L-Lysine Dihydrochloride: A Close Look at Its Journey, Properties, and Outlook
Historical Development
Thinking back to the rise of amino acid technology, L-Lysine dihydrochloride tells a story rooted in human effort to understand and shape nutrition. Chemists in the early 20th century started isolating lysine to address shortcomings in protein nutrition, as many staple crops lacked enough of this essential amino acid. The commercial push came in the 1950s, as fermentation technology unlocked practical mass production. When I visit a feed mill and look at the ingredient lists, I see how much the industry leaned on L-lysine to plug those nutritional gaps. Poultry and swine production, in particular, jumped forward in efficiency thanks to this development. Over the decades, continual improvements in microbial fermentation, especially with strains of Escherichia coli and Corynebacterium glutamicum, shifted L-lysine from a niche product to a lifeline for global protein supply.
Product Overview
Today's L-Lysine dihydrochloride stands as a standard material in animal nutrition and human supplements. As the hydrochloride salt of lysine, it packs better solubility and stability than the base amino acid. Suppliers focus on content accuracy—usually promising 98.5% purity or higher. In the feed industry, this product bolsters cost-efficiency by allowing tailored amino acid balancing in every ration. Anyone working in livestock nutrition sees the marked difference in animal growth and health once lysine supplements become part of the daily mix.
Physical and Chemical Properties
Pure L-Lysine dihydrochloride appears as a white or nearly white crystalline powder. It feels a bit gritty and tastes sharply acidic. I remember the tang lingering if a bag splits during handling. The compound dissolves readily in water, giving a clear solution, which works out best for mixing into feed, drinking water, or pharmaceutical preparations. Its melting point sits around 263°C, beyond which decomposition takes over. The molecular formula C6H14N2O2 · 2HCl and a molecular weight of 242.2 g/mol distinguish it on any lab inventory sheet. Chemists appreciate its dual amine structure: one free, one in the α-position, setting up lysine's unique chemistry among amino acids.
Technical Specifications and Labeling
Manufacturers guarantee lysine content typically above 98%, with moisture levels hovering below 1%. Ash and heavy metal values stay well within food and pharma safety codes. Labels note compliance with standards set by organizations like USP, FCC, and various regional pharmacopoeias. On the warehouse floor, one always checks batch numbers, manufacture dates, and country of origin. The final product usually ships in multi-layer bags or fiber drums, lined with polyethylene to block moisture and contamination. Any break in that seal raises alarms about shelf life and possible regulatory headaches.
Preparation Method
Modern L-Lysine dihydrochloride mainly comes from microbial fermentation, using carbohydrates like corn or sugar beet as feedstock. Genetically optimized bacteria churn out lysine in high concentrations. After fermentation, purification steps remove by-products, and hydrochloric acid converts free lysine into its stable dihydrochloride form. Massive centrifuges and filtration systems do much of this work. Final drying crystallizes the product and ensures purity. This process cuts down the environmental footprint compared to older chemical methods, giving plant operators a chance to meet sustainability goals without sacrificing yield.
Chemical Reactions and Modifications
In lab settings, L-Lysine's ε-amino group provides a strong anchor for modifications. Reagents target this moiety to generate derivatives for medicine, food, or material science. For example, conjugating lysine to peptides, proteins, or polymers enhances solubility or bioavailability, opening doors in drug delivery. In advanced synthesis, chemists protect its α-amino group with common groups like Boc or Fmoc, so they can perform selective reactions without cross-reactivity. This sort of specificity makes lysine a workhorse in peptide synthesis and cross-linking protocols.
Synonyms and Product Names
L-Lysine dihydrochloride itself appears under several names, including Lysine HCl, Lysine hydrochloride, and in some EU catalogues as E640. In scientific literature, you might run into (S)-2,6-diaminohexanoic acid dihydrochloride, especially in research on metabolic pathways. Feed and food industries stick with shorter variants, mixing "L-Lysine HCl" into their formulas and labels. The diversity of names across countries sometimes confuses buyers, but product testing and certificates of analysis keep quality consistent worldwide.
Safety and Operational Standards
Workshops and labs working with L-Lysine dihydrochloride need no heavy-duty protective gear, yet general good practice matters. The fine powder dusts easily; inhalation or eye contact can irritate sensitive individuals. Warehouses keep it far from strong oxidizing agents or moisture. Reputable suppliers provide thorough safety data sheets, covering acute oral toxicity, environmental hazard data, and first aid responses for accidental exposure. Workers undergo regular training, especially in countries where animal nutrition additives face strict regulatory scrutiny. Companies following GMP or FAMI-QS protocols find it easier to pass audits and keep their products in international markets.
