Magnesium Carbonate: From Ancient Curiosity to Modern Workhorse
Unearthing the Past: Historical Development
Humans have taken an interest in natural minerals for thousands of years, and magnesium carbonate stands out as one of those overlooked yet vital discoveries. Long before the details of atomic weights and chemical formulae arrived, folks stumbled across mineral deposits in regions like Greece and North Africa. White, chalky, and soft to the touch, these carbonates made their way into construction, pottery, and basic medicine. By the 18th and 19th centuries, with advancements in chemistry, researchers like Joseph Black and Humphry Davy pieced together its makeup: a compound blending magnesium, carbon, and oxygen. Meaningful applications began to take off following the increased purity and controlled production in the late 1800s. Factories looked for ways to manufacture durable materials for industrial, agricultural, and medical needs, often relying on magnesium carbonate in roles both subtle and transformative.
Product Overview: Unpacking the Powder
Magnesium carbonate appears to most people as a soft, white, almost fluffy powder. Its most common natural form, magnesite, reveals a mineral that craftspeople once used as a pigment and now see on climbing walls, weightlifting competitions, and in pharmaceutical bottles. This compound often fills several shoes within a lab or factory, stepping in as an anti-caking agent, an acid-neutralizer, or a lightweight filler. You’ll run into different grades, too—technical, food, and pharmaceutical—all destined for particular shelves and standards. Raw or refined, its versatility bends to what end-users expect, whether ingestible, applicable to surfaces, or sturdy enough to anchor trail markers in outdoor gear.
Physical & Chemical Properties: More than Meets the Eye
Although it looks benign at a glance, magnesium carbonate boasts a profile that encourages both widespread utility and careful respect. It refuses to dissolve in water at room temperature, which blocks absorption but increases staying power for many uses. Exposure to acids invites a brisk fizz as carbon dioxide escapes, leaving behind magnesium salts. The powder’s softness invites tactile applications, but don’t let that fool you—when heated above 350°C, it decomposes, giving up its carbon dioxide and turning into magnesium oxide, a crucial refractory material. Density, hardness, and melting point vary by hydration level. There’s light magnesium carbonate, which holds water loosely, and denser, anhydrous forms that endure rougher handling.
Technical Specifications & Labeling
Magnesium carbonate packaging must show what’s inside, with manufacturers detailing magnesium content, potential heavy metal traces, particle size, purity, and hydration status. Pharmaceutical grades need to stay almost surgically clean—well below 100 ppm of heavy metals, and strictly monitored for arsenic and iron. Food and supplement products print ingredient lists and recommended amounts. Technical grades allow minor impurities, provided they pose no risk to downstream processes. Regulatory codes from agencies in the US, Europe, and Asia guide production, stating not just what percentage magnesium should sit in the final product, but also referencing acceptable methods of analysis and identification.
Making Magnesium Carbonate: Preparation Methods
Production often starts with natural magnesite ore pulled from the earth, which then gets crushed, purified, and reacted with carbon dioxide. Chemical synthesis does the job by tossing magnesium salts—such as magnesium sulfate—into a bath with sodium carbonate. The result: magnesium carbonate precipitates out, ready for further drying and grinding. To reach different hydration states, workers remove water either with gentle heat or by letting the product air-dry over longer periods. Large-scale manufacturing relies on closed systems to prevent contamination, while labs run tests batch by batch to confirm the right balance of magnesium and carbonate ions.
Chemical Reactions & Modifications
The most straightforward reaction—tossing magnesium carbonate into a strong acid—releases a batch of bubbles as it turns into soluble magnesium salts and carbon dioxide. This reaction underpins its use in antacids. Fire a sample in a kiln, and it shrinks to form magnesium oxide, locking in heat resistance and low electrical conductivity, making it invaluable for the steel and ceramics industries. Chemists sometimes tweak magnesium carbonate by adding other ions or water molecules to yield products with altered solubility, color, or reactivity. Such modifications rely on the demands of the customer: pharmaceuticals need ultra-pure, food-safe grades, while construction might opt for coarser or blended powders.
