L-Cysteine Monohydrochloride: Science, Safety, and the Road Ahead
Historical Development
People have worked with amino acids for a long time, but L-Cysteine Monohydrochloride’s story stands out. Chemists first isolated cysteine from animal horns back in the 19th century, and the compound quickly found a role in biochemistry. It wasn’t always easy to produce commercially. For decades, manufacturers depended on extraction from natural sources. Once fermentation and chemical synthesis grew more reliable in the 1970s and 80s, L-Cysteine Monohydrochloride began showing up in pharmaceuticals and food processing on a global scale. Somewhere along the way, ethical supply and synthetic manufacture entered the conversation. Today’s production houses tend to lean on microbiological fermentation, thanks to pressure from consumers and regulators wanting less reliance on animal parts.
Product Overview
L-Cysteine Monohydrochloride shows up today in a white crystalline powder, usually shipped in heavy-duty bags for food and pharmaceutical manufacturers. The hydrochloride form changes properties and makes the compound more stable. This simple tweak turns a reactive amino acid into a manageable ingredient that travels well and blends into production lines. On a day-to-day level, food technologists and pharmacists use this form to keep tasks consistent—think leavening bread, prepping parenteral nutrition solutions, or formulating high-performance hair care.
Physical & Chemical Properties
With a molecular formula of C3H7NO2S·HCl, this compound is water-soluble and melts at about 175°C (decomposition may kick in a bit lower). The pure powder looks almost snow-white, but moisture can make it clump or yellow over time. In storage, it calls for dry, cool spaces to avoid degradation. The smell isn’t overpowering, though some people notice a sulfur-like note, which makes sense given the presence of a thiol group. The hydrochloride makes it less volatile and a bit easier to handle in the lab, especially when precision matters for downstream applications.
Technical Specifications & Labeling
Labels on L-Cysteine Monohydrochloride packaging usually point to purity standards, heavy metals checks, and microbiological specs. Pharma-grade material runs super-high purity—sometimes over 99 percent. Food-grade specs accept a bit more by-product, but still stick with tight controls on lead, arsenic, and microbial counts. Most products arrive marked by their USP, JP, or EP compliance. For the finest work in medicine or injectables, companies reference sterile lots and batch traceability from raw material to finished product. Labelling regulations lean on global food and drug standards, and traceability measures now stretch down to the non-animal sources preferred by most Western and halal/kosher markets.
Preparation Method
Producers usually opt for microbial fermentation these days, often relying on E. coli or similar bacteria genetically primed to churn out L-Cysteine. After growing the cultures, technicians isolate and purify the product, before reacting L-Cysteine with hydrochloric acid to get the monohydrochloride. This avoids animal sources and reduces issues around contaminants. Some manufacturers still use extraction from feathers, hair, or other keratin-rich sources—a method dating back over a century—but supply-chain scrutiny and consumer expectations keep the microbial route in the lead.
Chemical Reactions & Modifications
L-Cysteine Monohydrochloride’s reactive thiol group makes it a lightning rod for chemical modification. Food technologists love how it can reduce gluten bonds in baked goods, softening dough, boosting elasticity, and speeding up mixing—big help in industrial bread labs. It also finds work in reduction reactions, sometimes acting as an antioxidant in cell culture or holding vitamin blends stable. Manufacturers can derivatize the amino group too, which opens up more applications in peptide synthesis. In my own work with lab materials, I’ve seen it used as a building block for peptide coupling—its reactivity is both a plus and a challenge if side chains aren’t protected.
Synonyms & Product Names
Over the years, L-Cysteine Monohydrochloride traveled under plenty of names: 2-amino-3-mercaptopropionic acid monohydrochloride, L-Cysteine HCl, and Cysteine hydrochloride, just to name a few. Chinese supply chains sometimes refer to it as Cysteine Base Hydrochloride, and different grades make it to warehouses as “food additive E920” or as pharmaceutical substance for intravenous nutrition. End users scan for any of a dozen synonyms on spec sheets, especially when comparing international suppliers.