Application Area
Livestock nutrition remains the biggest draw for L-Lysine dihydrochloride. Managers of commercial pig and chicken operations use it to keep protein levels in feed at optimal efficiency. Producers lean on lysine to cut back on excess soybean or fishmeal, trimming feed costs and land use. In human health, physicians prescribe lysine supplements for specific deficiencies. Some athletes and bodybuilders tout lysine for muscle growth, though the science runs mixed. Manufacturers use L-Lysine as a flavor enhancer or processing aid in some processed foods; it can even help prevent bread staling. Pharmaceutical labs exploit its solubilizing power when formulating tablets or injectable drugs.
Research and Development
Science circles keep pushing the boundaries on L-Lysine. Fermentation scientists race to build faster, more resilient microbes that squeeze out higher yields with fewer inputs. Synthetic biologists program bacteria to turn waste-based carbohydrates into pure lysine, aiming to close loops in food production. Nutrition researchers probe links between subtle lysine balances and animal welfare, growth, or meat quality. Medical science explores new derivatives as anti-viral agents or delivery tags for novel drugs. In the lab, students keep using protected lysine forms for intricate peptide synthesis, pushing biochemical understanding each step further.
Toxicity Research
Most studies agree that L-Lysine dihydrochloride, in moderation, brings minimal health risk. Problems only show up at grossly elevated intake, far beyond dietary norms. Some animal tests report no measurable carcinogenic or mutagenic effects; chronic toxicity requires massive, sustained dosages. Feed regulators in the EU and US set maximum inclusion rates, reflecting this consensus. Even then, livestock producers find it difficult to accidentally overdose animals, as feed blends get precise composition checks. In personal experience, accidental exposure causes only minor discomfort—nothing beyond eye watering or mild cough.
Future Prospects
Sustainable agriculture promises further growth for L-Lysine dihydrochloride. As climate pressure pushes efficiency, more livestock operations will count on precision amino acid supplementation. Innovations in fermentation could bring down production costs or open up low-waste, circular-economy pathways. In human nutrition, as plant-based diets trend upward, lysine will see renewed value in balancing vegetarian protein blends. Medical applications could blossom too, as biochemists discover new ways to modify the lysine molecule for tailored drug delivery or tissue engineering. The next generation of researchers and nutritionists may find just as much reason to look closely at this humble but impactful compound.
What is L-Lysine Dihydrochloride used for?
More Than Just a Supplement
L-Lysine dihydrochloride doesn’t spark excitement at first glance. Most people never give a second thought to where their food or animal feed gets its extra boost of protein. I've spent years studying the science behind animal nutrition, and I see this amino acid pop up time and again. It shows up most often in feed used for pigs, chickens, and sometimes even cattle. That's no accident. Diets based heavily on grains tend to fall short on lysine, and without enough, animals don't grow well or use their feed as efficiently. You see the difference quickly. Growth slows down, and health troubles start layering up.
From Cornfields to Kitchen Tables
Most cereals miss out on lysine. Farmers, feed companies, and nutritionists learned long ago that supplementing feed with lysine dihydrochloride makes sense. Reports from the Food and Agriculture Organization confirm this—it lets producers decrease the amount of soybean meal without messing with animal performance. It’s efficient, too, and that matters. Feed represents up to 70% of the cost in animal farming. Lysine supports faster growth for less money. That trickles down to the consumer, who benefits from more affordable pork and chicken in the grocery store.
Use in Human Nutrition
People use L-lysine in a handful of ways themselves. It finds a spot in the supplement aisles, mainly in tablets or powders. Some take it hoping to lessen the frequency or severity of cold sores. Plenty of scientific studies suggest lysine hampers the replication of the herpes simplex virus, which causes these outbreaks. My grandmother swore by these tablets during stressful weeks. Whether it’s placebo or actual science, many stand by their routines. It’s also landed in protein shakes and meal replacement powders. Lysine supports muscle repair, making it useful for athletes and those recovering after injury or illness.
Benefits in Food Manufacturing
Food processing companies also rely on lysine to fortify flours and noodles, especially in regions where diets rely heavily on wheat and rice. People who eat mainly plant-based diets sometimes stumble into amino acid deficiencies. Adding lysine helps close that gap. You won’t taste or notice it in the final food, but it increases the protein quality of staples that millions depend on every day.
