Magnesium Citrate: A Commentary on Its Past, Progress, and Possibilities
Historical Development
Magnesium citrate hasn't always sat on store shelves in its familiar laxative bottles or health food supplement jars. Go back to the nineteenth century, and you'll find pharmacists mixing tartaric acid and magnesium carbonate as part of experimental remedies long before modern regulations or evidence-based medicine. The compound drew early attention—both for its ability to move the bowels and soothe acidic stomachs, and for the promise of delivering magnesium, an element that continues to baffle and delight nutritional researchers. Over decades, the story of magnesium citrate shifted from apothecaries to factories, from snake-oil perception to mainstream trust, largely driven by observable benefits for digestion and a growing understanding of magnesium’s role in cardiac and neuromuscular health.
Product Overview
Today, magnesium citrate comes in powder, tablet, and liquid forms. Walk into a pharmacy aisle, and there’s always a bottle promising relief from constipation or a tasteless powder meant to stir into water as a daily supplement. What grabs my attention isn’t just the marketing surrounding bioavailability, but also how magnesium citrate keeps popping up in packs labeled “gentle” or “fast-acting.” The compound occupies a unique niche—easy for the body to absorb, less likely to cause stomach upset compared to some other forms of magnesium, and cheap enough to show up in both prescription and over-the-counter packaging.
Physical & Chemical Properties
Magnesium citrate looks plain on the surface—white, crystalline, and barely different from sugar in appearance for most of us. Physically, it dissolves well in water, a property that gives it an edge for liquid supplements. Chemically, magnesium citrate acts as a salt, made from magnesium and citric acid, carrying the chemical formula C6H6MgO7. It doesn’t react wildly at room temperature or pressure, and it doesn’t emit funky odors or flavors in the quantities found in medicine. Because it's stable, manufacturers can blend it with other actives or excipients without worrying about unwanted side reactions.
Technical Specifications & Labeling
A look at any supplement or pharmaceutical bottle reveals meticulous attention to detail. Labels mention elemental magnesium content, warnings for kidney patients, recommended daily allowances, and batch traceability codes. In the United States, the Food and Drug Administration (FDA) establishes specific labeling rules to inform consumers about dosage, usage, and contraindications. These details aren’t just regulatory hurdles—they foster trust, and they help people using magnesium citrate for constipation, supplementation, or as part of a bowel prep before medical procedures. For anyone choosing a product, the absence of artificial flavors or sweeteners often signals a cleaner, well-documented ingredient sourcing process.
Preparation Method
Producing magnesium citrate in bulk involves reacting magnesium carbonate or magnesium hydroxide with citric acid under controlled conditions. The process takes place in large stainless steel reactors, leveraging water as a medium to dissolve both ingredients. Operators watch pH and temperature closely to ensure a pure, crystalline product. After the chemical reaction, the solution cools, and the crystals get separated, filtered, and dried. Careful drying prevents caking, and fine sieving gives the final product consistency across batches. I’ve seen manufacturers break down every step into quality control checkpoints—if the product clumps, fails solubility tests, or carries impurities, it doesn’t leave the site.
Chemical Reactions & Modifications
Magnesium citrate’s simplicity gives it a stability edge, but manufacturing tweaks can fine-tune the particle size, solubility, and even the ratio of components to suit specific products. For pharmaceutical applications, extra purification steps knock down unwanted traces of heavy metals. Modified versions with chelating properties sometimes find use in laboratory settings or as excipients in tableting. Manufacturers adjust the hydration state—anhydrous or trihydrate forms—to influence shelf life and flow characteristics. Balancing purity, shelf stability, and affordability pushes chemists to keep improving crystallization and drying protocols, always within tight regulatory bounds.
