L-Malic Acid: A Deep Dive Into a Versatile Ingredient
Historical Development
People might think malic acid came up with food technology’s latest trends. The truth goes back a few centuries. Sweden’s Carl Wilhelm Scheele first isolated malic acid from apple juice in 1785, driving a wedge into the field of organic acids. Chemists and industry players started realizing its strengths as food science matured. Over the decades, companies built manufacturing routes by harnessing old-school fermentations using fungi, then refining chemical synthesis methods to scale for commercial use. As snacks, drinks, and processed foods multiplied throughout the twentieth century, demand for malic acid rose, turning it from a curiosity to a staple in global ingredient lists.
Product Overview
Today, L-malic acid looks like a white, crystalline powder. It tastes tart — sharper than citric acid but less biting than fumaric acid. It dissolves smoothly in water and boosts the flavors of fruits, candies, and beverages. Many companies label L-malic acid as a pH adjuster or flavor enhancer. You’ll spot its name on labels for everything from chewing gum to sports drinks and even wine. Natural sources include apples, cherries, and blackberries — but the food industry relies almost entirely on manufactured L-malic acid for consistency and purity.
Physical & Chemical Properties
L-malic acid packs a punch at the molecular level. Its chemical formula, C4H6O5, hints at two carboxylic acid groups and one hydroxyl group. This combination explains its acidity and water solubility. Its melting point hovers around 131°C; it goes well with other acids and dissolves quickly in warm or cold water. The substance resists breakdown under typical food processing conditions. Occasionally, I see malic acid used in formulations to keep flavors fresh after pasteurization or even freezing. The distinctive L-isomer flows more naturally in human biochemistry than D-malic acid, and that’s what ends up in most commercial products.
Technical Specifications & Labeling
Companies measure L-malic acid in terms of assay (usually above 99% purity), heavy metal limits, moisture content, and the finer details listed in global pharmacopeias. Regional regulations differ, but European, American, and Asian agencies each require clear nomenclature and batch traceability. E296 is the renowned food additive code across the EU, while the U.S. FDA lists it as Generally Recognized As Safe (GRAS). Labels on finished goods don’t always say “L-malic acid”; they might use other synonyms, especially in international exports. Still, clarity in source, purity, and potential allergens builds consumer trust.
Preparation Method
Industrial production leans heavily on double fermentation processes, often involving fungal strains like Aspergillus species. Sugar substrates such as glucose get converted into L-malic acid, which further purifies through crystallization and filtration. Chemical synthesis works in a pinch, especially through hydration of maleic anhydride, but fermentation gets the nod for environmental sustainability and superior isomer purity. As a result, major ingredient suppliers invest millions in optimizing bioreactors, cultivation media, and process yields.
Chemical Reactions & Modifications
Once you have pure L-malic acid, the chemistry opens up. It reacts easily with bases to form salts, such as calcium or sodium malate, used in sports supplements and potassium fortification. Heating can dehydrate malic acid into maleic or fumaric acids, both with their own roles in food and pharma. Esterification creates various flavor additives. In the lab, malic acid can serve as a starting material for chiral syntheses or as an intermediate to more complex natural compounds. Some food technologists use it to help push preservative reactions or tie up unwanted metal ions that could spoil taste.
Synonyms & Product Names
Somewhere on a label, you might see “apple acid” or “hydroxybutanedioic acid” in place of L-malic acid. Across global markets, other familiar names show up: α-hydroxysuccinic acid, LE-malic acid, and even E296, depending on jurisdiction. For industry buyers, product codes and trade names — often tied to purity grade or particle size — matter more than the formal IUPAC nomenclature. All this means a manufacturer or chef can recognize what they’re getting, no matter the language or code used.
Safety & Operational Standards
L-malic acid often gets grouped as a food-safe ingredient, but that claim rests on rigorous production audits and batch testing. Facilities must meet ISO or GMP requirements, with raw material traceability and contaminant control keeping the risk of impurities or allergenic byproducts at bay. Worker safety needs real training, as exposure to concentrated acid powders could cause skin or respiratory irritation. Modern plants operate under tightly regulated industrial hygiene and process automation, which dramatically reduces spills or dosing errors. Proper storage — cool, dry, and sealed containers — preserves shelf life and quality.