Synonyms & Product Names
People in the business trade magnesium carbonate under a host of names: magnesite in its raw mineral form, hydromagnesite or dypingite for specific hydrated versions, and sometimes E504 in the world of food additives. Pharmaceutical labels call it magnesium carbonate, heavy or light, to spell out which hydration state sits inside. Names vary by region and application, with some blends described simply as “chalk” or, in sports circles, “gym chalk.” No matter the name, the foundational chemistry remains grounded in the union of magnesium’s cation paired to carbonate’s anion.
Safety & Operational Standards
Leaving a dusty pile of magnesium carbonate on a workshop table usually doesn’t invite dramatic hazards. Still, a responsible approach puts dust control, eye protection, and gloves on the checklist—long-term inhalation of fine powders strips comfort from lungs and can stir up mild irritation. For food and pharma applications, strict microbial and chemical controls anchor each lot. Transport codes rarely put it in the hazardous category, but workers store and handle it in dry, cool spaces to prevent caking and contamination. Regulatory authorities—FDA, EFSA, and others—impose strict limits on heavy metals like lead and arsenic.
Application Area: Where Does It Go?
Magnesium carbonate tags along in more daily products than most people notice. As an anti-caking agent in table salt and spices, it keeps shakers flowing smooth. Medicine harnesses it in antacid tablets, cramping remedies, and food supplements. Sports enthusiasts rely on it to dry sweaty palms, boosting grip on bars or stones. Construction and refractory sectors count on its heat resistance, feeding it into bricks, tiles, and fireproof coatings. Lab workers pick it up as a basic reagent, while specialty chemists experiment with newer uses in polymers and environmental cleanup. Agriculture leans on it to buffer acidic soils.
Research & Development: Digging Deeper
Current research focuses on tweaked forms of magnesium carbonate for controlled drug delivery, improved fire resistance in composites, or carbon capture applications. Scientists seek ways to lower energy footprints in its production or find alternative feedstocks, aiming to shrink environmental impact. Food scientists explore roles as texturizers or mineral enhancers in modern formulations. Collaboration between university chemists and industry engineers often leads to smarter synthesis methods, better blends with other compounds, and broader screens for impurities or performance under extreme conditions.
Toxicity Research: Getting the Facts Straight
Health authorities in the US and Europe put magnesium carbonate on the safe list for consumption in moderate amounts. Studies testing oral, dermal, and inhaled exposure in animals show limited toxicity, provided dosages don’t scale far past normal dietary intake or occupational exposure. Swallowing large quantities does invite digestive upset, especially diarrhea; people with kidney problems watch intake, since magnesium elimination faces a tougher road. Dust—if left unchecked—might irritate respiratory tracts, but no links to cancer or reproductive harm have held water in the literature. Ongoing research double-checks safety for new uses, especially nanoforms or blends not seen before in the market.
Future Prospects: Onward and Upward
Magnesium carbonate keeps turning up fresh opportunities as industries change. Carbon capture and storage draws attention, since the mineral can bind carbon dioxide under the right conditions. Lightweight composites built from processed magnesium carbonate stand on the cutting edge for green building and infrastructure. Supplement companies keep an eye on cleaner, more bioavailable forms, which means more advanced separation and purification. Environmental needs push producers towards reducing waste, closing production loops, and blending with other sustainable materials. Investment in smarter manufacturing will likely lower costs, boost purity, and cut energy use, opening more doors for this centuries-old powder in fields yet unimagined.
What is Magnesium Carbonate used for?
Beyond the Gym: Everyday Uses
Spend a bit of time in a rock climbing gym or gymnastics studio, and you’ll spot the white powder—magnesium carbonate—coating hands and equipment. It shows up wherever sweaty palms ruin grip. Folks count on it to cut down moisture, so you don’t slip on the rock face or while swinging from the bars. But magnesium carbonate goes further than sports.