Synonyms & Product Names
The shelves rarely call it by one name. In pharmacies, magnesium citrate sometimes appears as “Citromag,” “Milk of Magnesia (citrate variant),” or “Mag Citrate.” In chemical supply catalogs, names like “trimagnesium dicitrate” or “magnesium (II) citrate” fill technical datasheets. European and Asian suppliers might push brands under local trade names, but it’s the chemical composition on the bottle that speaks to the careful buyer or practitioner. Some supplement companies capitalize on branding—adding flavor tags or extra minerals—but the base compound remains unchanged, driving home the idea that efficacy doesn’t always track branding.
Safety & Operational Standards
Magnesium citrate’s inclusion in food supplements and medicines relies on a finely tuned web of safety protocols. From raw material sourcing to packaging, the entire production adheres to Good Manufacturing Practices (GMP). Plants test water and air quality just as scrupulously as finished bottles. For the operator, personal protective gear keeps raw powder out of airways, and contained systems minimize dust. On the consumer side, dosing clarity matters—especially for people with kidney problems, kids, or anyone on certain medications. Adherence to FDA, EFSA, and Health Canada guidelines isn’t just about boxes checked; these rules respond to real risks like magnesium overdose or contamination.
Application Area
Magnesium citrate’s long-standing application as a laxative predates grumbling about modern diets. Many who struggle with slow digestion know its gentle nudge, especially shielding themselves from harsher alternatives. Beyond the gut, clinicians turn to it for bowel prep before procedures like colonoscopies—a critical use, given how vital complete evacuation becomes before visualizing the intestine. In nutrition circles, athletes and health-enthusiasts embrace magnesium citrate for muscle recovery, nerve function support, and a persistent hope of boosting mood or sleep. Dentists, oddly enough, sometimes note its role in oral rinses or as a buffering agent in clinical settings. Its role in food fortification and as an ingredient in effervescent drinks keeps expanding, giving it a utility across more aisles than most realize.
Research & Development
The scope of research into magnesium citrate covers everything from absorption rates to its capacity for drug delivery. Scientists use isotope tracing to figure out how well magnesium citrate enters the bloodstream versus magnesium oxide or sulfate. Recent years saw a boom in research on magnesium’s impact on mood disorders, hypertension, and glycemic control. Formulators try new combinations—curcumin plus magnesium, for instance—to see if synergistic effects can lift both compounds’ bioactivity. Advances in nutritional genomics hint that individuals process magnesium differently, pressing researchers to personalize supplementation recommendations. The focus on sustainable production and biodegradable packaging keeps growing, as labs race to reduce waste and energy demands.
Toxicity Research
Though magnesium citrate brings many benefits, toxicity isn’t abstract. People with impaired kidney function can’t excrete magnesium efficiently, so dangerous levels can sneak up without warning—marked by muscle weakness, heart arrhythmias, or slow reflexes. Acute overdose, while rare in healthy adults due to the self-limiting effect of diarrhea, still occurs in laxative abuse or accidental consumption. Health agencies keep up surveillance, reporting side-effects and putting out alerts on improper use, particularly in pediatric and geriatric populations. Ongoing animal and clinical trials probe long-term safety, and some researchers investigate interactions between high magnesium supplementation and the absorption of other minerals like calcium and iron.
Future Prospects
Magnesium citrate likely won’t lose relevance anytime soon. Trends in preventive health and personalized nutrition set the stage for more targeted forms—think microencapsulated magnesium, or blends meant to bypass sensitive stomachs. Regulations grow tighter, but so do technologies for traceability and real-time quality assurance. The sustainability drive sends companies looking for greener synthesis pathways, minimal waste solutions, and smart packaging. On the research end, the interplay of magnesium with gut bacteria, sleep cycles, and even cognitive aging continues to attract grants and headlines. As understanding deepens, what seems a humble supplement could play a part in broader strategies for managing chronic disease, optimizing well-being, and—maybe most importantly—empowering people to take small but meaningful steps toward their own health.
What is Magnesium Citrate used for?
