Magnesium Gluconate: In-Depth Commentary
Historical Development
Magnesium has tugged at the attention of chemists for centuries. Story goes, folks recognized magnesium for its many benefits well before supplement aisles overflowed with bottles. Early pharmacists reached for magnesium salts—primarily magnesium sulfate—for both laxative and health-promoting effects. Later on, as understanding of human metabolism and nutrition stepped up, gluconic acid made a splash; it’s produced through the fermentation of glucose, a process that gained commercial traction as industrial chemistry matured at the tail end of the 19th century. By the time magnesium gluconate came around, manufacturers brought together the knowledge behind two familiar substances, giving rise to a supplement said to offer better absorption and gentler effects on the stomach compared with some older magnesium salts. Scientists favored magnesium gluconate for studies and product formulation for this very reason. So magnesium gluconate isn’t some shot-in-the-dark product – it’s a result of trial, error, and a steady look at what actually delivers tangible health results.
Product Overview
Plenty of folks take magnesium gluconate as a dietary supplement, but its reach stretches beyond just pill bottles. Tablets, powders, and syrups contain magnesium gluconate for both human and animal consumption, and in the food world, it props up mineral balance in sports drinks and helps fortify cereals. This magnesium salt dissolves well in water—unlike some of its chalkier cousins—so food and supplement companies like working with it in liquid formulations. Even in pharmaceutical circles, you’ll spot magnesium gluconate stacked high for its reliable performance, predictable behavior under testing, and good safety record across diverse age groups. Veterinary uses haven’t fallen by the wayside, either: specialized mixes for pets and livestock lean on magnesium gluconate to address shortfalls with precision.
Physical & Chemical Properties
Magnesium gluconate’s structure brings together a magnesium atom chelated with two gluconate molecules. It shows up as a fine, white powder or crystalline granules, not much odor to speak of and a relatively bland taste—making it easy to blend into food and medicines. It’s freely soluble in water, forming a clear solution, and barely budges in alcohol. With a molar mass of about 448 g/mol, it drifts easily through filtration and mixing processes. Chemically stable at room temperature and non-reactive with most food ingredients, magnesium gluconate won’t break down easily when stored in dry, cool conditions. That’s handy whether you’re making a health supplement or formulating a new beverage. Sensitivities to ambient moisture do matter, though, so airtight packaging serves an important purpose.
Technical Specifications & Labeling
Regulators keep a close eye on magnesium gluconate, setting tight standards for purity and labeling. Industry-grade material usually contains more than 97% pure substance, with strict limits on heavy metals like lead and arsenic. Residual solvents fall under serious scrutiny, and the salt must meet microbiological safety benchmarks. Labeling on commercial products must clearly show magnesium content per serving—either in milligrams of magnesium gluconate or, more informatively, elemental magnesium. Ingredient lists follow either the “magnesium gluconate” or “magnesium (as gluconate)” convention, which helps consumers understand exactly what they’re getting. Supplement manufacturers also stick compliance badges on product labels to indicate third-party quality checks or adherence to pharmacopeial standards. The U.S. Pharmacopeia (USP), European Pharmacopeia (Ph. Eur.), and Food Chemicals Codex (FCC) all provide tailored specifications aimed at consistent quality and accurate consumer information.
Preparation Method
Commercial processes for magnesium gluconate stick close to efficiency and safety. They usually start with gluconic acid, often from controlled fermentation of glucose using species like Aspergillus niger. This gluconic acid gets neutralized by adding a magnesium base like magnesium oxide or magnesium carbonate. The neutralization step must be tightly controlled—too much or too little magnesium throws off stoichiometry, and incomplete reactions can saddle the batch with unwanted impurities or off-colors. Following mixing, filtration strips away insoluble residue, then crystallization brings out pure magnesium gluconate. Technicians tweak parameters like pH and temperature to pull out a product with ideal particle size and solubility. Sometimes, spray drying or vacuum drying follows, knocking out moisture to lock in stability for transport and storage. Industrial facilities monitor the process with near-constant quality checks, especially where end-users expect pharmaceutical-grade purity.