Peace for the Stomach
People deal with heartburn and indigestion in all sorts of ways. For decades, antacids have borrowed from magnesium carbonate’s chemistry. Take a common chewable tablet after a meal, and you’ll find this mineral helping calm acid. It doesn’t work as fast as some others, but it has a gentle touch. No one wants stomach pain to linger. Magnesium carbonate tackles excess stomach acid and often avoids side effects that come from harsher substances.
Food and Drink
Even folks who blink at food labels probably consumed magnesium carbonate at one time. It pops up in table salt, baked goods, and powdered foods as an anti-caking agent. Salt shakers get clogged in humid kitchens, but this mineral keeps things flowing. That’s a small victory for cooks and eaters trying to shake just the right amount at the table.
Personal Experience in the Classroom
During my school years, I spent hours at the blackboard using “chalk.” Teachers would scrawl math formulas or history dates in white across green or black surfaces. Real chalk breaks easily and makes a mess. Most “chalk sticks” today come from compressed magnesium carbonate. They draw smoothly, erase easily, and don’t squeak as much as the old stuff. Even art students prefer it, since the powder blends into soft pastels.
Manufacturing and Medicine
In factories, workers use magnesium carbonate as a filler in making rubber and plastics. Product designers rely on it for paint and ceramics too. The stuff enhances hardness, adds texture, and stops clumping in powdered coatings. It’s not flashy, but it keeps wheels turning in basic industries. In medicine, doctors trust it in some pills because of its neutral taste and gentle effect on the gut. Pure magnesium carbonate doesn’t dissolve in water, which makes it a reliable carrier for certain medicines that shouldn’t get absorbed right away.
Athletes Count On It
Magnesium carbonate helps keep athletes safe. I used to play tennis on muggy summer courts, and sweaty hands turned rackets slippery. A pinch of powder boosted grip, cut down on errors, and sometimes prevented blisters. In the weight room, strict lifters can’t imagine deadlifting without a dusting of "chalk"—the mineral soaks up palm moisture and keeps barbells from twisting mid-lift. It’s simple but effective.
Looking Ahead
Magnesium carbonate works best because it’s reliable, affordable, and nearly everywhere. Some people worry about microdust in gyms, so better ventilation and safer packaging make sense. Eco-conscious folks have called for sustainable mining and more recycling of magnesium-rich materials. Consumer awareness pushes companies to clean up their sourcing, so future generations get the benefits without hidden costs. Magnesium carbonate proves that even a humble mineral can play a big role in daily life—health, industry, education, and sport all get a lift from this versatile compound.
Is Magnesium Carbonate safe for consumption?
Digging Into Everyday Uses
People eating processed foods or popping multivitamins encounter magnesium carbonate all the time. Bakeries toss it in flour to keep things fluffy, and supplement brands pack it into vitamin pills. Chalky yet tasteless, this white powder lands in the kitchen and gym thanks to its ability to soak up moisture and prevent clumping. Restaurants buy it in bulk for similar reasons—it keeps food looking fresh and easy to handle.
What Scientists Actually Know
Magnesium carbonate carries a long history of approval. Food safety authorities in the United States, Europe, and Japan all list it as safe. The Food and Drug Administration backs this up, labeling magnesium carbonate as ‘generally recognized as safe’ (GRAS) when used in normal quantities. The European Food Safety Authority goes a step further, confirming there’s no evidence showing harmful effects at usual intake levels.
Digging into research, toxicity studies date back decades. No record of people or animals getting sick from eating magnesium carbonate at levels found in food and supplements. Our bodies handle it pretty well. When swallowed, it breaks down in stomach acid, releasing magnesium ions that either get absorbed or flushed out.