Application Area
You’ll spot L-malic acid in everyday foods: hard candies, sodas, preserves, and pie fillings brighten up with its tang. It takes the lead in low-calorie sweetener blends pretending to be fruit-forward. In winemaking, this acid helps balance harsh flavors or triggers malolactic fermentation for smoother reds. Sports nutrition brands rely on malates to buffer acidity and promote quick ATP production for endurance. Pharmaceuticals use it as a pH modifier to stabilize active ingredients. Even cleaning products sneak in malic acid as a chelating agent for mineral deposits. Cosmetics grab it as a gentle exfoliant, contributing to the boom of fruit-acid-based skincare.
Research & Development
R&D teams keep seeking higher-yield fermenters, greener feedstocks, and continuous process monitoring. Genetic engineering pushes boundaries, aiming for improved fermentation strains or more robust cell-free synthesis. Analytical chemists develop more sensitive assays for trace contaminants or stereoisomer purity, keeping up with regulations that grow stricter every year. The interplay between malic acid and flavor compounds gets plenty of academic attention, especially in shelf-stable beverages or novel plant-based products. Researchers now look at using waste biomass—citrus peels, potato skins—as alternative feedstocks to make the process even leaner and less resource-intensive.
Toxicity Research
Plentiful animal and human studies track malic acid’s safety profile. At everyday food-use levels, it passes as non-carcinogenic and rapidly metabolized by human enzymes. High dosages may cause minor digestive discomfort, but no lasting toxicity pops up, even over repeated exposures. Researchers occasionally tie malic acid to improved mitochondrial function in fatigue studies, though claims need clearer evidence. Regulatory panels, including JECFA and EFSA, continue to evaluate new experimental data, setting rigorous acceptable daily intake standards to preempt any overlooked long-term effects.
Future Prospects
Demand keeps rising as the global palate shifts to tangy, “authentic” fruit tastes. Sustainable bioproduction sits front and center for future supply chains. New biotech startups pitch improved fermentation strains or closed-loop reactors that slash water and energy use. As plant-based meat and dairy expand their market, L-malic acid finds fresh roles as a flavor booster and acidulant. High-end winemakers and craft beverage artisans look to malic acid to fine-tune product profiles without synthetic aftertastes. International trade, eco-labeling demands, and food fraud detection all keep ingredient transparency and traceability under the spotlight. All signs show L-malic acid will remain a fixture across industries, its role evolving as science, farming, and consumer tastes bring new challenges and opportunities every season.
What is L-Malic Acid used for?
A Closer Look at L-Malic Acid’s Real-World Uses
Ask any food technologist about the sourness in green apples or the tang in sour candy, and they’ll throw around the term “malic acid.” L-malic acid plays a bigger role than just shaping flavors. Living in a world flooded with processed snacks, fresh drinks, and specialty health products, you’ll stumble onto its label more often than you’d expect.
The Food and Beverage Game Changer
Spend time working in a commercial kitchen or factory floor, and you’ll spot powdered L-malic acid as a staple. Chefs and manufacturers reach for it to punch up flavors that need a crisp, tart kick — think fruit chews, lemonade, jams, or even reduced-calorie sodas. Since L-malic acid gives a sharp, persistent sourness without overwhelming sweetness, it helps balance everything from shelf-stable applesauce to teas. There’s also a practical reason folks in food production use it: it helps keep food fresher by keeping the pH low, slowing down the growth of bacteria and molds. In practice, this means longer-lasting pies, juices, or even fruit fillings.
Taking L-Malic Acid Beyond the Kitchen
The health and fitness crowd will recognize it in energy drinks, chewable vitamin tablets, or sports supplements. It plays a critical part in giving some of these products a more refreshing taste—masking chalky or off-putting flavors from minerals, protein, or leafy greens. Since it naturally occurs in the body as part of the Krebs cycle (that’s the energy-making engine inside every cell), supplement brands tout L-malic acid as one more ingredient that supports stamina and recovery. Sports nutrition brands highlight its use, although research still explores just how much it impacts muscle fatigue or performance.
Pharmaceutical and Personal Care Uses
L-malic acid steps into a different role in pharmaceuticals. It adjusts the acidity of syrups, lozenges, or effervescent tablets so medicines go down smoother and taste bearable. In toothpaste and mouthwash, it brings a soft tartness, but it also helps maintain a healthy oral environment; it can assist with tartar control. Some dermatologists recommend skin peels containing L-malic acid for gentle exfoliation, especially for those who might get irritation from stronger acids.