Chemical Reactions & Modifications
Magnesium gluconate doesn’t show dramatic reactivity during ordinary use but can take part in a handful of useful reactions. Under acidic conditions, it dissociates to give up magnesium ions and gluconic acid, which can feed into biological pathways or industrial syntheses. In food chemistry, it acts as a source of both elements without adding bothersome tastes or gumming up the texture. As a chelating agent, gluconate holds onto minerals and keeps them soluble, so modification of the gluconate backbone—or pairing it with different cations—yields new products with tailored properties for specific applications. Scientists sometimes experiment with co-crystallization or blending technology to produce multi-mineral salts, giving consumers choices that meet niche nutritional needs. Looking at chemical compatibility, magnesium gluconate avoids precipitating with most dietary acids or bases, reducing worries about shelf-life surprises or unwanted changes during processing.
Synonyms & Product Names
Walk through a supplement catalog and magnesium gluconate shows up under a surprising mix of aliases: “magnesium(II) D-gluconate,” “magnesium digluconate,” and just “magnesium gluconate” on most packaging. Some pharmaceutical labs list it by the CAS Number 3632-91-5 or EINECS Number 222-848-2. In food circles, you’ll find E576 spread across ingredient lists for products shipped into the EU. Trademarked blends and proprietary names show up in multi-mineral tablets and liquid health tonics, but they always anchor back to the same core chemical structure.
Safety & Operational Standards
Magnesium gluconate rates as one of the safer choices among magnesium supplements. Toxicologists report that, as long as people stick close to recommended dosages, risk remains low for both healthy adults and most vulnerable groups like seniors and children. The compound doesn’t cause the harsh laxative effects seen with magnesium sulfate or oxide—one reason doctors recommend it for patients with sensitive stomachs or ongoing supplement needs. Industrial safety protocols treat magnesium gluconate with respect: workers use gloves and dust masks when handling fine powder, and good ventilation prevents accidental inhalation. Packages carry clear hazard statements for bulk quantities, detailing first-aid measures in case of accidental exposure. In most finished products, levels of contaminants stay well below regulatory limits, and strict testing covers every batch. Proper disposal avoids environmental impact—although both magnesium and gluconate break down safely, good manufacturing practice means not leaving anything to chance.
Application Area
Nutrition headlines dominate discussion of magnesium gluconate, but its usefulness doesn’t end there. Food scientists lean on it to adjust mineral balance in meal replacements, energy bars, and specialty drinks. Clinical nutrition relies on it when intravenous or enteral feeding is needed, especially for patients who can’t handle more reactive magnesium formulations. Magnesium gluconate even finds roles in topical solutions for certain skin formulations, though it does not penetrate as deeply as some other forms. Animal nutrition has plenty of uses: farms and zoos turn to magnesium gluconate for precise dosing, particularly where mineral deficiencies threaten animal health or reproduction rates. Beyond that, some niche uses appear in water softening and agricultural supplements, with research ongoing into more advanced chemical and biomedical applications.
Research & Development
Researchers continue to dig into magnesium gluconate’s absorption mechanics and interaction with the human body. Some studies suggest it absorbs more efficiently than traditional magnesium oxide, especially for people with digestive issues or compromised GI tracts. Ongoing clinical trials dig into whether magnesium gluconate outperforms other salts for long-term cardiovascular and metabolic health. On the formulation side, food scientists are looking for ways to boost taste and texture without driving up costs, so magnesium gluconate stays popular within R&D departments at major supplement and beverage companies. Investigators in pharmacology and geriatrics remain busy evaluating optimal dosing for older adults, who often face issues with both magnesium loss and poor absorption. Recent advances in particle engineering and encapsulation could change how magnesium gluconate ends up in functional foods—making it possible to add meaningful mineral content without clashing with flavor or mouthfeel.
Toxicity Research
Decades of safety studies make magnesium gluconate a dependable choice for both human and animal nutrition. Acute and chronic toxicity studies show high tolerance in rodent and non-rodent models, with few reports of negative outcomes unless doses hit extreme levels. Even among sensitive populations, side effects like diarrhea or mild GI upset only appear at well above recommended intakes. Regulatory agencies keep a close watch on potential risks tied to contaminants and impurities rather than magnesium gluconate itself. Researchers have also looked at interactions with pharmaceuticals and other dietary components, with no spikes in adverse events compared to other magnesium salts. The overall risk profile looks stable, and ongoing epidemiological work helps track rare sensitivities or allergic reactions, although those cases turn up with very low frequency.