Paying Attention to Who’s Eating It
Most people get way less magnesium from food additives than from nuts, whole grains, or green vegetables. For those with healthy kidneys, the body filters out any excess. Extra magnesium just leaves through urine. Athletes using magnesium carbonate for grip chalk in the gym rarely see side effects even with skin contact or accidental swallowing.
Problems start if kidneys don’t work right. People with kidney failure struggle to get rid of magnesium, so adding more—through food or supplements—can tip the balance. High levels cause nausea, muscle weakness, or irregular heartbeat. Doctors usually warn kidney patients to watch out for magnesium-based antacids or supplements, and that warning covers magnesium carbonate too.
Labeling and Regulation
Magnesium carbonate never ranks up there with food chemicals that raise alarms, like artificial dyes or trans fats. Still, watchdogs take a closer look anytime companies add minerals to processed foods. Each batch of food-grade magnesium carbonate has to meet purity standards, with limits on other minerals or contaminants like heavy metals. These rules make sure nobody ends up eating tainted chalk.
Food companies have rules to follow. Ingredients get listed on packaging. Customers with deep allergies or rare conditions can spot magnesium carbonate on the label, making it easier to steer clear if needed. The push for clearer labeling has helped some families avoid triggers, especially when buying snacks for kids.
How Much Is Too Much?
No one expects people to get their minerals from food additives. The amount added to bread flour or table salt usually sits far below daily dietary needs. Most folks pick up enough magnesium from foods like spinach or nuts. Too much from supplements or antacids, especially for people with health issues, brings risk. Teachers and trainers sometimes need to remind gymnasts or rock climbers not to eat chalk, but even there, small accidental doses slip through without trouble.
Sticking to foods and products where magnesium carbonate serves a real purpose, and keeping an eye on overall magnesium intake for those with medical conditions, helps keep things safe. For nearly everyone else, safety checks, trusted food science, and common sense keep magnesium carbonate in the clear.
What are the side effects of Magnesium Carbonate?
Why Magnesium Carbonate Gets Attention
Magnesium carbonate turns up in all kinds of places—chalk for athletes, some antacids, and even snack foods as an additive. Doctors sometimes suggest it as a supplement, especially for people who don’t get enough magnesium from food. The body counts on magnesium to keep nerves, muscles, and bones working smoothly. Still, the story doesn’t end with topping up mineral levels. Taking magnesium carbonate brings its own patch of side effects that deserve a clear look.
Digestive Roadblocks
Stomach troubles usually show up first. Most folks notice loose stools or even outright diarrhea if they use too much. The gut works like a tightrope act. Tipping magnesium balance throws things off. Upset stomach, cramps, and bloating add to the list. Even antacids with magnesium carbonate aren’t totally free of these headaches. One study published in the American Journal of Clinical Nutrition found that high doses of magnesium often mean running to the bathroom more often. Sensitive stomachs feel it fastest.
Rare But Serious Complications
Most people process out the extra magnesium they don’t need, especially with healthy kidneys. Problems start for those with kidney disease or slowed kidney function. Magnesium can start to build up, leading to something called hypermagnesemia. Signs aren’t always obvious at first—sometimes just feeling more tired or weak. If magnesium climbs too high, reflexes start to drop off, breathing slows, and the heart can get into real trouble. This risk means people with reduced kidney function should only use magnesium carbonate under medical supervision.
Unexpected Allergic Reactions
Though rare, allergic reactions happen with magnesium compounds. Rash, itching, swelling of the face or throat, and shortness of breath need a trip to the doctor right away. Allergies don’t appear in every case, but it’s worth mentioning because reactions can become serious in a hurry.
Interactions and Compounding Risks
Magnesium carbonate doesn’t keep to itself. It can clash with medications like some antibiotics, blood pressure pills, and osteoporosis drugs. In my own life, taking magnesium the same day as certain antibiotics meant less effect from the medicine. Research from Harvard Medical School backs this up—magnesium can bind with other drugs in the stomach and keep them from absorbing properly. People taking regular prescriptions should always double-check combinations with a pharmacist or doctor.