Safety & Operational Standards
Handling amino acids like this one comes with specific rules. Dust can irritate workers’ lungs and eyes if inhaled, so staff don gloves and masks for any large-scale processing. Material safety data sheets focus on avoiding ingestion and eye contact, good ventilation, and containment protocols for spills. In food and pharma production, plant audits track cleaning cycles and cross-contamination, especially when dealing with allergen-free lines. European and US regulators issue strict standards for contaminants—lead, arsenic, sulfates—so clean processing pays off in every sector. In research environments, one learns to keep stocks dry and sealed, since humidity can ruin the powder’s flow and shelf life.
Application Area
Bakers often rely on L-Cysteine Monohydrochloride to speed up dough work, making loaves rise better and crumb turn tender—this cuts batch times and saves labor. Nutritional formulations use it in intravenous and oral supplements for patients who cannot eat solid food, especially when the body’s glutathione needs a boost. Dermatologists value it for skin and hair repair formulas, because it supports keratin and antioxidant balance. Biotech scientists put it to work in cell cultures, stabilizing vitamins and acting as an antioxidant under stress. Across all these groups, consistent product quality makes the difference between success and major batch failures. I’ve seen food labs grind to a halt when a shipment was off-spec, showing how ingredient integrity directly affects whole industries.
Research & Development
The R&D push in L-Cysteine Monohydrochloride centers on purity, traceability, and reducing costs. Teams chase down more efficient fermentation strains, trying to produce bigger yields from less feedstock. Chemists are mapping out sustainable pathways that minimize waste and cut energy drain during purification. Some recent progress involves synthetic biology—using engineered bugs to generate not just cysteine, but related amino acids or specialty peptides as well. Consumer pressure also encourages work on vegan and non-GMO sourcing, which sends labs searching for new microbial platforms. In medicine, researchers are looking at L-Cysteine’s role in redox regulation and disease management, hoping to develop next-gen therapies from what was once seen as just a food additive.
Toxicity Research
Most evidence shows L-Cysteine Monohydrochloride to be safe when handled appropriately in food and drug settings, though high concentrations may cause nausea or gastrointestinal distress. In rodent studies, extremely high doses led to liver changes and metabolic shifts, underscoring the need for careful dosing. Industrial hygiene programs flag the dust inhalation risk for workers, pushing for engineering controls or personal protective equipment. Over the years, public concern has sometimes questioned the ingredient’s sourcing, especially in products destined for vegetarians or certain religious groups, but most health authorities support current use levels as safe for humans when compliant with international standards.
Future Prospects
Looking ahead, L-Cysteine Monohydrochloride’s roadmap veers toward better sustainability and broader function in both food systems and biomedicine. Innovations in microbial fermentation will likely drop production costs and lower carbon footprints. Biotech outfits expect to tailor amino acid profiles for customized nutrition, pivoting cysteine from a background player into a key health supporter for aging populations and patients with chronic conditions. With ongoing regulatory scrutiny and public awareness about ingredient provenance, transparent sourcing and green chemistry will matter even more across the next decade. New research linking L-Cysteine to antioxidant pathways and cell health suggests that medical and personal care products could expand even further. All these changes depend on honest, science-forward collaboration between researchers, manufacturers, and the folks actually using these compounds, whether they’re baking a loaf of bread or mixing hospital nutrition drips.
What is L-Cysteine Monohydrochloride used for?
What Sets L-Cysteine Monohydrochloride Apart
L-Cysteine Monohydrochloride keeps popping up on food labels, but that’s not where the story ends. This amino acid plays a role in both the food industry and medicine. In bakeries, it turns out to be a go-to ingredient for dough conditioners. A lot of us have eaten bread and not realized L-Cysteine has helped create that soft, stretchy texture in the loaf. The reason it matters is simple—yeast alone can’t give commercial bread the same shelf life or silkiness people expect. With bread on kitchen tables almost every day, the right conditioning keeps waste low and consistency high.