Future Prospects
With health-conscious consumers chasing after balanced mineral intakes and more doctors recommending magnesium for heart, brain, and metabolic benefits, the future looks solid for magnesium gluconate. Growth in the personal nutrition and supplements market remains strong, and innovations in food technology promise new formats and combinations. Emerging research into gut health and personalized medicine could drive demand for bioavailable magnesium sources, especially in populations facing high rates of deficiency. Biotechnology startups are exploring fermentation tweaks and eco-friendly production, hoping to bring down costs and lighten the environmental footprint of the industry. In the world of pharmaceutical R&D, nanotechnology and targeted delivery systems offer new hopes for those who need specific dosing regimens. At every step, consumers benefit from greater transparency, sum-total safety, and the possibility of more affordable, accessible magnesium solutions in everyday life.
What are the health benefits of Magnesium Gluconate?
Magnesium Gluconate—More Than a Supplement
Magnesium doesn’t usually top the list of nutrients people discuss, yet it plays an essential role in everyday health. The form called magnesium gluconate gets attention for being easily absorbed by the body, which is something anyone who’s struggled with other supplements would appreciate. For most people—especially those with a diet heavy on processed foods or little green vegetables—getting enough magnesium makes a difference.
Supporting Heart and Nerve Health
Heart health often grabs headlines, and magnesium gluconate quietly supports this area. I’ve seen individuals with occasional muscle twitches or irregular heartbeats benefit from improved magnesium intake. Scientific research links magnesium with maintaining normal heart rhythm and keeping blood pressure within a healthy range. Doctors sometimes recommend magnesium for minor heart rhythm concerns, pointing to studies in journals like the “American Journal of Clinical Nutrition.” This form of magnesium steps in where diet falls short, filling a gap that can keep blood vessels relaxed and the heartbeat steady.
Beating Fatigue and Muscle Cramps
Waking up with tight calves or struggling through a workout because of muscle cramps isn’t just a nuisance—it signals something missing. People working long shifts on their feet, athletes, or those dealing with stressful periods know these aches all too well. Magnesium helps send messages between nerves and muscles, so getting enough supports normal function. Magnesium gluconate offers a gentler way on the stomach compared to harsher options like magnesium oxide. By restoring these levels, many notice less cramping, improved sleep, and reduced fatigue, making work or exercise feel less like an uphill climb.
The Connection With Stress and Sleep
It’s not hard to spot signs of stress these days—tight shoulders, trouble winding down at night, and restless minds. I’ve found magnesium gluconate helpful for these issues, and the background research backs it up. The mineral helps regulate cortisol, a hormone tied to stress. Magnesium also plays a role in producing melatonin, which is key for getting a restful night’s sleep. When you spend hours staring at screens or staying up late, magnesium levels can drop lower. Raising them can shift restless nights into deeper, more restorative sleep.
Deficiency: Why It Deserves Attention
Not everyone thinks about magnesium deficiency, but it can sneak up on people without much warning. Symptoms like irritability, anxiety, poor appetite, or unexplained fatigue sometimes point to this gap. Blood tests don’t always catch mild deficiencies since only about 1% of the body’s magnesium circulates in the blood. Paying attention to diet—leafy greens, nuts, seeds, whole grains—sets the foundation, but reality often gets in the way. Magnesium gluconate steps in as a supplement that’s generally well-tolerated and less likely to cause stomach upset.
What Helps Alongside Magnesium Gluconate
Taking magnesium isn’t the only part of the equation. Staying hydrated, cutting back on caffeine or alcohol, and eating more produce all make a big difference. Taking time to read supplement labels also matters, since not all forms of magnesium offer the same absorption. Magnesium gluconate stands out for being gentle and effective. If you have a chronic condition or take certain medications, checking in with a healthcare provider is wise before adding any new supplement.
A thoughtful approach to magnesium goes a long way—again and again, I’ve seen small dietary changes and smart supplement choices lead to more energy, fewer aches, and steadier moods. Magnesium gluconate’s benefits reach past the pill bottle, supporting the real rhythms and challenges of daily life.