Think Before You Supplement
Choosing any supplement brings up questions of quality and need. Magnesium carbonate in food usually doesn’t cause problems, but supplements and regular use change the story. High doses rarely create more benefits and often dial up digestive problems instead. The National Institutes of Health set 350 mg per day as a safe upper limit for most adults from supplements. Getting most magnesium through dark greens, nuts, whole grains, and seeds keeps risks low unless a doctor says otherwise. Safe use matters more than just grabbing something off the shelf.
Real Solutions and Smarter Choices
People don’t have to ditch magnesium carbonate, but a little care keeps side effects from becoming a daily nuisance. Reading product labels for hidden sources helps. For anyone with chronic health issues, even over-the-counter choices are best cleared with a health professional. Gut-friendly forms of magnesium, such as magnesium glycinate, often work with fewer stomach symptoms for sensitive people. Looking out for clothes or supplements that promise “extra energy” with magnesium works best paired with trusted advice, not just marketing speak. At the end of the day, magnesium gives benefits, but balance and sensible choices keep the body working its best.
How should Magnesium Carbonate be stored?
Understanding Magnesium Carbonate
Magnesium carbonate appears in gyms as chalk for weightlifters, and in laboratories for various experiments. At home, it might show up as an ingredient in antacids or supplements. You’ll notice it’s a white powder, lightweight, and it turns clumpy with moisture. Most people overlook storage, but those who have seen ruined gym chalk or spoiled lab chemicals know how much storage matters.
Environmental Factors Cause Real Problems
Magnesium carbonate doesn’t dissolve easily in water, but let it sit in a damp room too long and every scoop will cake together. Humidity sneaks up on open containers, turning what should be a loose powder into stubborn bricks. I’ve watched this happen in old gyms where nobody bothered with lids, as well as in kitchens where containers sat near the sink. Moisture doesn’t just change the texture, it affects purity and makes dosing unreliable.
Safe Storage Starts With Dryness
Everyone benefits from a dry, cool spot away from bathrooms or dishwashers. Think high shelves, far from steaming kettles, steamy showers, or windows where rain gets blown inside. Seal it up tight after every use—choose screw-top jars or plastic tubs with good snaps. Bags with weak zip seals let air inside. Silica gel packets, the kind found in new shoes or electronics, can be tossed into your storage container for extra insurance against humidity. This tiny step saves a lot of frustration later.
Clean Containers Make a Huge Difference
Old containers with food crumbs, soap residue, or dust aren’t safe. I once saw leftover flour in a supposedly “clean” chalk jar—nobody noticed until the contents wouldn’t come out of the spout. Always wash storage containers with hot water, rinse, and let them dry completely before pouring in new powder. Skipping this leaves behind unwanted chemicals or bacteria, which anyone using magnesium carbonate as a supplement really can’t ignore.
Keep It Out of Reach—Curious Kids and Pets Get Into Everything
Children and pets always seem to find their way into cupboards. Magnesium carbonate is generally safe for most adults in supplements or as gym chalk, but it isn’t meant for unsupervised consumption. It’s smart as a parent, pet owner, or just a careful person to store chemicals high and out of sight, ideally in a locked cabinet if kids roam the house.
Label Everything Clearly
Labeling prevents mix-ups. More than one person in my circle has grabbed the wrong container for baking or spilled chalk into a drink. A marker and a strip of tape, or a printed sticker, work fine as long as the text stands up to daily handling. Add the purchase date somewhere on the label if you keep large quantities—stale stock might not hurt you, but why risk degraded material?
Rotating Stock and Checking for Contamination
Buy in quantities you’ll use within a year. Before tipping the jar or bag, take a look inside for odd colors, strange smells, or water droplets. If it looks or smells off, start fresh. Safe storage isn’t just about dryness; it includes basic checks to avoid problems down the line.