Understanding the Role of Magnesium Citrate
Magnesium citrate catches a lot of attention in medicine cabinets, often for reasons most folks would rather not talk about at dinner. I’ve seen it prescribed plenty of times while working in a health clinic, mainly to prep people for colonoscopies or to help someone who can’t seem to get their digestive system moving along. But writing this, I realize many people don’t really know why professionals reach for it or what it does inside the body beyond its reputation as a powerful laxative.
Supporting the Basics: Muscle and Nerve Health
People use magnesium citrate for more than emptying their bowels. Lots of us fall short on magnesium because processed foods on supermarket shelves don’t give us enough. The mineral works in over 300 bodily processes — think muscle movements, steady heartbeats, supporting bones, and keeping nerves firing. Without enough, you might notice twitchy muscles, leg cramps, a racing heart, or just feeling worn out for no clear reason.
Magnesium citrate offers a form your digestive system handles fairly well. It’s more absorbable than some other supplements on the market. In my own experience, runners and gym fans who push themselves physically sometimes miss the mark on magnesium, and a supplement in this form fills in the gap. One study published in the journal "Nutrients" described that about 48% of Americans get less magnesium than their bodies need. That gap adds up in long-term health issues.
Why Doctors Recommend It
Magnesium citrate shows up right before a colonoscopy for a reason: it reliably empties the bowels. That part is undeniable. It works by bringing water into the intestines, making stools easier to pass. Anyone who has had to drink the stuff will tell you — the results are not subtle. Sometimes it shows up in prescription for short-term constipation too, helping out when fibers and prunes haven’t done the trick.
Some research points to benefits in calming restless legs, taming migraine headaches, and even easing anxiety. Magnesium connects to brain chemistry, and when levels look low, nerves can misfire and muscles tense up. Long night shifts in emergency rooms taught me magnesium helped settle some of these problems, especially when dealing with folks under stress or recovering from illness.
Staying Safe With Supplements
Like with most things, more isn’t always better. Too much magnesium citrate pulls water into the gut and leads to diarrhea, dehydration, and sometimes low blood pressure. People dealing with kidney problems can run into bigger trouble if they don’t clear out the extra mineral. Over-the-counter sales sometimes tempt people to take it regularly, but doctors don’t recommend using it day in and day out without a plan.
Good primary care takes a look at the bigger picture. Blood tests can spot a true deficiency. Dietary changes, like adding leafy greens, beans, nuts, and seeds, help many people restore normal levels without needing a supplement. For those who need extra help, medical advice builds sure footing, rather than tossing pills at symptoms. The FDA classifies magnesium citrate as generally safe, but guidance always matters.
Better Health Through Understanding
Learning what magnesium citrate does, why doctors suggest it, and how to use it carefully puts people in charge of their health. People know their own bodies best and talking openly with care teams about symptoms, diet, and supplements keeps things on track. Magnesium citrate plays several roles in health, and using it with care makes the difference between relief and unnecessary risk.
How should I take Magnesium Citrate?
Why Magnesium Matters for Me
Magnesium runs the show in my body, from helping my muscles relax after a workout to making sure my mind settles enough at night to sleep. Many foods—from leafy greens to nuts and whole grains—give me some, but not always enough. Busy routines, stress, and a less-than-perfect diet can drain my magnesium. I came to appreciate magnesium citrate especially since it absorbs into my system better than other types.
What I Watch for Before Taking It
Before bringing any supplement home, I talk with my doctor. Not everyone should grab a bottle. Some with kidney trouble or heart issues may feel worse after using magnesium citrate. Mixing it with certain medicines like antibiotics or blood pressure pills can work against what those meds are trying to do. My doctor checked all this with me before I started.
How I Take Magnesium Citrate
Magnesium citrate usually comes as a flavored liquid or tablet. The liquid works fast, often used for clearing out the bowels before treatments. In my daily routine, tablets or capsules fit better.
I stick to the dose marked on the label. More doesn’t mean better, especially with magnesium. Too much sends me running to the bathroom with diarrhea or cramps. Others might face upset stomach or even low blood pressure.