How should I take Magnesium Gluconate and what is the recommended dosage?
The Basics of Magnesium Gluconate
Magnesium isn’t just another mineral you hear about in commercials for multivitamins. It helps nerves fire the way they should, keeps muscles from cramping, and lets the heart do its steady job. Most people don’t realize that magnesium also plays a hand in everything from bone strength to keeping blood sugar under control. So, if a doctor suggests magnesium gluconate, there’s probably a good reason.
How to Take Magnesium Gluconate
Looking at the bottle or prescription label gives the most direct answer, but the real trick is about routine and timing. Doctors usually tell people to take magnesium gluconate with a meal or right after they eat. Taking it on an empty stomach sometimes leads to an upset stomach or some pretty uncomfortable diarrhea. I’ve seen this play out after trying to take magnesium supplements for leg cramps on an empty stomach — it made me regret skipping breakfast just to “stay on schedule.”
Swallow the tablet whole with a full glass of water. Crushing or chewing the pill doesn’t help and might make it harder for the body to absorb it evenly. If someone struggles to swallow pills, some magnesium gluconate products come as liquid or powder. In this case, measuring the dose carefully saves a lot of trouble—pouring a “guesstimate” dose might sound like it couldn’t hurt, but it opens the door to overdosing.
Recommended Dosage: Fact Over Guesswork
Taking extra supplements only helps if the amount lines up with what the body needs. For adults, the dietary dosage for magnesium sits between 310 to 420 milligrams per day, depending on age and sex, according to the National Institutes of Health. Most pills vary in strength—magnesium gluconate contains less elemental magnesium than some other forms, so a 500 mg tablet of magnesium gluconate delivers only about 27 mg of pure magnesium.
Doctors often adjust the dose based on why someone needs more magnesium. A person with a simple dietary shortfall might get a smaller dose than someone whose medicine flushes magnesium out too fast (like certain diuretics). I’ve seen older relatives with heart problems taking two to three pills daily only after their doctors reviewed their blood test results.
Why Following Advice Matters
Going off script, taking too much supplement, or trying “remedies” found online can backfire. Magnesium overdose doesn’t play around—it causes low blood pressure, heart rhythm troubles, even trouble breathing if things go far enough. People with kidney problems walk an even finer line; their bodies struggle to get rid of extra magnesium, so it builds up dangerously fast. If someone takes other medicines, like antibiotics or blood pressure drugs, speaking with a doctor or pharmacist first helps avoid hidden interactions.
Lifestyle Fixes and Final Thoughts
Supplements fill gaps, but real food matters most. Green veggies (spinach, broccoli), nuts, seeds, and whole grains bring magnesium plus other nutrients into the body in a natural, balanced way. I started cooking more beans and brown rice at home for that reason—my energy improved, and leg cramps stopped waking me up. Pairing these changes with the right supplement dose often works better than popping handfuls of pills alone.
No pill can outmuscle a wildly unbalanced diet or poor medical advice. Real results come from smart choices, some planning, and checking in with skilled professionals who have your back.
Are there any side effects or risks associated with Magnesium Gluconate?
Everyday Use and Real Networks of Trust
Magnesium gluconate lines up on pharmacy shelves right next to other supplements claiming to fix cramps, boost heart health, and steady nerves. People sometimes grab it hoping to trade restless nights or muscle twitches for a little bit of peace. I’ve spoken with plenty of people searching for that quick fix, only to wonder a week later if their gut troubles or headache snuck in with the bottle. The truth about any supplement, magnesium gluconate included, starts with facts rather than promises.
What Can Go Wrong with Magnesium Gluconate?
Our bodies only need so much magnesium. Take too much, and things can twist out of balance. Soft stools or an upset stomach show up first. For most healthy adults, these complaints stay mild. The real trouble enters the picture for those with kidney disease. Kidneys act as a gatekeeper, deciding what gets kept and what leaves. Poor kidney function doesn’t clear extra magnesium well. Levels rise higher than intended and bring on low blood pressure, irregular heart rhythms, or, in rare cases, confusion and difficulty breathing.