Protecting Value and Safety
Careless storage shortens the shelf life and messes up the consistency, whether you’re mixing supplements or prepping workout supplies. Taking a few moments to seal, label, and lift containers out of reach means fewer headaches and safer usage every time.
What is the recommended dosage for Magnesium Carbonate?
Magnesium’s Role in the Body
You probably see magnesium on supplement labels at the store and wonder whether it makes a real difference. Magnesium supports muscle function, nerve signals, the production of energy, and helps keep the heart’s rhythm steady. Many people don’t get enough magnesium through food, and magnesium carbonate shows up in pills, powders, and even as a chalky additive in sports drinks.
Dosage for Adults and Children
A lot of us want a direct answer — how much magnesium carbonate is safe, and how much works? For healthy adults, the National Institutes of Health sets the upper daily limit at about 350 mg of magnesium in supplement form. Magnesium carbonate on a label refers to the compound form, not the elemental magnesium itself. It contains about 29% magnesium by weight. So, a 1000 mg tablet of magnesium carbonate delivers about 290 mg of pure magnesium.
Teenagers and younger children have lower recommended maximums. For instance, kids aged 9 to 13 can take up to 240 mg daily from supplements. Too much magnesium at once often means a trip to the bathroom, as it can cause diarrhea and cramps. Sticking to these limits, and getting most of it from food, keeps things simple.
Why Getting Enough Magnesium Matters
I learned the importance of magnesium from family experience. My uncle’s nighttime muscle cramps vanished after his doctor suggested magnesium. Studies back this up: magnesium supports over 300 enzyme reactions in the body. Low levels sometimes show up as muscle cramps, fatigue, and even high blood pressure.
Not everyone needs a supplement. Foods like pumpkin seeds, almonds, and leafy greens do a great job, offering magnesium in a way the body absorbs easily. Supplements like magnesium carbonate help for those who skip out on these foods regularly or have a condition that lowers magnesium.
Discussing Health Conditions
Folks with kidney problems need extra caution. The kidneys handle magnesium balance. If they slow down, magnesium can build up, causing more harm than good. In my years writing about nutrition, I’ve heard doctors stress the need for blood tests and medical supervision in such cases.
Certain medications like diuretics or proton pump inhibitors can lower magnesium, sometimes requiring careful supplementation. Anyone with ongoing health issues should go over magnesium plans with their care team.
Choosing How to Supplement
Magnesium carbonate works best dissolved in water or mixed with food for easier absorption. Some forms absorb better, like magnesium citrate, but carbonate’s gentle action suits sensitive stomachs or those using it to relieve occasional constipation.
Reading supplement labels makes a difference. Look for products listing both the compound amount and the elemental magnesium. Quality brands stick close to science-backed dosages and steer clear of unnecessary fillers.
Better Habits for Magnesium Balance
No one likes taking unnecessary pills. My advice: focus on diverse meals, aim for more whole grains and seeds, and use supplements as a backup, not the main event. Routine health checkups give you a sense of whether your body stores enough minerals. If magnesium stays low despite a good diet, a healthcare provider helps sort out why.
Sharing real-life experiences, watching for symptoms, and sticking with evidence-based daily amounts keep magnesium—whether from carbonate or food—safe and useful.