Industrial and Food Applications
Ask anyone who has worked behind the scenes in a big bakery: L-Cysteine Monohydrochloride helps keep production reliable. It breaks down gluten, making dough easier for machines to handle. As a result, bread, pizza, and pastries rise evenly. Restaurants, school cafeterias, even frozen food manufacturers rely on this amino acid to prepare dough quickly, especially where mass production rules. The applications don’t stop there. Snack foods like crackers and tortillas get the same treatment.
Besides improving texture, L-Cysteine cuts down mixing time. Bakeries save money by making batches faster. Businesses juggling high volumes during busy periods depend on ingredients that keep production efficient. Even at home, anyone baking with cheap, ready-mix dough knows the difference between soft, bendy bread and rock-hard leftovers.
Health and Pharmaceutical Benefits
In medicine, L-Cysteine plays a more serious role. Hospitals use it to make acetylcysteine, a common drug for treating paracetamol overdoses and chronic lung disorders. Cysteine helps break down mucus, making it easier to clear airways. Doctors trust it because the science checks out—patients with cystic fibrosis feel relief with its use. The World Health Organization includes acetylcysteine in its list of essential medicines. Pharmaceutical companies also include this compound in nutritional supplements for people who can’t get enough amino acids from food.
A healthy body builds cysteine naturally, but certain illnesses, high stress, or a poor diet drain reserves. Supplements aim to help in those cases, sometimes under medical supervision. Scientific studies have linked cysteine’s antioxidant properties to lower levels of cell damage, which ties back to healthier aging.
Concern over Sourcing and Labeling
Talk about this ingredient gets complicated once people learn some manufacturers source it from duck feathers or pig bristles. That raised flags for vegetarians, vegans, and others with religious dietary requirements. For a long time, labels left out details about the animal source. Groups raised awareness, pushing for clear sourcing information and alternatives made by fermenting plants or microbes. Now, major food producers in parts of Europe and the United States have listened, switching to non-animal sources where possible.
Consumers pay closer attention to ingredient lists. Parents with children who have severe allergies or dietary restrictions can avoid unexpected animal products. For people following kosher or halal rules, certification gives much-needed peace of mind.
Looking at Solutions
With food technology advancing quickly, alternative sources become more cost-effective. Manufacturers who move to plant-based fermentation can appeal to a broader audience. Clear labeling builds trust and avoids consumer backlash. In medicine, further clinical studies continue to prove safety and develop more targeted treatments using L-Cysteine. As someone who watches food trends, I’ve noticed brands that take clear stances on ingredients and transparency often build a loyal following. People want to know what they’re eating and trust matters more than ever.
Is L-Cysteine Monohydrochloride safe for consumption?
Understanding L-Cysteine in Everyday Foods
Walk through any grocery store aisle and pick up a loaf of bread or a pack of crackers. Scan the ingredient list, and L-Cysteine Monohydrochloride might catch your eye. This amino acid pops up in many bakery products, serving as a dough conditioner. Most folks rarely think about where this ingredient comes from or what it does. I did not either, until I started reading more ingredient lists. For me, curiosity always leads to questions about food safety.
Sourcing and Use in Industry
L-Cysteine occurs naturally in protein-rich foods like eggs, chicken, and dairy. In food processing, manufacturers add a synthetic version to improve dough texture and shelf life. Years ago, sourcing methods raised eyebrows. Companies often extracted L-Cysteine from human hair or poultry feathers. These days, the industry is shifting to microbial fermentation using plant materials and sugar. Some providers label their L-Cysteine as vegan or plant-based, a clear sign that sourcing matters to consumers.
Looking at the Research and Safety Data
The Food and Drug Administration includes L-Cysteine Monohydrochloride on its GRAS (Generally Recognized As Safe) list. The European Food Safety Authority and food agencies in Japan and Australia have reviewed this compound as safe, within the limits allowed in food applications. Studies show that healthy adults process small amounts of L-Cysteine without trouble. This amino acid already exists in many proteins we eat every day, and our bodies use it for essential processes. High intake — far more than you’d get in food products — can lead to stomach pain or nausea, but packaged foods typically contain much less than that threshold.