Why L-Malic Acid Matters
With stricter labeling laws and the demand for clean, honest ingredients, consumers have gotten savvier. The history of L-malic acid traces back to real foods like apples and cherries. That origin story gives it favor over synthetic alternatives. As plant-based products grow, more companies seek naturally fermented L-malic acid, steering away from versions made with petrochemicals.
Food safety watchdogs, including the FDA and EFSA, label L-malic acid as safe when used correctly. Manufacturers still need to pay attention to how much ends up in each product. Too much sourness or acidity can turn beloved foods into pucker-inducing mistakes, and in rare cases, trigger sensitivities in some people. Building transparency around source, processing, and how it fits into foods and supplements helps build real trust with shoppers.
Paving the Way for Responsible Use
Having spent time talking with food scientists, it’s clear that simple testing and honest labeling practices make a real difference. Industry professionals need to keep refining how and why they use L-malic acid — not just to hit a sour note, but to elevate quality, extend shelf-life, and meet changing dietary trends. Good training and oversight in factories matter as well; every worker, from QA analyst to line supervisor, should know why an acidulant like this matters and how to use it right.
Is L-Malic Acid safe for consumption?
What L-Malic Acid Does in Food
Go to any supermarket and check the label of a tart candy, a fruit-flavored drink, or even a pre-mixed salad dressing. You’ll spot “malic acid” sitting among the ingredients. L-malic acid, found naturally in apples and cherries, boosts tartness. Food scientists lean on it for the crisp, sour taste that hits your tongue in lemonade or sour gummies. Some folks wonder if consuming something that sounds this “chemical” can really be fine for the body.
How the Body Handles L-Malic Acid
L-malic acid doesn’t just come from a factory. Your own cells make it. During exercise, muscles break down sugars for energy in a series of steps scientists call the Krebs cycle, and malic acid is right in the middle of it. So eating foods with L-malic acid really means you’re swallowing something the body expects. The acid gets absorbed in the gut and goes straight to work fueling your cells or breaking down into water and carbon dioxide. Anyone eating apples, tomatoes, or even grapes gets a natural dose every time.
What Researchers Say About Safety
Plenty of food safety tests and reports from groups like the U.S. Food and Drug Administration and the European Food Safety Authority consider malic acid “Generally Recognized As Safe.” No big health group suggests people should limit exposure to it in reasonable amounts. Even in higher doses, short studies in healthy adults only reported mild stomach irritation, the sort you’d get after too much citrus or vitamin C.
Who Might Want to Take Care
Some people do notice discomfort after too much sour candy or high-acid drinks. Folks with sensitive stomachs, acid reflux, or ulcers might react to any kind of acid, not just L-malic. For them, less intense, low-acid foods could make sense. But for most people, including children, the occasional splurge on malic acid-infused treats won’t set off health alarms.
Natural vs. Added Sources
The body doesn’t really care if malic acid landed in a salad from a green apple or got sprinkled in during food processing. It reacts the same way in both cases. The difference really comes from what surrounds it. Additives in processed food can come with heavy sugar, extra salt, and artificial dyes. That’s the bigger health question, not necessarily malic acid on its own.
Finding a Balance in the Diet
A good diet draws on plenty of fruits and vegetables, which bring malic acid along with beneficial fiber and vitamins. Focusing on whole foods limits how much added acid you’ll pick up from other sources. For anyone who loves sour flavors or sports drinks, it helps to check labels and not go overboard. Moderation keeps everything in line.
The Takeaway for Everyday Eating
L-malic acid shows up naturally in much of what most people eat and runs a key job inside every living cell. Scientific reviews back up its safety for nearly everyone. A balanced diet, full of real produce instead of heavy-handed candy or soda, leaves little reason to worry about what malic acid brings to the table. If sour candy gives you heartburn, stick with apple slices for that tart kick instead.
What are the health benefits of L-Malic Acid?
What L-Malic Acid Brings to the Table
L-Malic acid pops up in everyday foods like apples, cherries, and grapes. This is the stuff behind that tart kick your tongue catches in Granny Smith apples. Its job doesn’t end with flavor. Science says it plays a real role in how our bodies make energy. The compound helps at the cellular level, keeping things like stamina and muscle recovery on track. Those late-afternoon slumps after lunch feel a lot less rough when your cells are burning fuel efficiently, and malic acid helps with that.