Anyone who regularly uses antacids, laxatives, or other medicines often finds their body’s chemical mix shifts in unexpected ways. Combining those with magnesium gluconate can sneak up on you. I remember a patient who took magnesium for leg cramps alongside a water pill. She started feeling weak and dizzy, and her doctor caught the imbalance just in time. These stories show why blind faith in “just another supplement” can backfire.
Where Evidence Meets Hype
Magnesium itself plays key roles inside muscle, bone, and nerves. Research backs up its importance for heart health and blood sugar regulation. Still, health claims sometimes run ahead of what studies prove. People living with chronic diseases swallow supplement promises hoping for relief, but solid evidence for fixed conditions remains thin. Medical guidelines in the U.S. suggest using supplements only to correct a known deficiency. The risks stack up if people start treating tablets like multivitamins—something to toss in the cart just in case.
Magnesium gluconate does get absorbed well. It usually sidesteps the intense diarrhea seen with the cheaper oxide kind, but even the “gentle” forms turn rough with high doses. The National Institutes of Health puts the upper limit of magnesium from supplements at 350 milligrams for adults. Go past this, and you start gambling with your health, especially without a doctor’s oversight.
Safe Supplement Use: Real Steps to Protect Yourself
Before starting magnesium gluconate, talk to a doctor or a licensed dietitian. Blood work tells you if extra magnesium is even needed—most people can fill their quota by eating greens, nuts, and beans. People living with chronic illness or those on several prescription medications need extra caution. A pharmacy consult helps untangle the knot of drug-supplement interactions.
Buying from reputable sources matters. Third-party tested supplements—those stamped by organizations like USP or NSF—give confidence that what’s on the label is in the bottle, with no unwelcome extras. One more thing: pay attention to how you feel once you start. New headaches, gut changes, or changes in heart rhythm deserve respect and a call to a healthcare provider.
Looking for Answers and Accountability
Magnesium gluconate sits in that gray space between food and medicine. It can help some people, but comes with its own risks. Facts, regular check-ins with doctors, and smart self-advocacy form a better safety net than any bottle on the shelf.
Can I take Magnesium Gluconate with other medications or supplements?
Understanding How Magnesium Fits into a Daily Routine
Magnesium gluconate, often picked for muscle cramps, low magnesium, or as part of a general wellness plan, can seem harmless enough to mix into any routine. Magnesium helps nerves fire, muscles flex, and keeps hearts beating steadily. Yet adding it into the mix with other medications or supplements doesn't always go as smoothly as its benefits imply. I've seen people overlook the details and regret it later.
Magnesium Gluconate Isn’t Always a Simple Add-On
Doctors and pharmacists usually check your list of meds when you ask about new vitamins, because certain combinations just don’t play nicely. Magnesium, for all its naturalness, falls in that group. People on antibiotics, blood pressure pills, or certain osteoporosis medications run into trouble if they just toss magnesium into the mix. Magnesium can block your body from absorbing some antibiotics, especially those in the tetracycline and quinolone groups, like ciprofloxacin and doxycycline. So, if you take both at the same time, the antibiotic may not work as well and the infection could drag on longer than needed.
Blood pressure drugs, like certain calcium channel blockers, also react with magnesium. Instead of getting more relaxed blood vessels, you might drop your pressure more than you wanted, leading to lightheadedness or danger for people who already run low. And let’s not forget medications for osteoporosis such as alendronate. Magnesium can prevent your stomach from soaking up these pills, making them almost useless. Sometimes timing fixes that—taking magnesium at least two hours apart—but not everyone follows through perfectly.
It’s Easy to Lose Track of Hidden Interactions
Supplements crowd the shelves, with magnesium hiding in multi-mineral and electrolyte blends, meal shakes, sleep aids, and natural laxatives. Too much magnesium, even from good sources, acts as a laxative and brings on diarrhea. That can drain other important minerals. If you’re taking vitamin D or calcium at the same time, your body might rely on extra magnesium to process them, so the balance matters.
Older adults or those with kidney problems can run into trouble handling too much magnesium. The kidneys do most of the work filtering out the extra, and anyone with slower kidney function can stack up magnesium to risky levels without realizing.