To stay comfortable, I take magnesium citrate with a meal, especially breakfast or dinner. Food, in my experience, makes it less likely I’ll feel queasy. Sticking to the same time each day helps me remember.
How I Know It’s Working—And When to Stop
Getting older, pushing myself at the gym, or skimping on vegetables—my legs cramp up or my sleep turns restless. Magnesium helps. If I feel better-rested, less tense, and calmer, I know it’s doing something.
Still, not every day calls for a supplement. If my diet looks better, or if I notice bathroom trips getting too frequent, I ease up. I look for signs like diarrhea, muscle weakness, or too much sluggishness. I always check in with my doctor if anything feels off.
Seeking Out Quality
Supplements can be a gamble. I look for options vetted by trusted labs, like NSF or USP testing. Quality means no surprise additives or contamination. Reading labels and checking the company’s reputation online helps me skip the shady stuff.
Simple Steps for Better Results
Hydration plays a big part, since magnesium draws water into the gut. I drink extra water with my tablet. I spread out nutrients through the day instead of loading up at once. Taking vitamin D with magnesium helps me absorb more, so sometimes I combine them in my breakfast.
I always listen to my body. If I feel off or have worries, I speak with my doctor before changing anything. Magnesium citrate can play a role, but it acts as an assistant to a balanced diet and regular movement, not a quick fix.
Are there any side effects of Magnesium Citrate?
What Happens After That Glass of Magnesium Citrate?
Magnesium citrate shows up on pharmacy shelves for good reason. People buy it to help with constipation or get their magnesium levels back on track. Doctors sometimes recommend it before procedures like a colonoscopy. Still, drinking that bottle—even half of it—does more than just move the bowels.
After taking magnesium citrate, the bathroom might turn into a second home for a few hours. That’s the point, really. The stuff draws water into the intestines and helps loosen everything up. But the rush to the restroom usually comes with some stomach cramps, rumbling guts, and maybe a bit of nausea. Diarrhea is to be expected, not just possible.
Not Just a Simple Laxative
Some people say they feel lightheaded or tired after magnesium citrate. A big clear-out can leave them wiped, especially if they haven’t eaten much. Losing water because of diarrhea, and losing salts like potassium or sodium, can bring muscle cramps or weakness. The body doesn’t like losing too much of these at once.
Anyone with kidney problems needs to be careful. Healthy kidneys can clear extra magnesium, but people with kidney disease risk toxic levels. Signs like confusion, slow heartbeat, or trouble breathing point to something more serious than stomach cramps. Medical help shouldn’t wait if any of those pop up.
Simple Facts Backed by Science
Magnesium citrate’s side effects show up regularly in studies and in local clinics. The Mayo Clinic, for example, lists common issues: abdominal discomfort, gas, and an urgent need to use the bathroom. Over-the-counter medicine labels warn about it. It’s all documented in plain terms.
The risk of dehydration increases for the elderly, very young, and those already on water pills or blood pressure meds. Diarrhea pulls water out faster, and some folks don’t feel thirst until they’re already low. That’s how people get dizzy or even faint.
Experience Helps People Learn
Many folks, including myself, have tried magnesium citrate before a procedure. The taste doesn’t stick around, but the hours stuck near a bathroom do. Some people feel fine once their gut settles. Others end the day feeling wrung out. Drinking extra fluids—clear broth, water, or juice—makes it more manageable. People who forget to hydrate may get headaches or notice darker urine.
Anyone looking to use magnesium citrate for regular constipation needs to talk with a healthcare provider. Sometimes it’s better to find a gentler solution. If constipation lasts more than a few days or keeps coming back, it’s not just a laxative problem. Diet changes—like adding fiber, more water, and moving around—can help long-term.
Practical Solutions
Start by listening to the body. If heart, kidney, or stomach issues are already part of life, talk with a doctor before reaching for magnesium citrate. Keep a glass of water handy and don’t stray far from the bathroom. It’s safer to stick to the dose on the label. Take it only as needed—never for fun or out of boredom.