Potential Pitfalls and Solutions
Nothing rolls out problem-free. Not all lysine supplements come from “natural” sources. Much of the bulk comes from fermentation using genetically engineered bacteria or fungi. That can spook some buyers, especially those wary of genetic modification. Clear labeling and transparent sourcing calm those fears. Another worry I hear about: Over-supplementation. Too much lysine in feed can burden animal kidneys or tip the balance of other amino acids. There’s no substitute for good science and experienced nutritionists who know how to balance these inputs.
Looking Forward
Researchers keep exploring different strains of bacteria and ways to make production less resource-intensive. Tighter regulations help keep quality high. Still, the role of L-lysine dihydrochloride in farming and food won’t fade anytime soon. It sits right in the middle of efforts to produce enough protein for a world that isn’t getting any smaller. Each scoop, tablet, or pellet carries a little more weight than people realize.
Is L-Lysine Dihydrochloride safe for humans and animals?
Why L-Lysine Dihydrochloride Exists in Foods and Supplements
L-Lysine Dihydrochloride isn’t some mystery chemical snuck into food. It starts as lysine, an amino acid that the body craves but can’t make on its own. Anyone who eats, or lives with animals, has probably seen its name pop up somewhere. Feed manufacturers value it as a way to close gaps in diets, and supplement makers use it to support health. Farmers pour it into feed mixtures because pigs and chickens actually need it for growth and muscle. As for people, you’ll spot lysine on vitamin shop shelves, usually pitched as a way to strengthen immunity or help cold sore sufferers.
Science and Safety: What Do the Experts Say?
To be blunt, powdered L-Lysine Dihydrochloride comes from refined production processes, often using fermentation from corn or cane sugar. The FDA classifies lysine as Generally Recognized as Safe (GRAS) for food use. Studies in the Journal of Nutrition or by the European Food Safety Authority point out that lysine has a long history of safe usage at recommended amounts. The World Health Organization even set guidelines for lysine intake decades ago, mostly focused on keeping nutritional gaps filled, especially where diets run short on complete proteins.
Most of us—whether human or animal—don’t overload our systems through regular eating or responsible supplement use. L-Lysine Dihydrochloride just becomes a source of lysine once digested. I remember my veterinarian explaining this when my family’s dog was put on a lysine supplement for eye health. “It’s just giving the body what it gets from good protein anyway, unless you overdo it,” she said. That idea holds up for humans, too: clinical trials using as much as three grams a day for months hardly noted any serious issues. The most common stories involve mild belly upset or running to the bathroom, much like with nearly any excess amino acid. Toxic doses sit far, far above what most folks would ever take by accident—or on purpose.
Animal Feed Additive: More Than Just Safe
L-Lysine Dihydrochloride made feeding livestock more efficient. Cattle, pigs, chickens—these animals once needed huge grain rations, but corn and soy don’t provide the lysine they need. By topping up these feeds, farmers raise healthier herds on less land. I saw a pig operation in Iowa where young animals bounced with energy on lysine-supplemented feed. The National Research Council and dozens of studies report no harmful effects as long as feed follows labeling guidelines. It lets farmers avoid waste and actually improve the nutritional quality of meat and eggs.
What Could Go Wrong?
No supplement is a cure-all. Taking way too much lysine—several times the recommended amount per day—can stress out kidneys or raise cholesterol, but those are rare cases and often involve ignoring label instructions or combining with unrelated medications. Animals may suffer if they eat nothing but powdered lysine and little else, but in the real world, animal nutritionists keep rations balanced. Anyone with kidney problems or special dietary needs should ask their doctor before diving into heavy supplementation.
Finding Balance: Sensible Use and Oversight
L-Lysine Dihydrochloride plays a real role in nutrition, both for people and animals. Its safety profile tracks with decades of research, expert consensus, and widespread use. Like so many nutrients, it earns trust by doing its job quietly, with trouble only showing up at extremes. Reading the label, keeping doses reasonable, and listening to your doctor or vet works for lysine just as it does for most things we put in our bodies—or theirs.
What is the recommended dosage of L-Lysine Dihydrochloride?