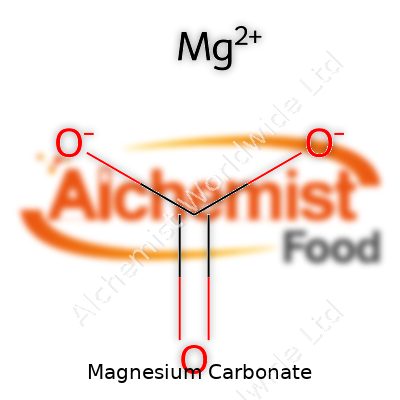
| Names | |
| Preferred IUPAC name | Magnesium carbonate |
| Other names |
Magnesite Magnesium salt Carbonic acid magnesium salt MgCO3 |
| Pronunciation | /maɡˈniːziəm ˈkɑːbəneɪt/ |
| Preferred IUPAC name | Magnesium carbonate |
| Other names |
Magnesia alba Magneisum mono carbonate Magnesium(II) carbonate |
| Pronunciation | /maɡˈniːziəm ˈkɑː.bə.neɪt/ |
| Identifiers | |
| CAS Number | 546-93-0 |
| Beilstein Reference | 127681 |
| ChEBI | CHEBI:32599 |
| ChEMBL | CHEMBL1201742 |
| ChemSpider | 5531 |
| DrugBank | DB09462 |
| ECHA InfoCard | ECHA InfoCard: 029-019-00-4 |
| EC Number | 209-943-4 |
| Gmelin Reference | Gmelin 1618 |
| KEGG | C06839 |
| MeSH | D008279 |
| PubChem CID | 24856 |
| RTECS number | OM3850000 |
| UNII | 7Z6E8C4O9S |
| UN number | UN1415 |
| CompTox Dashboard (EPA) | DJ8N69D36M |
| CAS Number | 546-93-0 |
| Beilstein Reference | 3596856 |
| ChEBI | CHEBI:32599 |
| ChEMBL | CHEMBL1201113 |
| ChemSpider | 5285 |
| DrugBank | DB09280 |
| ECHA InfoCard | 03edb6a3-23d9-44a6-a48a-c50e4e318f8e |
| EC Number | 208-915-9 |
| Gmelin Reference | 80551 |
| KEGG | C06636 |
| MeSH | D008271 |
| PubChem CID | 109333 |
| RTECS number | OM3850000 |
| UNII | 7Z6EE20A7M |
| UN number | UN 1457 |
| Properties | |
| Chemical formula | MgCO3 |
| Molar mass | 84.313 g/mol |
| Appearance | White, odorless powder |
| Odor | Odorless |
| Density | 2.16 g/cm³ |
| Solubility in water | Insoluble |
| log P | -0.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.8 |
| Basicity (pKb) | 6.23 |
| Magnetic susceptibility (χ) | +35.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.511 |
| Dipole moment | 0 Debye |
| Chemical formula | MgCO3 |
| Molar mass | 84.313 g/mol |
| Appearance | White, odorless powder |
| Odor | Odorless |
| Density | 2.96 g/cm³ |
| Solubility in water | Insoluble |
| log P | -0.79 |
| Vapor pressure | Vapor pressure: Negligible |
| Acidity (pKa) | 6.8 |
| Basicity (pKb) | 6.77 |
| Magnetic susceptibility (χ) | +120·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.509 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 65.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1095.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1113 kJ mol⁻¹ |
| Std molar entropy (S⦵298) | 65.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1095 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −1111 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A02AA01 |
| ATC code | A02AA01 |
| Hazards | |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Autoignition temperature | 350 °C (662 °F) |
| Lethal dose or concentration | LD50 (Oral, Rat): > 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 8,500 mg/kg |
| NIOSH | SCG |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 350 mg/day |
| Main hazards | May cause respiratory irritation; may cause eye and skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Precautionary statements | P260: Do not breathe dust/fume/gas/mist/vapours/spray. P262: Do not get in eyes, on skin, or on clothing. P264: Wash hands thoroughly after handling. P280: Wear protective gloves/protective clothing/eye protection/face protection. |
| NFPA 704 (fire diamond) | 1-0-1-W |
| Autoignition temperature | 350 °C |
| Lethal dose or concentration | LD50 oral rat 8,500 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 8,500 mg/kg |
| NIOSH | MGCO |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 420 mg |
| Related compounds | |
| Related compounds |
Magnesium oxide Magnesium hydroxide Magnesium bicarbonate Calcium carbonate Sodium carbonate |
| Related compounds |
Magnesium oxide Magnesium hydroxide Magnesium bicarbonate Calcium carbonate Sodium carbonate |