Digestive Health and Natural Detox
Digestive issues tie up millions of people. Malic acid gives a boost here as well. It helps the stomach break down food so nutrients get absorbed effectively. By helping the liver process toxins, malic acid supports natural detoxification without putting stress on your system. Fresh fruit snacks don’t just taste good—they pack helpful compounds like this one, easing the work your digestive tract does every day.
Pain Support for Those with Fibromyalgia
Chronic pain, especially from conditions like fibromyalgia, can bring even daily chores to a standstill. Studies show that L-malic acid, paired with magnesium, might help reduce muscle discomfort and fatigue. I’ve seen people manage their symptoms with dietary tweaks—more fruits, magnesium-rich nuts—and notice a real pick-up in how they feel. Every win counts when pain chips away at your quality of life. Choice of food isn’t just a matter of taste; it can shift pain levels, stamina, even outlook.
Keeping Oral Health in Check
Teeth and gums always need attention, yet sugary snacks and busy routines often get in the way of good habits. Malic acid has been researched for its role in dental health. It boosts saliva production, helping clean your mouth and resist bacteria growth. I’ve used natural gums and rinses with malic acid for a mild, refreshing lift that doesn’t bring the harshness or sugar other sweets do. It isn’t a replacement for brushing, but smart choices throughout the day add layers of support that show in dentist visits.
Boosting Exercise Performance
Athletes and anyone aiming to get active chase ways to bounce back from workouts. Malic acid helps with the breakdown of lactic acid, a byproduct that builds up during exercise and causes sore muscles. You don’t have to be running marathons to feel the impact. Even yard work or a long bike ride can bring aches the next day. Topping up with malic acid, through fruit or supplements, lines things up for less post-activity drag.
Sourcing Reliable L-Malic Acid
Trust is at the center of wellness choices. Not every supplement offers consistent quality, so checking labels and choosing known manufacturers can make all the difference. Registered dietitians recommend looking at third-party testing and opting for naturally derived options where possible. This path lets you skip unnecessary fillers and synthetic ingredients that sometimes cause more harm than good.
Finding a Place for Malic Acid in Your Routine
Health isn’t one-size-fits-all. Some notice an improvement just by swapping apples or cherries into their snacks or adding leafy greens to meals. Others discuss with their healthcare provider if malic acid supplements make sense for chronic pain, fatigue, or exercise. Taking stock of how you feel day-to-day, getting real about symptoms, then picking small, sustainable shifts in diet delivers results over time. Wellness works best as a steady, thoughtful process rather than a dramatic overhaul.
How should L-Malic Acid be stored?
Understanding L-Malic Acid’s Sensitivity
L-Malic acid may sound like a mouthful, but it shows up in smart places: think apples, grapes, and several processed foods. At home or in a work environment, storing this white crystalline powder requires some diligence. Experience tells me that even a small slip in its storage conditions can dull its flavor, reduce its effectiveness, or even cause it to clump up from absorbing moisture. Since L-malic acid supports a range of functions in the food, beverage, and supplement industries, keeping it fresh isn’t just a technicality—it’s a necessity.
Common Threats: Moisture, Light, Heat, and Air
The big trouble for L-malic acid comes from moisture in the air. Anyone who’s opened a bag after a rainy week and found clumped powder knows what happens. L-malic acid pulls in water easily and can become sticky pretty quickly. Mold is a rare but possible risk if the powder sits damp for too long. Heat also shortens its shelf life. Temperatures above 30°C (about 86°F) often encourage the acid to break down, which can hurt its typical tart profile. Air exposure brings another problem—freshness fades, and impurities from the environment sneak in.
Choosing the Right Container
A good, airtight container makes all the difference. I’ve learned that hard plastic HDPE jars, glass bottles with secure tops, or double-sealed aluminum bags go a long way. After every use, seal the container tightly. If you’re working with leftovers from a bulk package, break it up into smaller, opaque containers. This slows down how often the original supply gets opened and exposed. Some brands ship L-malic acid in resealable pouches lined with moisture-blocking film; using those keeps things simple.
Storage Environment Matters
Reliable storage spots keep temperature steady and humidity low. Dry, cool spaces—such as a pantry, laboratory cabinet, or storeroom—work well. Avoid storing L-malic acid near dishwashers, sinks, sunny windows, or radiators. These spots often trap moisture or see temperature swings, eroding the acid’s quality and shelf life.