What Works for Checking Safety
Clear communication with doctors and pharmacists helps catch dangerous combinations. Let them know every supplement you use, not just prescriptions. Bring bottles, or list ingredients so nothing gets missed. I once assumed something like a magnesium powder added after workouts was too “basic” to mess up my routine, but it made my blood pressure pill work a little too well. My hands got tingly, and I realized I needed to rethink timing or even if I needed that extra dose.
For most folks, spacing magnesium away from other medications by at least two hours cuts down on problems. Staying aware of all the places magnesium hides in your products matters too. Blood tests once in a while give a clearer picture of whether things stay in balance, especially for anyone on meds for heart, bone, or kidney conditions.
Smart Habits Create Real Safety
Reading ingredient lists, keeping a current list of all medications and supplements, and double-checking with trusted health experts help keep surprises away. It’s not a scare tactic, but a way to get the right benefits from magnesium while steering clear of unwanted problems.
Who should avoid using Magnesium Gluconate?
Understanding Magnesium Gluconate
Magnesium gluconate is popular as a dietary supplement. People take it to support muscle function, nerve health, and sometimes to correct low magnesium levels. Grocery aisles and online shops often line their shelves with these tablets and powders, promising better sleep or improved energy. Before anyone tosses a bottle into their cart, it’s smart to ask who really shouldn’t be using it.
Individuals with Kidney Issues
The kidneys regulate how much magnesium the body keeps or gets rid of. Folks dealing with kidney disease or reduced kidney function face a higher risk if they take extra magnesium. Their bodies struggle to filter excess minerals, which can lead to dangerous levels of magnesium in the bloodstream. Symptoms include low blood pressure, slow heartbeat, or even trouble breathing if it builds up. People with chronic kidney problems should check with their physician or dietitian before taking any magnesium supplement, including gluconate.
People on Certain Medications
Some prescriptions interact with magnesium. For instance, antibiotics from the tetracycline or fluoroquinolone families don’t work as well if taken together with magnesium. Magnesium can bind to the medication in the gut, making it less effective. Diuretics prescribed for high blood pressure or heart problems may change how magnesium moves through the body. Over-the-counter proton pump inhibitors used to manage acid reflux can also affect magnesium levels, sometimes lowering them. Mixing supplements with daily medicines without talking to a doctor or pharmacist easily causes trouble.
Those with Heart Block or Bradycardia
Magnesium influences the electrical rhythm of the heart. If someone deals with heart block (where the heart’s signal slows down or gets interrupted) or bradycardia (an unusually slow pulse), extra magnesium may do more harm than good. Too much can slow the heart even further, ramping up the risk of fainting or other irregular rhythms. Anyone with heart rhythm troubles should avoid adding supplements unless a cardiologist specifically recommends it.
People with Allergies to Magnesium Gluconate Ingredients
Most supplement bottles contain more than just magnesium gluconate. Binders, fillers, dyes, or preservatives often sneak into the formula. Anyone allergic to a listed ingredient should steer clear or find an alternative that suits their needs. If a rash, swelling, itching, or trouble breathing develops after taking it, get medical attention right away.
Pregnant or Breastfeeding Women
Expecting or nursing mothers juggle a lot. Magnesium serves as a vital nutrient during pregnancy, but supplementing without medical advice crosses into risky territory. Studies track possible complications and note how high doses of magnesium from supplements, rather than food, may lead to problems for a newborn. Prenatal vitamins typically include amounts tested for safety. If more is needed, an obstetrician is the right person to make that call.
Children without a Doctor’s Approval
Kids grow fast and need proper nutrition, including magnesium. As a parent, it’s tempting to reach for a supplement if a child seems low on energy or complains of cramps. The safest path includes asking a pediatrician to check for deficiency before relying on over-the-counter solutions. Children’s bodies have different requirements and tolerances, so dosing errors easily happen without professional guidance.
Keeping Safety Front and Center
Taking magnesium when it isn’t needed, or when health conditions exist, puts someone at risk. Regular blood tests and medical follow-up make a difference. People unsure about their levels or the need for a supplement can ask for these tests through their primary care provider. Food sources like nuts, leafy greens, seeds, and whole grains offer magnesium in safer, natural amounts. Education and conversation often help prevent problems before they start.