It helps to remember that over-the-counter doesn’t mean harmless. Magnesium citrate helps many people, but the side effects are real. No one likes unwelcome surprises halfway through the day, especially the ones that announce themselves in the gut.
Can I take Magnesium Citrate daily?
Looking Beyond the Hype
Magnesium citrate gets a lot of attention on pharmacy shelves. Some folks reach for it to stay “regular,” while others hope it helps them sleep or ease muscle cramps. As a writer with a lifelong curiosity about nutrition, I’ve watched the world of supplements explode. It’s easy to assume that more of a good thing is always better, but the story with magnesium citrate isn’t so simple. Regular use carries both benefits and risks—not a one-size-fits-all answer.
Our Bodies and Magnesium
Magnesium is an essential mineral. You can’t live without it. Muscles, nerves, and bones depend on it every hour of the day. Most people get enough through leafy greens, seeds, nuts, and grains. Still, surveys from the CDC show plenty of adults don’t hit the recommended intake. Fatigue, cramps, or an uneasy gut can follow if levels drop low enough. But the solution isn’t always a supplement, and magnesium citrate, in particular, comes with extra effects.
Why Magnesium Citrate Raises Eyebrows
One quick fact: magnesium citrate isn’t just a source of magnesium, it’s a proven laxative. Hospitals and clinics use it to empty intestines before surgery. Some folks discover this the hard way after taking it for “extra energy.” If you use it daily, loose stools, dehydration, and even a shift in electrolytes can sneak up on you. Too much magnesium from supplements, not food, can tax kidneys or throw off heart rhythms.
What Doctors and Research Say
Doctors rarely hand out magnesium citrate for long-term daily use without a good reason. Clinical guidelines, including those from Harvard Medical School and the Mayo Clinic, advise making food the foundation for your minerals. Supplements help if your doctor spots a medical need, like a documented deficiency or certain migraines. Even then, safer forms like magnesium glycinate or oxide often win out, especially for people managing chronic health conditions.
Plenty of randomized studies chart the risks of chronic reliance on magnesium-based laxatives. In a review found in the journal Magnesium Research, researchers highlight possible dangers like electrolyte imbalance and renal strain with routine use. People with kidney disease face higher risk. Nobody likes cramping or a podcast-interrupting bathroom trip, so regular daily dosing isn’t as innocent as it seems in supplement ads.
Better Ways to Boost Magnesium
I’ve chatted with dietitians from local clinics and they all agree: hit the produce aisle before the supplement aisle. Spinach, almonds, black beans, and whole grains pump up magnesium the natural way. For most healthy adults, daily needs fall between 300 to 400 milligrams—a number cleared with a single serving of nuts and greens. If a supplement’s required, checking in with a healthcare professional beats guessing. Blood work and medical history should guide the dose and type.
Practical Steps and Safer Alternatives
If constipation troubles your daily life, swapping in fiber-rich veggies and drinking enough water works better for most people than a laxative. Movement helps too—walk around the block; it can do more than you think. If sleep, muscle tension, or anxiety spark your interest in magnesium, stress management and gentle exercise deserve a look before pills. Doctors suggest magnesium in pill or powder form only when a food-first strategy fails.
Final Thought
Magnesium citrate has its place, but daily use without supervision doesn’t fit most situations. Building habits around real food, hydration, and activity pays off with more stability and fewer side effects than most supplements can offer.
Is Magnesium Citrate safe to use during pregnancy?
A Common Question in Everyday Life
Magnesium citrate pops up on pharmacy shelves and internet searches every day. Many people reach for it to get relief from constipation or to support daily mineral intake. Once pregnancy enters the picture, though, supplements start to raise bigger questions. I remember sipping on prenatal vitamins each morning, scanning labels, searching for reassurance that what I put in my body wouldn't cause harm. The balance between keeping things moving and keeping things safe feels a lot trickier.