Ethical Concerns and Transparency
Some concerns come not from safety, but from ethics and sourcing. If you follow strict dietary laws or are vegan, the origin of food additives matters. This ingredient once came from sources that did not align with everyone’s dietary beliefs. Calls for transparency and clearer labeling grew louder as more people started to care about what goes into their food. Today, several producers make vegan L-Cysteine, and more products include sourcing details on packaging.
Potential Solutions for Greater Consumer Confidence
Food companies have a responsibility to keep ingredient sourcing safe and ethical. Improved communication can address uncertainty. Clear labeling about both function and origin goes a long way — especially for people with dietary restrictions. Regulatory oversight and routine safety reviews keep food manufacturing practices in check. Retailers and brands should strengthen supplier audits and traceability, so consumers do not have to guess what they are eating. For those with allergies or health concerns, consulting a healthcare provider before trying new food ingredients makes sense.
Balancing Science and the Food Experience
Safe consumption of ingredients like L-Cysteine Monohydrochloride depends on both science and transparency. With more people scrutinizing labels and expecting honest sourcing, the conversation around food additives keeps evolving. Respect for consumer choice and clear, reliable safety data help everyone make better decisions at the table. From my own experience, learning about ingredient origins opened the door to more mindful eating and sparked conversations about what matters most in food.
What is the recommended dosage of L-Cysteine Monohydrochloride?
Understanding L-Cysteine Monohydrochloride
L-Cysteine Monohydrochloride has carved a place in supplement bottles, fortified foods, and even specialty bakery products. It’s an amino acid derivative, often talked about for supporting hair, nail, and skin health, sometimes tied to antioxidant effects, or used in dough conditioning. With all the buzz, many people want the magic number: how much should folks actually be taking?
Approaching Dosage the Right Way
Few things in life come with a one-size-fits-all label—nutrition is no exception. The recommended dosage for L-Cysteine Monohydrochloride usually falls in a range. Most supplement manufacturers point to daily intakes between 200 mg and 600 mg for adults. You won’t find standard recommended dietary allowances for L-Cysteine like you do for vitamins such as C or D. Research studies and food regulations help fill that gap, but many factors push and pull what’s safe and useful: age, body weight, health status, and the reason someone’s looking to use it in the first place.
Pharmacies, nutritionists, doctors, and food regulatory agencies all play roles in shaping guidance. For example, the Food and Drug Administration in the U.S. lists L-Cysteine as "Generally Recognized As Safe" when used in food, but they don’t publish specific upper limits for daily intake from supplements. Some studies highlight up to 1,200 mg per day without significant side effects in adults, while others suggest staying well below that unless under medical supervision. The margin for L-Cysteine is wider than, say, iron or vitamin A, but more is not always better.
Why Dosage Matters
Experience matters here. I’ve seen people assume supplements are harmless just because they’re easy to buy or promise natural benefits. It’s tempting to toss in an extra capsule or two. But L-Cysteine, like many amino acid supplements, can put unnecessary strain on kidneys or cause digestive upset in sensitive people at high doses. There’s also a chance of interactions with medications or with conditions like cystinuria. Chronic overuse makes things worse for those already dealing with kidney or liver problems.
Factoring in Diet and Safety
Most people eating a balanced diet get enough cysteine and similar amino acids from chicken, eggs, dairy, and legumes. Supplementation often fits best for those with special health needs—like certain metabolic or absorption issues—or under professional recommendation.
Checking with a healthcare provider before starting daily supplementation looks like the most sensible step. For healthy adults curious about supporting immune defenses or hair growth, starting at the lower end—200 to 300 mg per day—and paying attention to any body signals such as nausea or headaches stays safest. Adjusting as needed, based on guidance from someone with clinical training, goes further than copying what works for a friend or internet post.