L-Lysine Dihydrochloride and Daily Use
L-Lysine Dihydrochloride shows up in many pantries—often in supplement drawers, sometimes in animal feed bags. This amino acid matters for human health. The body can't make it, so everyone has to get it from food or supplements. Most daily requirements often get handled by a diet rich in poultry, red meat, eggs, beans, and dairy. In some health situations, or for folks with limited diets, the position shifts, and supplementing starts making more sense.
Dosage Ranges Backed By Medical Experience
A lot of doctors recommend aiming for around 1,000 mg (1 gram) per day for adults using lysine as a supplement, which research and safety studies support. Some conditions may call for more—for example, research in managing cold sores shows doses of up to 3,000 mg per day in divided servings often used for a short period, usually a week or two. A medical professional should always confirm higher amounts, as excessive intake might cause abdominal discomfort or diarrhea.
Diet keeps playing the lead role. Adults with balanced, protein-rich meals may already meet the World Health Organization’s minimum—about 30 mg per kilogram of body weight per day. For a person weighing about 70 kg (154 pounds), that’s close to 2,100 mg daily. Most people reach this through common foods and only use supplements to fill occasional gaps.
Special Populations and L-Lysine
Some folks—vegans, people with eating disorders, certain athletes—frequently land in shortage territory. In those cases, their health providers often check lysine levels and suggest doses to build up reserves. L-Lysine Dihydrochloride also works in veterinary diets. Swine and poultry feed often includes it to help animals grow strong, as plant-based feeds can lack enough amino acids to support proper development.
What the Science Says About Safety
Sticking to 3,000 mg per day or less appears safe for most healthy adults. Going far above this hasn’t shown added benefits and creates the risk of kidney tension, especially for those who already have kidney concerns. Reports about interactions show lysine can increase absorption of calcium and might raise calcium levels in the blood. People who supplement with both should inform their doctor.
Rarely, some experience cramps or gut issues from lysine supplements. Anyone on medication or with a chronic illness should run it by their clinical team before adding lysine, to avoid trouble. Pregnant and breastfeeding women are best off playing it safe and sticking to food for their needs, unless their OB-GYN recommends supplementing.
How to Make Good Decisions
Anyone considering L-Lysine Dihydrochloride should read labels, choosing trusted brands. Look for GMP-certified supplements to limit the risk of contamination. Dosing sticks best to small, spaced-out servings—too much at once can cause an upset stomach.
As someone who’s experimented with supplements during training and flu season, balance and moderation work better than overdoing it. Real food first, then supplements for real gaps—that approach fits most health needs, whether you’re managing an occasional outbreak or steering through a vegan diet.
Are there any side effects of taking L-Lysine Dihydrochloride?
What L-Lysine Dihydrochloride Does in the Body
L-Lysine dihydrochloride stands out as an essential amino acid supplement, most people know it from its role in collagen production, fighting off cold sores, or simply as something athletes and bodybuilders add to their routine. Lysine isn’t produced naturally by the body, so getting it from diet or supplements matters for health, muscle repair, and immune activity.
Typical Experiences with L-Lysine Dihydrochloride
Many people take L-Lysine dihydrochloride with no problems. I’ve met people (myself included) who’ve added it to their day to boost their protein intake, or to help reduce episodes of cold sores. For most, the experience stops at reading a supplement label or mixing a powder into a shake. No dizziness. No stomach upset. Just an extra boost.
Others haven’t been so lucky. Some users have felt minor stomach issues: belly discomfort, diarrhea, or bouts of nausea. If you’ve eaten a meal before taking lysine, you may be less likely to notice these issues. For folks who already have sensitive digestion, even a moderate dose might lead to stomach cramps.
There’s always a temptation to double up on dosing, especially for those hoping to speed up healing from a stubborn sore or muscle injury. Taking too much, though, can throw levels of other amino acids out of balance, especially arginine, which can lead to more frequent outbreaks of cold sores — the very thing users are trying to avoid. In large amounts, over several weeks, there have been cases of gallstones and kidney problems popping up, likely because lysine is processed by the kidneys and can become hard to clear if intake outpaces what your body needs.
Who Should Be Cautious
Anyone with kidney issues ought to talk to a healthcare professional before trying L-Lysine dihydrochloride. Since the kidneys clear excess amino acids, taking extra can put extra strain on compromised organs. People with lactose intolerance or vegans who get little lysine from their regular food sometimes reach for higher doses, but checking with a dietitian or doctor makes sense, especially if you’re mixing supplements along with other medications. There’s evidence that some antibiotics or drugs for osteoporosis can interact with high lysine levels, lowering absorption or causing unusual side effects like nausea and flushed skin.