Official Guidance on Magnesium Citrate During Pregnancy
Obstetricians and pharmacists field this question all the time. Magnesium plays a real role in muscle and nerve work, bone health, and heart rhythm. Pregnant women need steady magnesium intake, no doubt about that. Official recommendations suggest pregnant adults should get around 350-360 mg a day. Magnesium citrate floats in as one of several supplemental forms, often suggested for constipation that pops up in pregnancy.
Here's where the story gets specific. Magnesium citrate pulls water into the intestines and softens stools. For short-term use—meaning a day or two, not weeks—obstetricians sometimes recommend it, especially if iron supplements block up the works. The U.S. Food and Drug Administration gives magnesium citrate an “over-the-counter” nod, but that doesn't erase risk altogether.
Potential Risks and Considerations
Many pregnant women experience constipation, but not all constipation calls for a laxative. Gentle fixes like water, prunes, and fiber usually land at the top of an OB’s list. Magnesium citrate clears stubborn blockages quickly, but frequent use can cause dehydration or trigger diarrhea. Dehydration poses a serious risk during pregnancy, sometimes even raising the odds of preterm contractions.
Too much magnesium from supplements may also lead to low blood pressure, muscle weakness, or abnormal heart rhythms. Under normal circumstances, healthy kidneys take care of any extra magnesium. Pregnancy, though, shifts how the body handles almost everything. Preexisting kidney issues can tilt the risk balance even more.
Research and Real-World Experience
Plenty of women find temporary relief with a single dose, followed by several glasses of water. No strong research ties short-term, recommended magnesium citrate use to direct harm for the baby. Bigger worries show up with frequent use, high doses, or combining several magnesium-rich products. Babies born with too much magnesium on board sometimes deal with breathing problems or poor muscle tone right after birth. Studies show maternal intake from food, or occasional supplement for relief, does not usually cause this.
Expecting parents face a maze of advice and marketing. Sometimes magnesium citrate does the job no prune can manage, especially after surgery or travel. Those moments call for a real conversation with a prenatal doctor—not the internet, not the neighbor who swears by this or that.
Building Safe Habits and Smart Choices
Safe magnesium intake in pregnancy comes down to the source, the dose, and the reason behind seeking it out. Always read supplement labels, measure doses carefully, and ask a doctor before reaching for the bottle. Stories of overuse, or seeing a patient come in dehydrated and contracting, remind me how quick fixes sometimes backfire. Doctors want to help patients fix uncomfortable symptoms while keeping both sides of the pregnancy equation—parent and baby—protected.
Eating magnesium-rich foods helps the most: nuts, seeds, leafy greens, beans. If constipation turns unmanageable, temporary, thoughtful use of magnesium citrate after medical advice might nudge things along. Staying hydrated, tracking symptoms, and keeping honest communication with health care providers create the safety net every pregnant person deserves.
No supplement works in a vacuum. In pregnancy, the little details stack up and matter more than ever.