Making Sense of Product Labels
Many supplement bottles block out the fine print when it comes to purity, fillers, and additives. Reputable brands list L-Cysteine Monohydrochloride, serving size, and recommended dosage clearly. Certifications like USP or NSF show the product passed third-party testing for identity and purity.
No single supplement can take the place of whole diet and lifestyle. L-Cysteine Monohydrochloride can play a role for some, but moderation, professional advice, and sourcing from brands that value safety over hype make all the difference.
Are there any side effects of L-Cysteine Monohydrochloride?
Ingredients on Labels: What’s in the Food You Eat
The ingredients list on food packages often raises questions, especially when a name is long and scientific. L-Cysteine Monohydrochloride turns up in bakery goods, processed foods, and some supplements. It helps soften dough, making commercial bread fluffy and ready in less time. Plenty of folks spot it and wonder, “Is this safe? Are there side effects I need to know about?” I’ve seen this question asked in online forums and health groups, and I decided to dig into the facts.
Digestive Health: Upset Stomach, Nausea, and More
Common side effects related to L-Cysteine Monohydrochloride stem from its sulfur content. People with sensitive stomachs or those already taking other amino acid supplements sometimes feel queasy, deal with mild bloating, or get diarrhea. I’ve spoken with bakers who’ve handled bulk powders, and some say the smell alone is enough to cause mild nausea. The FDA lists it as “generally recognized as safe”—but too much of any additive has the potential to throw the gut off balance.
Allergy Concerns: Who Should Be Cautious
L-Cysteine can come from different sources. Plenty of it today is produced synthetically or via fermentation, but older production methods often relied on feathers or even human hair. That surprises many vegetarians and vegans. The risk for allergy mainly hits folks with sensitivities to sulfur compounds or additives. Breathing dust from the powdered form, especially in industrial bakeries, can aggravate asthma or trigger sinus irritation. I remember an acquaintance with chronic allergies who had to switch to a different baking powder after reacting to products containing L-Cysteine. It’s not common, but it does happen.
Big Doses: Effects Beyond Baking
At the doses used in food, most people don’t report problems. High doses in supplements raise other concerns. I checked published papers on cysteine supplements and high intake can sometimes cause headaches, fatigue, or a sulfur-like body odor. Doctors sometimes warn those with kidney or liver conditions to limit extra amino acid intake, as overloading these organs with anything—protein, amino acids, or even vitamins—can add stress. L-Cysteine plays a role in the body’s antioxidant system, but more doesn’t always equal better.
Safe Use and Label Reading
For most, grabbing bread or bakery goods with L-Cysteine virtually guarantees safe consumption. The real issue kicks in for those managing allergies, chronic health issues, or who simply want to limit intake of additives. The European Food Safety Authority and FDA review the data and set guidelines, but personal thresholds and reactions always vary. My own approach involves reading ingredient labels carefully, especially for friends with allergies. I take the time to call the bakery about sourcing if the ingredient list looks murky.
Improving Food Safety and Awareness
Clear labeling makes a world of difference. Food companies could do more to notify customers about the origins of complicated ingredients—synthetic or animal-derived. Better awareness means fewer allergy scares and more trust in the brands we choose. Doctors and dietitians encourage asking questions and reporting side effects, since only open feedback keeps the system honest and responsive.
Is L-Cysteine Monohydrochloride suitable for vegetarians and vegans?
Facing the Ingredient List: More Than Just a Name
Grocery shopping gets complicated these days, especially for anyone who pays close attention to what’s in their food. L-Cysteine Monohydrochloride pops up on ingredient lists for breads, pastries, and processed foods. At first glance, it looks like chemistry class. Still, nobody wants to eat something that clashes with their values or dietary choices.
Where L-Cysteine Comes From Makes All the Difference
L-Cysteine Monohydrochloride acts as a dough conditioner. Bakeries love it because bread rises better and stays soft longer on the shelf. The trouble begins with how L-Cysteine is made. Factories can produce it in labs or extract it from sources like human hair, duck feathers, or swine bristles. Human hair and feathers contain a lot of cysteine, so industrial manufacturers chased after the cheapest, most plentiful supply.