Pregnant and breastfeeding women won’t find much evidence of harm at regular dietary levels, but large supplemental doses haven’t been studied well. With most things you swallow, moderation usually wins out over pushing the dose.
Solutions and Safer Use
Read the label. Don’t shoot over the recommended amount. Lysine offers benefits in reasonable quantities, but huge amounts bring diminishing returns and possible problems. Try food sources first — beans, lentils, dairy, lean meats — before going straight for capsules or powders. Listen to your body and stop if your stomach starts sending warning signs.
Doctors, pharmacists, or registered dietitians can guide you if you’re on the fence or dealing with chronic illness. Keeping supplements in perspective is always smart; too many promises with too little information can cause more trouble than help. Looking for better skin, muscle strength, or immunity, there’s rarely a magic bullet in a bottle.
How should L-Lysine Dihydrochloride be stored?
Understanding L-Lysine Dihydrochloride
L-Lysine Dihydrochloride has become a regular ingredient in many feed and supplement formulations. Used widely in animal nutrition and sometimes in food processing, its importance keeps growing alongside demand for high-quality protein sources. With so much riding on this single ingredient, getting its storage right affects quality and safety for every batch down the line.
Why Proper Storage Matters
Anyone who has worked in a feed mill, a warehouse, or even a vet supply shop knows the frustration that comes when a powder cakes or clumps. L-Lysine Dihydrochloride absorbs moisture from the air, which causes it to harden and lose flow properties. Sometimes this clumping also signals a loss of purity, as excessive moisture leads to microbial contamination or chemical breakdown. Keeping this product dry does more than keep the powder loose; it preserves its nutritional value and prevents headaches during formulation.
Years of storing feed additives have taught me that even minor lapses—like leaving a bag open just a bit too long—invite spoilage. The costs of wasted product, rejected batches, or declined performance in feed go beyond the price of the raw material itself. For this reason, using proper storage methods makes a real financial difference over time.
Best Practices for Storage
L-Lysine Dihydrochloride needs a cool, dry place, away from direct sunlight and sources of heat. I keep it away from windows, furnace rooms, and warehouse doors. If stored above 25°C (77°F), clumping occurs more quickly and shelf life drops. Ideally, it should sit at room temperature, around 20°C (68°F), and in cases where things get humid, I run a dehumidifier to keep things under control. According to published guidelines, moisture content above 1% allows for rapid product breakdown. Even silica gel packets thrown into storage drums make a difference.
Packaging plays its part, too. Bags should never sit directly on concrete floors or near walls where condensation forms. Stacking them on clean, dry pallets helps air circulate. In practice, double-bagging, or using thick plastic liners, goes a long way in barrier protection, especially in older warehouses. Keeping bags sealed until use and folding the top tightly after opening prevents air and humidity from getting into the rest of the batch.
Labeling and Inventory RotationEven the most careful storage fails if inventory management slips. Every bag should have a clear manufacture and expiry date. I use a ‘first in, first out’ rule. Older stock goes first. This stops unnoticed degradation in the back row. L-lysine degrades faster once opened, so small containers for routine use beat pouring from a 25kg sack each day.
Looking for Solutions
Warehouses in tropical or coastal regions deal with chronically high humidity. In these places, investing in climate-controlled storage areas makes sense. Some operations use vacuum sealing for long-term storage, though this increases costs. Improved training for warehouse teams prevents mistakes, and regular inspections for moisture and pests keep losses down. In my experience, a routine checklist proves far more effective than occasional deep cleans.
L-Lysine Dihydrochloride goes farther for nutrition and business when these storage practices become habit. Reliable quality starts with the basic details—cool temps, dry conditions, sound packaging, and clear labeling. These lessons apply whether you’re running a global feed operation or managing a small animal clinic’s storeroom shelf.