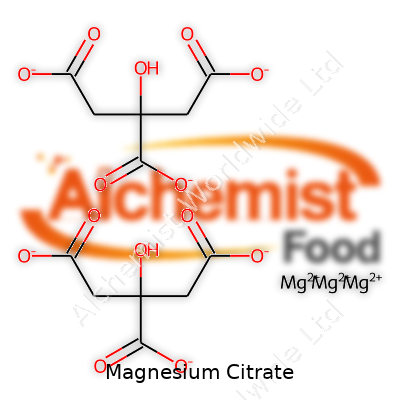
| Names | |
| Preferred IUPAC name | magnesium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Citrate of Magnesia Trimagnesium Dicitrate Citric acid magnesium salt Magnesium salt of citric acid |
| Pronunciation | /mæɡˈniːziəm ˈsɪtreɪt/ |
| Preferred IUPAC name | magnesium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Citroma Citrate of Magnesia Mag Cit Magnesium salt of citric acid |
| Pronunciation | /mæɡˈniːziəm ˈsɪtreɪt/ |
| Identifiers | |
| CAS Number | 3344-18-1 |
| Beilstein Reference | 87814 |
| ChEBI | CHEBI:31595 |
| ChEMBL | CHEMBL1201131 |
| ChemSpider | 5263290 |
| DrugBank | DB06716 |
| ECHA InfoCard | 01b7cfa4-8d4c-43bd-a0f1-1113fcb5c59d |
| EC Number | 222-093-9 |
| Gmelin Reference | 21767 |
| KEGG | C18351 |
| MeSH | D008271 |
| PubChem CID | 6047 |
| RTECS number | OM3850000 |
| UNII | 967Z5N26OD |
| UN number | UN2818 |
| CAS Number | 3344-18-1 |
| Beilstein Reference | 3597243 |
| ChEBI | CHEBI:31595 |
| ChEMBL | CHEMBL1203627 |
| ChemSpider | 5087149 |
| DrugBank | DB06778 |
| ECHA InfoCard | ECHA InfoCard: 03e0a8b7-2712-4dd8-9539-f5b5c52a371b |
| EC Number | 222-093-9 |
| Gmelin Reference | 69657 |
| KEGG | C18635 |
| MeSH | D017563 |
| PubChem CID | 65060 |
| RTECS number | OM3675000 |
| UNII | BD3536NA4J |
| UN number | UN2818 |
| Properties | |
| Chemical formula | C6H6MgO7 |
| Molar mass | 451.11 g/mol |
| Appearance | White to almost white, crystalline powder |
| Odor | Odorless |
| Density | 1.7 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -1.52 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa1 = 3.13, pKa2 = 4.76, pKa3 = 6.40 |
| Basicity (pKb) | 6.0 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.551 |
| Dipole moment | 4.05 D |
| Chemical formula | C6H6MgO7 |
| Molar mass | 214.41 g/mol |
| Appearance | White or almost white, fine, slightly hygroscopic powder. |
| Odor | Odorless |
| Density | 1.7 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -2.0 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 5.2 |
| Basicity (pKb) | 3.20 |
| Magnetic susceptibility (χ) | −15.9×10⁻⁶ |
| Refractive index (nD) | 1.343 |
| Viscosity | viscous liquid |
| Dipole moment | 2.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 365.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2312 kJ/mol |
| Std molar entropy (S⦵298) | 365.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2308 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3230 kJ/mol |
| Pharmacology | |
| ATC code | A12CC04 |
| ATC code | A12CC04 |
| Hazards | |
| GHS labelling | GHS07, Warning, H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| Precautionary statements | Keep out of reach of children. If pregnant, nursing, taking medication, or have a medical condition, consult your physician before use. Store in a cool, dry place. Do not use if seal is broken or missing. |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 1, Special: - |
| Flash point | No flash point |
| Lethal dose or concentration | LD50 (oral, rat): 2960 mg/kg |
| LD50 (median dose) | Mouse oral LD50: 4,960 mg/kg |
| NIOSH | MGCO3 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 350 mg |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. May cause skin irritation. |
| GHS labelling | GHS07, Warning, H319, P264, P280, P305+P351+P338, P337+P313 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: May cause respiratory irritation. |
| Precautionary statements | Keep out of reach of children. If you are pregnant, nursing, taking medication, or have a medical condition, consult your physician before use. Store in a cool, dry place. Do not use if seal is broken or missing. For adult use only. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | No flash point |
| Lethal dose or concentration | LD50 (oral, rat): 7010 mg/kg |
| LD50 (median dose) | 4000 mg/kg (oral, rat) |
| NIOSH | MN9600000 |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 400 mg per day |
| Related compounds | |
| Related compounds |
Magnesium sulfate Magnesium chloride Magnesium oxide Citric acid |
| Related compounds |
Magnesium carbonate Magnesium chloride Citric acid Magnesium oxide Magnesium sulfate |