Hard facts reveal that until recently, most major L-Cysteine production relied on human hair collected from barbershops in China or elsewhere. Over time, supply shifted, and now duck feathers claim a big share in the market. Many people feel uncomfortable about that, even if nobody ever tastes the ingredient itself in the finished loaf of bread.
Plant-Based Methods: Slow Spread, Big Opportunity
Demand for plant-based ingredients keeps rising. More companies now offer “vegan L-Cysteine” using fermentation with bacteria fed on non-animal sources, such as corn glucose. This approach sidesteps animal products, creating possibilities for bread and snack manufacturers to go animal-free. Yet, real-world choices at the store often lag behind the hype. Not every bakery, pizzeria, or snack brand has made the switch.
Some food makers clearly label if they use vegan or synthetic L-Cysteine, but not all. Legally, the label only has to show the compound name—not the source. This makes things tricky for vegetarians and vegans looking to avoid animal ingredients. Emails to big bread brands don’t always get a straight answer. Some companies keep details under wraps, pointing to trade secrets or cost.
Why It Matters: Ethics, Beliefs, and Health
Choosing between animal-based or synthetic L-Cysteine turns into a question of principles, transparency, and health. Massive numbers of people aim to avoid animal suffering on ethical grounds, yet hidden ingredients can derail even the most careful shopper. For some, kosher or halal status gets thrown into the mix—animal origin can also mean these products get ruled out.
The impact ripples out. According to Mintel, plant-based launches in food products have tripled in a decade. Consumers rely on accurate information. The path from factory to plate should not hide animal inputs under scientific names. Better label rules, honest answers from food-makers, and more affordable plant-based choices could untangle this mess.
What Works: Making the Right Call
Direct calls to bakeries, reading up on favorite brands, or leaning on small-batch producers known for transparency help. Some bakeries already bake with only plant-based ingredients, skipping L-Cysteine entirely. Natural fermentation with longer proofing times can replace chemical shortcuts for better texture and flavor. Anyone aiming for a vegan or vegetarian diet has a right to know the facts. Until every label spells out the difference, asking questions and sharing information remains the best tool in the consumer’s hands.
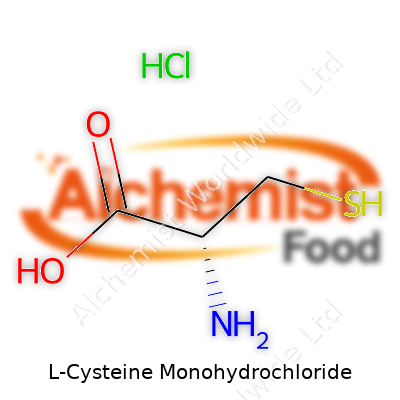
| Names | |
| Preferred IUPAC name | (2R)-2-amino-3-sulfanylpropanoic acid;hydrochloride |
| Other names |
L-Cysteine HCl Monohydrate 2-Amino-3-mercaptopropanoic acid monohydrochloride Cysteine hydrochloride monohydrate Cysteine HCl L-Cysteine hydrochloride hydrate |
| Pronunciation | /ˌɛlˈsɪstiːn ˌmɒn.oʊˌhaɪdrəˈklɔːraɪd/ |
| Preferred IUPAC name | (2R)-2-amino-3-sulfanylpropanoic acid;hydrochloride |
| Other names |
L-Cysteine hydrochloride monohydrate Cysteine HCl monohydrate L-2-Amino-3-mercaptopropanoic acid hydrochloride monohydrate L-Cysteine HCl·H2O |
| Pronunciation | /ɛl-sɪsˈtiː.iːn ˌmɒn.oʊˌhaɪ.drəˈklɔː.raɪd/ |
| Identifiers | |
| CAS Number | 52-89-1 |
| 3D model (JSmol) | `3D model (JSmol)` string for **L-Cysteine Monohydrochloride** (C3H8ClNO2S) is: ``` C[C@H](S)C(=O)N.Cl ``` |
| Beilstein Reference | 82028 |
| ChEBI | CHEBI:61344 |
