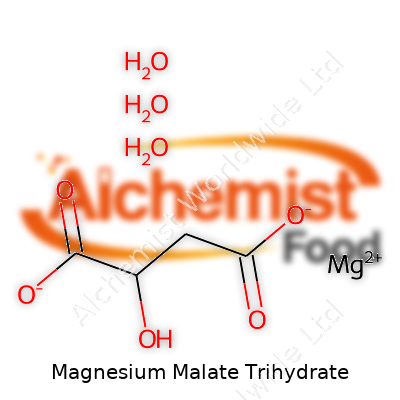
| Names | |
| Preferred IUPAC name | Magnesium 2-hydroxybutanedioate trihydrate |
| Other names |
Malic acid magnesium salt trihydrate Magnesium malate hydrate Magnesium 2-hydroxybutanedioate trihydrate |
| Pronunciation | /maɡˈniːziəm ˈmæleɪt traɪˈhaɪdreɪt/ |
| Preferred IUPAC name | magnesium 2-hydroxybutanedioate trihydrate |
| Other names |
Malic acid magnesium salt trihydrate Magnesium 2-hydroxybutanedioate trihydrate Magnesium malate hydrate Magnesium(2+) malate trihydrate DL-Malic acid magnesium salt trihydrate |
| Pronunciation | /maɡˈniːziəm ˈmæleɪt traɪˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 869-06-7 |
| Beilstein Reference | 68399 |
| ChEBI | CHEBI:131189 |
| ChEMBL | CHEMBL4308837 |
| ChemSpider | 555040 |
| DrugBank | DB14574 |
| ECHA InfoCard | 10d4687c-cf76-4ea0-a630-d7c1c1b3e76c |
| EC Number | 01-2119967298-17-0006 |
| Gmelin Reference | 119242-56-5 |
| KEGG | C14436 |
| MeSH | D017674 |
| PubChem CID | 162139166 |
| RTECS number | OM2626000 |
| UNII | X3M9O4GX53 |
| UN number | UN3077 |
| CAS Number | 18107-26-5 |
| Beilstein Reference | 0118733 |
| ChEBI | CHEBI:139747 |
| ChEMBL | CHEMBL4165475 |
| ChemSpider | 53390983 |
| DrugBank | DB14546 |
| ECHA InfoCard | 01e1e7d2-1bba-476e-b223-29c2e934b201 |
| EC Number | 209-942-9 |
| Gmelin Reference | 56320 |
| KEGG | C15130 |
| MeSH | Dihydroxymagnesium Malate |
| PubChem CID | 159201 |
| RTECS number | OM4494000 |
| UNII | 58H6Z64G2Q |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C4H8MgO7·3H2O |
| Molar mass | 277.49 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 2.0 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -2.6 |
| Acidity (pKa) | 3.40 |
| Basicity (pKb) | 8.1 |
| Magnetic susceptibility (χ) | -12.0e-6 cm³/mol |
| Refractive index (nD) | 1.580 |
| Dipole moment | 1.3 D |
| Chemical formula | C8H12MgO10 |
| Molar mass | 277.51 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 2.0 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.6 |
| Acidity (pKa) | 3.4 |
| Basicity (pKb) | 8.7 |
| Magnetic susceptibility (χ) | -1.1×10⁻⁴ |
| Refractive index (nD) | 1.505 |
| Dipole moment | 2.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 324.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1622.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2340 kJ/mol |
| Std molar entropy (S⦵298) | 344.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1787.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −2062.7 kJ/mol |
| Pharmacology | |
| ATC code | A12CC05 |
| ATC code | A12CC04 |
| Hazards | |
| Main hazards | May cause respiratory irritation, eye irritation, and skin irritation. |
| GHS labelling | GHS07, GHS hazard statements: H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "Wash hands thoroughly after handling. Do not eat, drink or smoke when using this product. IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth. |
| NFPA 704 (fire diamond) | 1-1-1-W |
| Lethal dose or concentration | LD50 Oral Rat: > 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 4,660 mg/kg (rat, oral) |
| NIOSH | MFCD00149688 |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 350 mg |
| IDLH (Immediate danger) | Not established |
| Main hazards | Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | No flash point |
| Lethal dose or concentration | LD50 (Oral, Rat): > 5000 mg/kg |
| LD50 (median dose) | > 8,200 mg/kg (Rat, oral) |
| NIOSH | Not listed |
| PEL (Permissible) | 50 mg/m³ |
| REL (Recommended) | 1200 mg |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Malic acid Magnesium oxide Magnesium carbonate Magnesium citrate Magnesium sulfate Magnesium chloride |
| Related compounds |
Magnesium Malate Malic Acid Magnesium Oxide Magnesium Citrate Magnesium Aspartate |