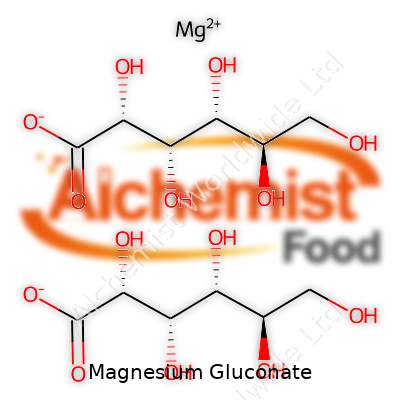
| Names | |
| Preferred IUPAC name | magnesium bis[(2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate] |
| Other names |
Magnesium(II) gluconate D-gluconic acid magnesium salt Magnesium digluconate |
| Pronunciation | /ˌmæɡˈniːziəm ˈɡluːkoʊneɪt/ |
| Preferred IUPAC name | magnesium bis[(2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate] |
| Other names |
Magnesium digluconate Gluconic acid magnesium salt |
| Pronunciation | /mæɡˈniːziəm ˈɡluːkəneɪt/ |
| Identifiers | |
| CAS Number | 15283-23-1 |
| Beilstein Reference | 4126808 |
| ChEBI | CHEBI:31595 |
| ChEMBL | CHEMBL1201080 |
| ChemSpider | 23321 |
| DrugBank | DB11206 |
| ECHA InfoCard | 050000003479 |
| EC Number | E345 |
| Gmelin Reference | 68285 |
| KEGG | C07560 |
| MeSH | D008271 |
| PubChem CID | 65168 |
| RTECS number | OM2725000 |
| UNII | M36O6UO9PO |
| UN number | UN2813 |
| CAS Number | 3632-91-5 |
| Beilstein Reference | 140873 |
| ChEBI | CHEBI:31595 |
| ChEMBL | CHEMBL1201120 |
| ChemSpider | 23009 |
| DrugBank | DB09462 |
| ECHA InfoCard | 13de1f1f-34e3-4afe-8864-017a6c8457a1 |
| EC Number | E579 |
| Gmelin Reference | 85894 |
| KEGG | C01793 |
| MeSH | D008268 |
| PubChem CID | 65061 |
| RTECS number | OM3325000 |
| UNII | 9G1IQ3003U |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C12H22MgO14 |
| Molar mass | 414.6 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.44 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -2.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.28 |
| Basicity (pKb) | 10.4 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.56 |
| Dipole moment | 1.85 D |
| Chemical formula | C12H22MgO14 |
| Molar mass | 414.6 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.44 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -2.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.39 |
| Basicity (pKb) | 4.95 |
| Magnetic susceptibility (χ) | -6.9×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.59 |
| Dipole moment | 0.00 D |
| Pharmacology | |
| ATC code | A12CC04 |
| ATC code | A12CC02 |
| Hazards | |
| Main hazards | May cause respiratory tract, eye, and skin irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | No signal word |
| Hazard statements | May cause respiratory irritation. Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Use with adequate ventilation. If swallowed, get medical advice/attention. |
| NFPA 704 (fire diamond) | 1-1-0 |
| LD50 (median dose) | > 4,500 mg/kg (rat, oral) |
| NIOSH | Not Listed |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 250 mg |
| IDLH (Immediate danger) | Not listed |
| Main hazards | May cause respiratory irritation; causes serious eye irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Use with adequate ventilation. |
| NFPA 704 (fire diamond) | 1-0-1 |
| Autoignition temperature | 450 °C |
| Lethal dose or concentration | LD50 (oral, rat): 8,120 mg/kg |
| LD50 (median dose) | LD50 (median dose): 4,000 mg/kg (rat, oral) |
| NIOSH | Not Listed |
| PEL (Permissible) | 50 mg/m³ |
| REL (Recommended) | 250 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Calcium gluconate Ferrous gluconate Potassium gluconate Zinc gluconate Magnesium sulfate Magnesium citrate |
| Related compounds |
Calcium gluconate Potassium gluconate Zinc gluconate Sodium gluconate Ferrous gluconate |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 439.7 J·mol⁻¹·K⁻¹ |