| ChEMBL | CHEMBL1201472 |
| ChemSpider | 14021 |
| DrugBank | DB00141 |
| ECHA InfoCard | 03d1fe62-477d-431c-b053-4376f6cf1c32 |
| EC Number | 205-204-5 |
| Gmelin Reference | 76474 |
| KEGG | C00283 |
| MeSH | D-Cysteine |
| PubChem CID | 61153 |
| RTECS number | WL1400000 |
| UNII | 66Y4TH14QV |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID1020183 |
| CAS Number | 52-89-1 |
| 3D model (JSmol) | `3D model (JSmol)` string for **L-Cysteine Monohydrochloride**: ``` CSCC(N)C(=O)O.Cl ``` |
| Beilstein Reference | 3569531 |
| ChEBI | CHEBI:40937 |
| ChEMBL | CHEMBL1201521 |
| ChemSpider | 79913 |
| DrugBank | DB00141 |
| ECHA InfoCard | 100.007.756 |
| EC Number | 200-157-7 |
| Gmelin Reference | 3917 |
| KEGG | C00283 |
| MeSH | D-Cysteine |
| PubChem CID | 61171 |
| RTECS number | WLQ7000000 |
| UNII | 78XXS39WSI |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID9062452 |
| Properties | |
| Chemical formula | C3H8ClNO2S |
| Molar mass | 175.63 g/mol |
| Appearance | White crystalline powder |
| Odor | Faint odor of hydrochloric acid |
| Density | Density: 1.36 g/cm³ |
| Solubility in water | Freely soluble in water |
| log P | -3.2 |
| Acidity (pKa) | -1.71 |
| Basicity (pKb) | -0.5 |
| Magnetic susceptibility (χ) | -46.7×10⁻⁶ cm³/mol |
| Dipole moment | 1.14 D |
| Chemical formula | C3H8ClNO2S |
| Molar mass | 175.63 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 0.74 g/cm³ |
| Solubility in water | Freely soluble in water |
| log P | -2.5 |
| Vapor pressure | Negligible |
| Acidity (pKa) | -1.71 (pKa1), 8.33 (pKa2), 10.78 (pKa3) |
| Basicity (pKb) | -0.5 (for 25 °C, water) |
| Magnetic susceptibility (χ) | -53.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.620 |
| Dipole moment | 2.14 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 157.3 J K⁻¹ mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -537.1 kJ/mol |
| Std molar entropy (S⦵298) | 221.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -210.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1170.3 kJ/mol |
| Pharmacology | |
| ATC code | A16AA17 |
| ATC code | A16AA02 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid breathing dust. Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. In case of contact, flush with plenty of water. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | 300°C |
| Lethal dose or concentration | LD50 (oral, rat): 1890 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 1890 mg/kg |
| NIOSH | KM2975000 |
| PEL (Permissible) | PEL (Permissible) of L-Cysteine Monohydrochloride: Not established |
| REL (Recommended) | 30 mg/kg |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H302 Harmful if swallowed. H319 Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid breathing dust. Wash thoroughly after handling. Use personal protective equipment as required. Avoid release to the environment. |
| NFPA 704 (fire diamond) | NFPA 704: 2-0-0 |
| Autoignition temperature | 300°C |
| Lethal dose or concentration | LD50 (oral, rat): 1890 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral >3980 mg/kg |
| NIOSH | KM2975000 |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 30 mg/kg bw |
| Related compounds | |
| Related compounds |
Cysteine N-Acetylcysteine L-Cystine DL-Cysteine S-Carboxymethyl-L-cysteine |
| Related compounds |
Cysteine L-Cysteine DL-Cysteine N-Acetylcysteine Cystine Homocysteine Glutathione Methionine |