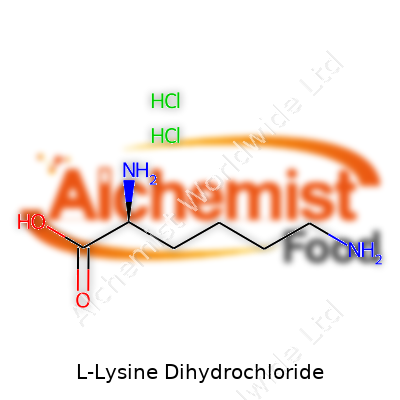
| Names | |
| Preferred IUPAC name | (2S)-2,6-diaminohexanoic acid dihydrochloride |
| Other names |
L-Lysine monohydrochloride L-Lysine HCl L-Lysine hydrochloride 2,6-diaminohexanoic acid monohydrochloride L-Lysine 2HCl |
| Pronunciation | /ˌelˈlaɪsiːn daɪhaɪˈdrɒklaɪd/ |
| Preferred IUPAC name | (2S)-2,6-diaminohexanoic acid dihydrochloride |
| Other names |
L-Lysine·2HCl L-Lysine hydrochloride L-Lysine dihydrochloride L-2,6-Diaminohexanoic acid dihydrochloride |
| Pronunciation | /ˌelˈlaɪsiːn daɪˌhaɪdrəˈklɔːraɪd/ |
| Identifiers | |
| CAS Number | 657-27-2 |
| Beilstein Reference | 1720807 |
| ChEBI | CHEBI:86663 |
| ChEMBL | CHEMBL1201478 |
| ChemSpider | 14022 |
| DrugBank | DB11353 |
| ECHA InfoCard | 38e7b779-dc5e-4cb1-aa44-8996910e6253 |
| EC Number | 205-758-4 |
| Gmelin Reference | 15803 |
| KEGG | C00463 |
| MeSH | D019215 |
| PubChem CID | 6093227 |
| RTECS number | OJ0700000 |
| UNII | HN5X4J0HVA |
| UN number | UN3335 |
| CAS Number | 657-27-2 |
| Beilstein Reference | 1712616 |
| ChEBI | CHEBI:64261 |
| ChEMBL | CHEMBL1201352 |
| ChemSpider | 19651 |
| DrugBank | DB00114 |
| ECHA InfoCard | 06e2c622-1c29-4dda-98d7-920209249360 |
| EC Number | 205-750-7 |
| Gmelin Reference | 71999 |
| KEGG | C00250 |
| MeSH | D008232 |
| PubChem CID | 162112 |
| RTECS number | OJ6300000 |
| UNII | 6Z9F85SRIG |
| UN number | UN3332 |
| CompTox Dashboard (EPA) | DTXSID3040003 |
| Properties | |
| Chemical formula | C6H16Cl2N2O2 |
| Molar mass | 182.65 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.28 g/cm3 |
| Solubility in water | Freely soluble in water |
| log P | -6.2 |
| Acidity (pKa) | 9.59 |
| Basicity (pKb) | 9.06 |
| Magnetic susceptibility (χ) | -35.2·10⁻⁶ cm³/mol |
| Viscosity | Viscous powder |
| Dipole moment | 2.4 D |
| Chemical formula | C6H16N2O2·2HCl |
| Molar mass | 182.65 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | Density: 0.854 g/cm³ |
| Solubility in water | Freely soluble in water |
| log P | -4.1 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10.79 |
| Basicity (pKb) | 7.9 |
| Magnetic susceptibility (χ) | -9.5×10^-6 cm³/mol |
| Dipole moment | 1.14 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 176.0 J/(mol·K) |
| Std enthalpy of formation (ΔfH⦵298) | −552.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4165.6 kJ/mol |
| Std molar entropy (S⦵298) | 181.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1266.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4036.2 kJ/mol |
| Pharmacology | |
| ATC code | A16AA08 |
| ATC code | A16AA21 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. |
| GHS labelling | GHS07, Warning, exclamation_mark |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| Precautionary statements | Store in a dry place. Store in a closed container. Avoid breathing dust. Wash hands thoroughly after handling. Do not eat, drink or smoke when using this product. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 4,670 mg/kg |
| NIOSH | NIOSH: *MI5950000* |
| PEL (Permissible) | PEL: 15 mg/m³ |
| REL (Recommended) | 1500 mg |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS irritant, Warning, H315, H319, H335 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Use with adequate ventilation. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 5,000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat) = 4,980 mg/kg |
| NIOSH | SN4320000 |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 1500 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
L-Lysine L-Lysine Monohydrochloride L-Lysine Sulfate L-Lysine Acetate L-Lysine Hydrochloride |
| Related compounds |
L-Lysine L-Lysine Monohydrochloride DL-Lysine L-Lysine Sulfate L-Lysine Acetate L-Lysine Hydrochloride L-Lysine Monohydrate |