Labeling and Rotation
Accurate, up-to-date labeling prevents confusion. Every new container gets a date of opening and the original manufacturer’s expiration date, based on my routine. This makes it easy to rotate older batches to the front and catch signs of old, caked-up powder before it ends up in a product or recipe. If you notice an odd smell, unusual color, or any signs of contamination, it’s safer to discard the batch.
Small-Scale Versus Commercial Storage
Home kitchens usually go through smaller quantities, and foil-lined spice jars or well-sealed glass jars get the job done. Workplace settings demand more rigorous controls. Food plants and supplement manufacturers might use food-grade drums with desiccant packets inside to absorb stray moisture. Quality checks and records also help track downside events like accidental spills or packaging faults.
Simple Habits, Big Difference
Paying consistent attention to these details keeps L-malic acid intact, both in taste and function. My own experience with ingredient storage shows small habits—fast resealing, picking stable storage places, avoiding wet scoops—make a big impact on keeping powdery acids in their prime for longer.
Looking at the Bigger Picture
Food safety experts recommend periodic cleaning of storage areas and containers. Leftover residues or spilled acid can attract pests or kickstart unwanted reactions. Regular stock checks allow for early intervention, and well-maintained inventory logs keep chaos at bay. Keeping these habits in place gives anyone relying on L-malic acid reliability every time the container is opened.
Is L-Malic Acid suitable for vegans and vegetarians?
What L-Malic Acid Really Is
L-Malic acid shows up on all sorts of food ingredient lists. It gives tartness to sour candies, adds tang to fruit juices, and boosts flavor in packaged baked goods. Some folks spot it on the back of a label and wonder: is it animal-based, or does it fit a vegan or vegetarian lifestyle?
Sources and Manufacturing
Naturally, malic acid occurs in fruit. Apples, especially, pack a good amount of it — gives them a distinct sharpness. Commercial production tends to favor fermentation. Here, certain microorganisms convert sugars, producing L-malic acid in an environment checked for purity and safety. Older methods turned to chemical synthesis, but fermentation produces the “L” isomer, which people’s bodies handle easily.
Many manufacturers use sugar sources such as corn or sugar beets, along with specific strains of bacteria. There’s rarely animal input in the core process. I’ve looked through several industry technical datasheets and food chemistry guides, and haven’t come across a standard process that draws from animal products. Still, cross-contamination can’t be totally ruled out in less-controlled environments. Certified vegan brands usually put out statements about their supply chain to settle those concerns.
Food Additive Regulations and Labeling
Groups like the U.S. Food and Drug Administration and the European Food Safety Authority list malic acid as Generally Recognized as Safe. No animal-derived processing aids show up in legal requirements or typical food science textbooks. If a company wants to call something vegan, it’s under more scrutiny than ever. Third-party vegan labels drive trust.
Potential Red Flags
Not every food company carries the same standards. L-malic acid itself rarely sees animal sources, but the end product that contains it sometimes does. Think of gelatin in chewy sweets or dairy in yogurts. Ingredient lists won’t say where each substance comes from. Emailing a company or checking for vegan logos on the package gives extra peace of mind.
Sometimes, less-transparent suppliers might process malic acid using filtration systems that have animal-derived parts (like bone charcoal in some sugar refining). I haven’t seen strong evidence supporting this risk for malic acid, but it serves as a reminder: food production gets complicated fast, especially with mass manufacturing.
Importance for Vegans and Vegetarians
Diet shapes identity and health. People putting in the effort to stick to a vegan or vegetarian approach deserve clear information and honest labeling. Malic acid makes its way into foods that seem plant-based at a glance, so it’s worth asking questions and expecting honest answers. Most mainstream malic acid comes from plant sugars through microbial fermentation, so the risk stays low.
A few companies help out by publishing documentation of their production methods. Ingredient transparency isn’t just helpful; it fosters trust. Manufacturers benefit from being upfront, and shoppers feel more confident making choices that fit their values.
Building Trust Through Certification and Communication
Personal experience as someone who checks every label: nothing feels better than seeing a clear “vegan” or “vegetarian” symbol right on the box. Brands that pursue reputable certification earn customer loyalty. For anyone uncertain about a product, reaching out to manufacturers often gets the quickest, most accurate response.
L-malic acid can fit into vegan and vegetarian lifestyles, especially if shoppers keep a close eye on extra ingredients and sourcing details. In a world full of food choices, sticking to one’s values sometimes boils down to attention, research, and a bit of healthy skepticism.
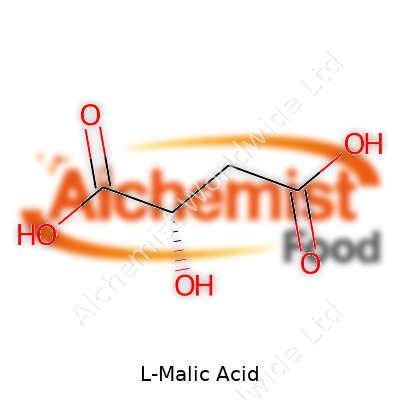
| Names | |
| Preferred IUPAC name | (2S)-2-hydroxybutanedioic acid |
| Other names |
(S)-Malic acid L-2-Hydroxysuccinic acid Apple acid Hydroxybutanedioic acid L-Malate |
| Pronunciation | /ˈɛl ˈmælɪk ˈæsɪd/ |
| Preferred IUPAC name | (2S)-2-hydroxybutanedioic acid |
| Other names |
Apple acid L-2-Hydroxysuccinic acid Hydroxybutanedioic acid Malic acid (L-form) L-MAE |
| Pronunciation | /ˈɛl ˈmælɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 97-67-6 |
| Beilstein Reference | 82222 |
| ChEBI | CHEBI:25115 |
| ChEMBL | CHEMBL682 |
| ChemSpider | 55384 |
| DrugBank | DB01360 |
| ECHA InfoCard | 100.067.841 |
| EC Number | EC 200-293-7 |
| Gmelin Reference | 6078 |
| KEGG | C00149 |
| MeSH | D008287 |
| PubChem CID | 525 |
| RTECS number | OJ7896000 |
| UNII | JKM4C9S32W |
| UN number | UN1789 |
| CAS Number | 97-67-6 |
| Beilstein Reference | 82163 |
| ChEBI | CHEBI:17896 |
| ChEMBL | CHEMBL12311 |
| ChemSpider | 776 |
| DrugBank | DB03702 |
| ECHA InfoCard | ECHA InfoCard: 100.007.311 |
| EC Number | EC 200-293-6 |
| Gmelin Reference | 7876 |
| KEGG | C00149 |
| MeSH | D008285 |
| PubChem CID | 525 |
| RTECS number | OJ7875000 |
| UNII | 817L1N4CKP |
| UN number | “UN1789” |
| Properties | |
| Chemical formula | C4H6O5 |
| Molar mass | 134.09 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.601 g/cm³ |
| Solubility in water | Miscible |
| log P | -1.26 |
| Vapor pressure | <0.1 hPa (20 °C) |
| Acidity (pKa) | 3.40 |
| Basicity (pKb) | 3.22 |
| Magnetic susceptibility (χ) | -13.2×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.585 |
| Dipole moment | 2.72 D |
| Chemical formula | C4H6O5 |
| Molar mass | 134.09 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.601 g/cm³ |
| Solubility in water | Miscible |
| log P | -1.26 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.40 |
| Basicity (pKb) | 3.22 |
| Magnetic susceptibility (χ) | -9.9·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.554 |
| Dipole moment | 2.6957 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 166.4 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -886.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −1348.3 kJ/mol |
| Std molar entropy (S⦵298) | 157.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -886.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1341.7 kJ/mol |
| Pharmacology | |
| ATC code | A16AA12 |
| ATC code | A16AA12 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 99 °C |
| Autoignition temperature | 220 °C |
| Lethal dose or concentration | LD50 (oral, rat): 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1600 mg/kg |
| NIOSH | SN1225000 |
| PEL (Permissible) | 200 mg/m³ |
| REL (Recommended) | 400 - 2400 mg |
| IDLH (Immediate danger) | Unknown |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 220°C |
| Autoignition temperature | 220°C |
| Lethal dose or concentration | LD50 (oral, rat): 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (rat): 3,200 mg/kg |
| NIOSH | MA8300000 |
| PEL (Permissible) | PEL: 5 mg/m³ |
| REL (Recommended) | 100 mg/kg |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Fumaric acid Succinic acid Maleic acid Tartaric acid Citric acid |
| Related compounds |
Fumaric acid Succinic acid Tartaric acid Malonic acid Maleic acid Citric acid |

